Zdunkowski W., Trautmann T., Bott A. Radiation in the atmosphere: A course in Theoretical Meteorology
Подождите немного. Документ загружается.

7.1 The shape of single spectral lines 205
(1) Natural broadening: owing to the finite natural lifetime of a molecule in an excited
state, according to Heisenberg’s uncertainty principle the emitted energy is distributed
over a narrow frequency interval ν.
(2) Collision broadening: during the emission of radiation the molecule will collide with
other molecules. This interaction disturbs the emission process resulting in a broadening
of the emission line. This process is also called pressure broadening.
(3) Doppler broadening: the Doppler effect caused by the thermal motion of the molecule
yields a broadening of the line. Often it is also called thermal broadening.
The natural lifetime of the excited state of a molecule is of the order 10
−2
to
10
−1
s, (Houghton and Smith, 1966). This is much larger than the time between
collisions in a gas at normal atmospheric pressures. Therefore, the first effect is
much smaller than the second and the third so that we are justified to disregard
it in our discussion. In the lowest 30 km of the atmosphere the line broadening
due to molecular collisions is much more important than the Doppler broadening.
At altitudes higher than about 50 km, however, the Doppler broadening becomes
more and more important as compared to the collision broadening. Certainly, this is
caused by the vertical decrease of the air density yielding a reduction of the number
of molecular collisions with height while at the same time the mean free path
length of the molecules is increasing. In a region of about 30–50 km the collision
broadening as well as the Doppler effect should be taken into account.
7.1 The shape of single spectral lines
In this section we will determine the shape of the mass absorption coefficient κ
abs,ν
of an absorbing gas resulting from the line broadening by collisions of molecules
and from the Doppler effect. We start with the description of the isolated effects. At
the end of this section the collision and Doppler broadening effects will be combined
yielding the so-called Voigt profile. For ease of notation, the mass absorption coef-
ficient will henceforth also be denoted by k
ν
, that is k
ν
= κ
abs,ν
as given in (1.41).
Thus we omit the reference to the density of the absorbing gas. Furthermore, k
ν
will simply be called the absorption coefficient.
7.1.1 The Lorentz line
The simplest approach describing the collision broadening effect is due to Lorentz
who assumed that at each collision the interaction of radiation with a molecule
is momentarily halted and a random phase change is introduced. This is called a
strong encounter. First we will proceed to give a mathematical description of the
collision or Lorentz broadening.

206 Transmission in spectral lines and bands of lines
Let us consider an electromagnetic wave with circular frequency ω
0
which is
incident on an absorbing atmospheric gas molecule. The time interval during which
this molecule absorbs the wave is given by −t
0
/2 ≤ t < t
0
/2. The time signal of
this wave can be expressed by
f (t) =
exp(−iω
0
t) −t
0
/2 ≤ t < t
0
/2
0 otherwise
(7.2)
Using the Fourier transformation we may switch from the time domain to the
frequency domain of the wave. If g(ω) denotes the Fourier transform of f (t) then
g(ω) and f (t) are related by
g(ω) =
1
2π
∞
−∞
f (t)exp
(
iωt
)
dt, f (t) =
∞
−∞
g(ω)exp
(
−iωt
)
dω (7.3)
Inserting (7.2) into the first equation of (7.3) we obtain
g(ω) =
1
2π
t
0
/2
−t
0
/2
exp
[
i(ω − ω
0
)t
]
dt =
sin
(ω−ω
0
)t
0
2
π(ω − ω
0
)
(7.4)
Instead of ω we may also use the frequency since ω = 2πν so that
g(ν) =
sin
[
π(ν − ν
0
)t
0
]
2π
2
(ν − ν
0
)
(7.5)
The modulus or the absolute value of g(ν) is called the amplitude spec-
trum. Since the square of the amplitude of a wave is proportional to the energy
of the oscillation, the quantity G(ν) =|g(ν)|
2
is the so-called power spectrum.
Figure 7.1 illustrates the time signal f (t) of the real part of the finite wave, its
Fourier transform and its power spectrum.
