Zdunkowski W., Trautmann T., Bott A. Radiation in the atmosphere: A course in Theoretical Meteorology
Подождите немного. Документ загружается.

7.7 Problems 275
radiation use
B =
κσT
4
π
with κ = 0.25, σ = 5.6697 × 10
−8
Wm
−2
K
−4
.
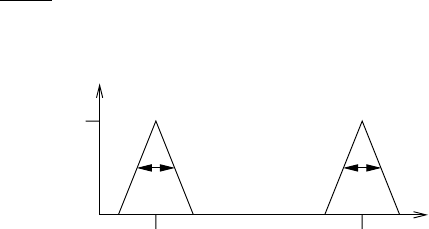
7.14: Consider two idealized lines in triangular form as shown in the figure.
,max
αα
k
ν
k
ν
ν
1
ν
2
ν
The center lines are located at ν
1
and ν
2
. The distance between the lines
exceeds the half-width. (Note that here the half-width is twice as large
as in the usual definition.) Find an analytic representation of the spectral
absorption coefficient, the equivalent width W
1
, W
2
of each line and the
equivalent width W of the line spectrum. Moreover, consider the limiting
cases u → 0 and u →∞.
7.15: In an isothermal atmospheric layer an absorbing gas is homogeneously
distributed, that is the concentration c
gas
is constant. Calculate the equivalent
width of a spectral line for a vertical path in the region of the weak line
approximation. Assume the Lorentz line shape.
8
Absorption by gases
8.1 Introduction
In this chapter we are going to discuss some of the more elementary ideas in
connection with the absorption spectra of gases. The energy E of a molecule may
be expressed as the sum of the rotational energy E
rot
, vibrational energy E
vib
and
electronic energy E
el
. Of these three types of energy, E
rot
is generally the smallest,
typically a few hundredths of an electron Volt.
1
Vibrational energies are of the order
of a few tenths of one electron Volt, while the largest energies are of the electronic
type which generally amount to a few electron Volts.
The absorption (emission) spectrum arising from the rotational and vibrational
motion of a molecule which is not electronically excited will be located in the
infrared region. In infrared absorption experiments light from a suitable source
penetrates an absorption chamber containing the gas to be studied and then enters a
spectrograph. If the instrument is of low resolving power, a series of wide bands is
observed which correspond to the vibrational transitions. If an instrument of high
resolving power is used, these bands are seen to consist of numerous spectral lines
resulting from the energy levels of rotation.
In the next section we are going to discuss the vibrational motion of two relatively
simple molecules (CO
2
and H
2
O) which are particularly important in the study
of radiative transfer in the atmosphere. The forces between the atoms making
up the molecule may be crudely approximated by forces exerted by weightless
springs which hold the atoms relative to each other in the neighborhood of certain
configurations. The forces due to stretching or compression of the springs are
assumed to follow Hooke’s law.
If a molecule contains n atoms, there are 3n modes of motion. Of these, three
correspond to translation, and three to rotation (or two for a linear molecule). The
1
If an electron falls through a potential difference of one Volt it attains a kinetic energy of 1.602 × 10
−19
joule
which is used as the definition of one electron volt, i.e. 1 electron volt = 1.602 ×10
−19
joule.
276

8.2 Molecular vibrations 277
x
1
x
2
Fig. 8.1 Two coupled harmonic oscillators with equilibrium positions at x
1
= 0,
x
2
= 0.
remaining 3n − 6 (or 3n − 5) correspond to the normal vibrational modes of the
molecule.
8.2 Molecular vibrations
8.2.1 Two coupled harmonic oscillators
Before studying the vibrational motion of the carbon dioxide and water molecules,
we are going to discuss a fairly simple example by considering two particles, each
of mass m, connected by light springs of stiffness k, see Figure 8.1. The particles
are constrained to move in a straight line. The distances x
1
and x
2
stand for the
displacements of particles 1 and 2 from their equilibrium positions.
The equation of motion of each particle can be obtained quite easily by using
Lagrange’s equation of motion. The Lagrangian function L is defined as the dif-
ference of the kinetic energy K and the potential energy V of the system, that is
(a) L = K − V
(b) K =
1
2
m
˙
x
2
1
+
1
2
m
˙
x
2
2
(c) V =
1
2
kx
2
1
+
1
2
k(x
1
− x
2
)
2
+
1
2
kx
2
2
(8.1)
The second term on the right-hand side of (8.1c) refers to the potential energy
stored in the spring connecting the two masses. If q
k
and
˙
q
k
stand for the generalized
coordinate and the generalized velocity of the particles, then Lagrange’s equation
of motion of a conservative system is given by
d
dt
∂ L
∂
˙
q
k
=
∂ L
∂q
k
(8.2)
Substituting for q
k
= x
1
, x
2
equation (8.1) into (8.2) yields the equations of motion
for the two particles
d
dt
∂ L
∂
˙
x
1
=
∂ L
∂x
1
or m
¨
x
1
=−kx
1
+ k(x
2
− x
1
)
d
dt
∂ L
∂
˙
x
2
=
∂ L
∂x
2
or m
¨
x
2
=−kx
2
+ k(x
1
− x
2
)
(8.3)

278 Absorption by gases
Let us find the possible common frequencies of vibration of the two particles.
These frequencies are known as eigenfrequencies. The associated vibrational states
are the corresponding eigenvibrations or normal modes of vibrations. We are going
to solve the system (8.3) by means of the trial solutions
x
1
= A
1
cos ωt, x
2
= A
2
cos ωt (8.4)
which requires that both particles vibrate with the frequency ω. Had we used a sine
function or the combination of a cosine and a sine function we would still obtain the
same equations expressing the conditions for the frequency. Substitution of (8.4)
into (8.3) gives two linear homogeneous equations for the amplitudes A
1
and A
2
(−mω
2
+ 2k)A
1
− kA
2
= 0
−kA
1
+ (−mω
2
+ 2k)A
2
= 0
(8.5)
Nontrivial solutions for the amplitudes exist only if the determinant of the coeffi-
cients vanishes. The expansion of the determinant results in the frequency equation
−mω
2
+ 2k −k
−k −mω
2
+ 2k
= (−mω
2
+ 2k)
2
− k
2
= 0 (8.6)
The positive roots
ω
1
=
%
3k
m
, ω
2
=
%
k
m
(8.7)
are the eigenfrequencies of the system.
In order to get some idea about the type of the normal vibrations, we substi-
tute (8.7) into the system (8.5) and find the conditions for the symmetric and the
antisymmetric modes
antisymmetric mode: A
1
=−A
2
for ω
1
=⇒ x
1
=−x
2
symmetric mode: A
1
= A
2
for ω
2
=⇒ x
1
= x
2
(8.8)
The number of normal vibrations is equal to the number of coordinates which are
required for a complete description of the system. The equally large amplitudes
of this example result from the assumption that the two masses are equally large.
The general motion of the mass points will be given by superimposing the normal
vibrations with different amplitudes and phases.
8.2.2 Review of physical principles
Theoretical calculations of molecular vibrations often are carried out in a coordinate
system in which the center of mass of the particles is at rest. To prevent the molecule
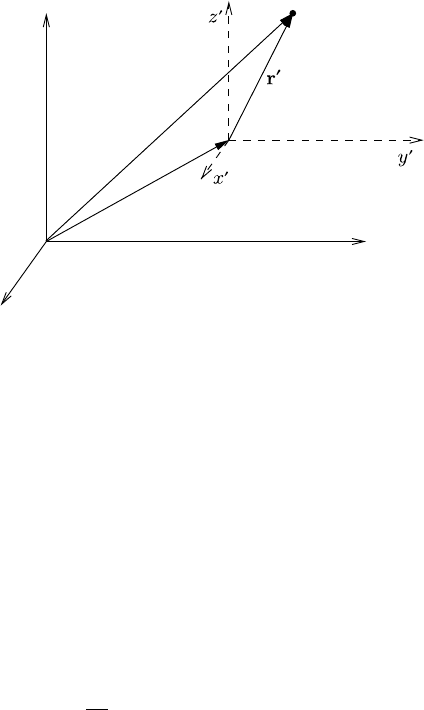
8.2 Molecular vibrations 279
x
y
z
m
i
r
c
i
r
i
Fig. 8.2 Center of mass system.
from rotating we must require that the angular momentum of the molecule vanishes.
A brief review of the physical principles involved in the solution technique now
follows.
We ignore the translational motion of the system as a whole since for spectro-
scopic considerations only the motion of the atoms relative to the center of mass is
of importance. As shown in Figure 8.2, the origin of the primed coordinate system
is the center of mass whose position in the unprimed system is given by the vector
r
c
.
The position of the particle m
i
in the primed and unprimed (laboratory) system
is r
i
and r
i
, respectively. In general, the center of mass is defined by
r
c
=
1
M
n
i=1
m
i
r
i
with M =
n
i=1
m
i
(8.9)
where M is the total mass. Replacing r
i
by the vector sum r
c
+ r
i
results in
Mr
c
=
n
i=1
m
i
r
c
+ r
i
= Mr
c
+
n
i=1
m
i
r
i
(8.10)
From this equation it follows that
n
i=1
m
i
r
i
= 0,
n
i=1
m
i
v
i
= 0 (8.11)
whereby the second equation results from the time differentiation of the first one.
Hence in the center of mass system the sum of the mass moments m
i
r
i
as well as
the sum of the linear moments m
i
v
i
vanish.

280 Absorption by gases
321
ll
m
A
m
B
m
A
Fig. 8.3 Linear symmetric triatomic molecule in equilibrium position.
The total angular momentum of the system is defined by
J =
n
i=1
m
i
(
r
i
× v
i
)
=
n
i=1
m
i
r
c
+ r
i
× (v
c
+ v
i
)
= M
(
r
c
× v
c
)
+
n
i=1
m
i
r
i
× v
i
= J
c
+
n
i=1
J
i
(8.12)
showing that the angular momentum J can be expressed as the sum of two terms. The
first term represents the angular momentum of the center of mass J
c
having mass
M, while the second term gives the sum of the angular momenta of the individual
particles about the center of mass.
8.2.3 Linear triatomic molecules
In the following we calculate the normal vibrations of the linear symmetric CO
2
molecule whose equilibrium position is shown in Figure 8.3. The atoms m
A
are
separated from the atom m
B
by the equilibrium distance l. We assume that the
potential energy of the molecule depends only on the distances between the atoms
and on the angle of bending. The motion of the atoms takes place in the (x, y)-plane.
First we are going to discuss longitudinal vibrations.
If the vector x
i
with components (x
i
, y
i
) stands for the displacement of atom i
from its equilibrium position r
0,i
then the momentary position of this atom is given
by
r
i
= r
0,i
+ x
i
(8.13)
The forces holding the atoms together, in first approximation, follow Hooke’s law.
For longitudinal vibrations the Lagrangian function L of the system is expressed by
L = K − V =
m
A
2
˙
x
2
1
+
˙
x
2
3
+
m
B
2
˙
x
2
2
−
k
l
2
[(x
1
− x
2
)
2
+ (x
3
− x
2
)
2
] (8.14)
where k
l
is the spring constant for the longitudinal motion. To eliminate one
coordinate, say x
2
, we make use of
n
i=1
m
i
r
i
=
n
i=1
m
i
r
0,i
(8.15)
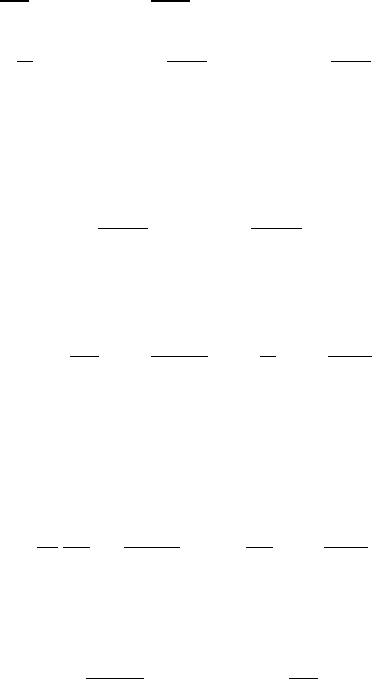
8.2 Molecular vibrations 281
which is a statement for the conservation of the center of mass. Application of
(8.13) and (8.15) yields
m
A
(x
1
+ x
3
) + m
B
x
2
= 0 (8.16)
so that L is given by
L =
m
A
2
˙
x
2
1
+
˙
x
2
3
+
m
2
A
2m
B
(
˙
x
1
+
˙
x
3
)
2
−
k
l
2
x
2
1
+ x
2
3
+
2m
A
m
B
(
x
1
+ x
3
)
2
+
2m
2
A
m
2
B
(x
1
+ x
3
)
2
(8.17)
We introduce the new set of coordinates (η, ξ ) by means of
η = x
1
− x
3
, ξ = x
1
+ x
3
=⇒
x
1
=
ξ + η
2
, x
3
=
ξ − η
2
(8.18)
Utilizing the new coordinates the Lagrangian function may be written as
L =
m
A
4
˙η
2
+
m
A
m
T
4m
B
˙
ξ
2
−
k
l
4
η
2
−
k
l
m
2
T
4m
2
B
ξ
2
(8.19)
with m
T
= 2m
A
+ m
B
. In contrast to (8.17), in this form L does not contain any
cross terms.
Differentiation of the Lagrange function (8.19) according to (8.2) yields
d
dt
∂ L
∂
˙
ξ
=
m
A
m
T
2m
B
¨
ξ,
∂ L
∂ξ
=−
k
l
m
2
T
2m
2
B
ξ (8.20)
so that the equations of motion for the (η, ξ) coordinates are given by
¨
ξ +
k
l
m
T
m
A
m
B
ξ = 0, ¨η +
k
l
m
A
η = 0 (8.21)
From these equations it is seen that due to the introduction of the coordinates (η, ξ)
the differential equations of motion are decoupled which was not the case in the
example of two coupled harmonic oscillators, see (8.3). The coordinates (η, ξ ) are
known as the normal coordinates. It is a characteristic feature of normal coordinates
that the differential equations of motion are automatically separated, there being
one differential equation for each normal coordinate.
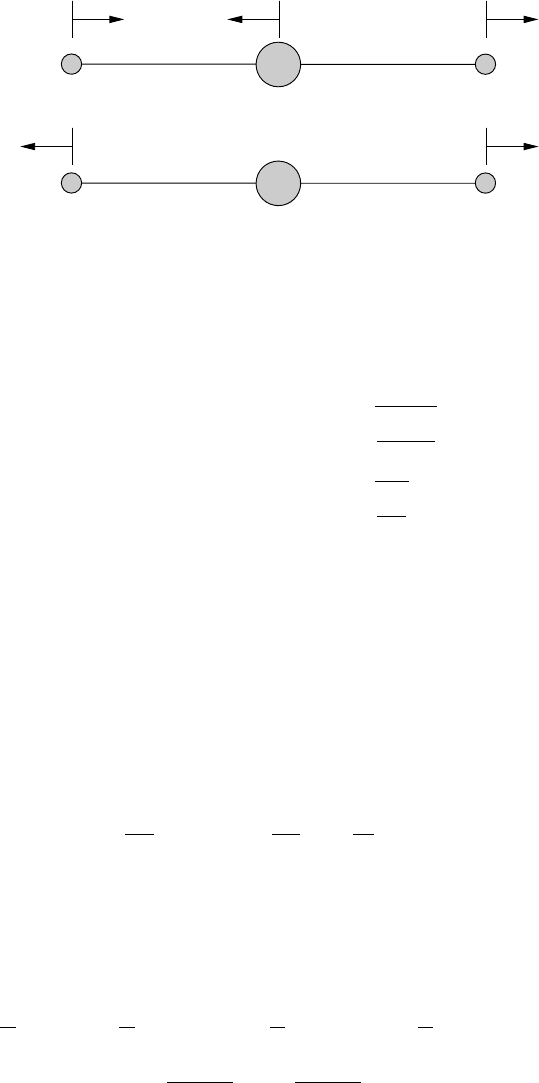
282 Absorption by gases
m
A
m
B
m
A
m
A
m
B
m
A
(a)
(b)
Fig. 8.4 (a) Antisymmetric mode of the longitudinal vibrations, η = 0, ν
3
-
vibration. (b) Symmetric mode of the longitudinal vibrations, ξ = 0, ν
1
-vibration.
Substituting the trial solution exp(iωt) into (8.21) we immediately find the
frequencies
antisymmetric vibration: ω
a
=
&
k
l
m
T
m
A
m
B
symmetric vibration: ω
s
=
&
k
l
m
A
(8.22)
Let us examine the vibrations more closely and consider case (a) of Figure 8.4.
If x
1
= x
3
then η = 0 resulting in the antisymmetric vibrational mode. This is the
reason we have added the suffix a to the circular frequency in equation (8.22). This
type of motion is usually called the ν
3
-vibration. In case (b) we set x
1
=−x
3
so
that ξ = 0 resulting in the symmetric ν
1
-vibration (suffix s in (8.22)).
So far we have restricted the motion of the atoms to one direction. A nonrigid
triatomic molecule, such as CO
2
, vibrates not only longitudinally but also transver-
sally as shown in Figure 8.5. In this case the Lagrangian function is given by
L =
m
A
2
˙
y
2
1
+
˙
y
2
3
+
m
B
2
˙
y
2
2
−
k
T
2
(lδ)
2
(8.23)
where the constant k
T
of the potential energy part of L refers to the transversal
displacement. The angle δ stands for the deviation from 180
◦
. The meaning of the
angles α
1
and α
2
follows from the figure. Since the angle δ is assumed to be very
small, we may replace it by the sine functions as shown in
δ =
π
2
− α
1
+
π
2
− α
2
≈ sin
π
2
− α
1
+ sin
π
2
− α
2
= cos α
1
+ cos α
2
=
y
2
− y
1
l
+
y
2
− y
3
l
(8.24)

8.2 Molecular vibrations 283
m
A
m
B
m
A
y
1
y
2
y
3
α
1
α
2
ll
Fig. 8.5 Coordinates of the transversal vibration of a linear triatomic molecule.
As before, we eliminate one coordinate by using the conservation of the center of
mass. The result is
m
A
(y
1
+ y
3
) + m
B
y
2
= 0 (8.25)
In order to exclude the rotation of the molecule, the total angular momentum
must vanish. The mathematical form of the angular momentum is given by (8.12).
Since the deviation from the equilibrium position is small, we may approximate
the vector r
i
by r
0,i
. Thus the total angular momentum is approximately given by
J =
3
i=1
m
i
(r
i
× v
i
) ≈
3
i=1
m
i
(r
0,i
× v
i
) =
d
dt
3
i=1
m
i
(r
0,i
× x
i
) = 0 (8.26)
which is satisfied by
3
i=1
m
i
(r
0,i
× x
i
) = 0 (8.27)
This equation can be used to show that y
1
= y
3
so that the Lagrangian function
may be written as
L =
m
A
m
B
4m
T
(l
˙
δ)
2
−
k
T
2
(lδ)
2
(8.28)
After a few steps we find the eigenfrequency of the transversal vibration
ω
T
=
&
2k
T
m
T
m
A
m
B
(8.29)
Details of the derivations will be left to the exercises.
The transversal vibration shown in Figure 8.6(a) is called ν
2
-vibration. At the
beginning of the chapter we have stated that this molecule should have four vibra-
tional modes. Our calculations, however, provided only three of these. The fourth
vibrational mode of the CO
2
molecule results from a twofold degeneracy, i.e. the
direction of one vibrational mode is perpendicular to that of the other as shown in
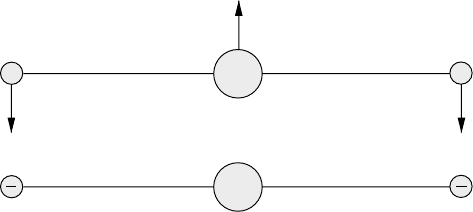
284 Absorption by gases
(a)
(b)
m
A
m
B
m
A
+ −
Fig. 8.6 Transversal vibration of a linear triatomic molecule.
Figure 8.6(b). This does not provide any new information. The + attached to m
B
and the − attached to m
A
simply indicate that they vibrate in opposite directions in
a plane perpendicular to the plane of the paper.
As will be shown later, the interaction of an electromagnetic wave with the
molecule to produce absorption or emission results from the interaction of the ele-
ctric field vector E with the (variable) dipole moment M of the system. The dipole
moment of an electrically neutral molecule is a vector whose direction is along the
line joining the center of charge of the negative charges to the center of charge of
the positive charges. The magnitude of the dipole moment is the length of that line
multiplied by the total negative or positive charge, these being equal. An atom or a
molecule is said to be polarized by an electric field when the displacements of the
charges caused by the electric field produce or alter the dipole moment.
In a Cartesian coordinate system the components of M are given by
M
x
=
k
e
k
x
k
, M
y
=
k
e
k
y
k
, M
z
=
k
e
k
z
k
(8.30)
where e
k
is the charge of the particle k at the position (x
k
, y
k
, z
k
). If the particles
are the atoms of a molecule, the charges e
k
must be considered as effective charges.
Some molecules have a permanent dipole moment such as the heteronuclear
diatomic molecule CO. The dipole moment results from the asymmetric charge
distribution. In contrast, homonuclear diatomic molecules such as N
2
have no elec-
tric dipole moment due to the symmetric charge distribution. Similarly, in the
equilibrium configuration the CO
2
molecule has no permanent dipole moment due
to the symmetric distribution of charges. Further details may be found, for example,
in Wilson et al. (1955).
Let us re-examine Figure 8.4. The symmetric longitudinal stretching of the CO
2
molecule, usually called the ν
1
-vibration, does not produce any dipole moment
so that this type of vibration is inactive in the infrared spectrum. The remain-
ing vibrations are classified as parallel or perpendicular according as the change