Let us now briefly discuss how the shape of a spectral line is influenced by the
collisions with other molecules. Let p
c
stand for the number of collisions per unit
time so that p
c
t is the number of collisions within the time increment t. Thus,
q = 1 − p
c
t is the probability that the molecule experiences no collisions within
t. Let the wave have a duration of t
0
= nt. Assuming that the collisions during
each time increment t are independent of each other, we obtain the probability
that the molecule experiences no collisions within the time span t
0
as
q
n
= (1 − p
c
t)
n
= (1 − p
c
t)
t
0
/t
= [(1 − p
c
t)
−1/(p
c
t)
]
−p
c
t
0
(7.6)
Since lim
x→0
(1 − x)
−1/x
= e, for t → 0 we obtain from (7.6)
lim
t→0
q
n
= exp
(
−p
c
t
0
)
= exp
−
t
0
¯τ
(7.7)
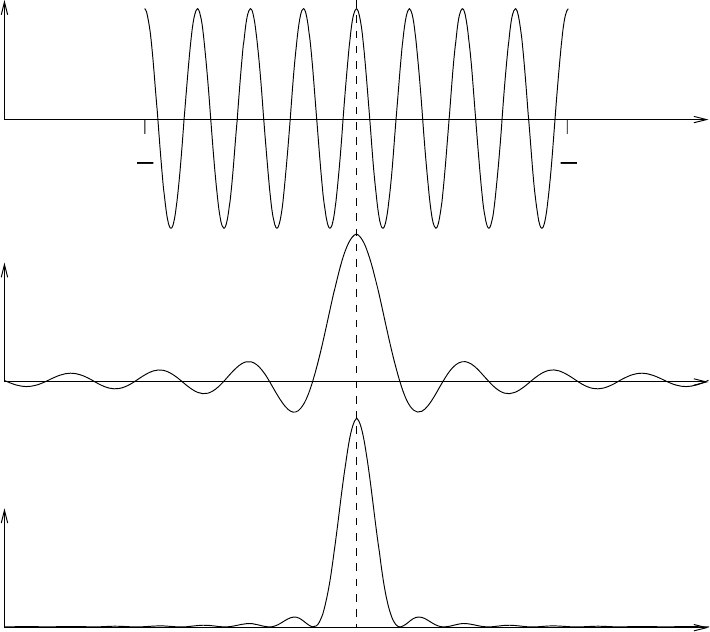
7.1 The shape of single spectral lines 207
f(t)
g
ω
ω
ω
ω
(ω)
G()
t
0
2
t
0
2
t
0
−
Fig. 7.1 Illustration of a finite wave f (t) in the time domain, its Fourier transform
g(ω) and its power spectrum G(ω).
Here, ¯τ = 1/ p
c
is the average time between two collisions and t
0
/¯τ is the total
number of collisions within t
0
.
The absorption coefficient of the Lorentz line is proportional to the probabil-
ity exp(−p
c
t
0
) for the time interval t
0
between collisions and to the power spec-
trum |g(ν)|
2
describing the spectral energy distribution. Furthermore, in order to
obtain the total absorption, we need to integrate over all possible time spans t
0
during which the molecule absorbs radiation. Hence k
ν,L
may be written in the
form
k
ν,L
= A
∞
0
|g(ν)|
2
exp
(
−p
c
t
0
)
dt
0
(7.8)
208 Transmission in spectral lines and bands of lines
where A is a constant. Substituting (7.5) into this equation the definite integral may
be evaluated yielding
k
ν,L
=
A
4π
4
(ν − ν
0
)
2
∞
0
sin
2
[
π(ν − ν
0
)t
0
]
exp
(
−p
c
t
0
)
dt
0
=
A
2π
2
p
c
p
2
c
+ 4π
2
(ν − ν
0
)
2
(7.9)
From this equation it is seen that the absorption coefficient is largest at ν = ν
0
.
Denoting k
ν
0
,L
= k
0
we find from (7.9) that A = k
0
2π
2
p
3
c
. Substituting this expres-
sion into (7.9) yields
k
ν,L
=
p
2
c
k
0
p
2
c
+ 4π
2
(ν − ν
0
)
2
(7.10)
We will now introduce the half-width of the Lorentz line α
L
. In general, the
half-width of a spectral line is defined by the distance from the line center ν = ν
0
to the points ν
1,2
where the absorption coefficient has decreased to one-half of
its maximum value. Hence we may write α
2
L
= (ν
1,2
− ν
0
)
2
. Evaluating (7.10) at
ν = ν
1,2
yields
α
L
=
p
c
2π
=
1
2π ¯τ
(7.11)
showing that the Lorentz half-width is inversely proportional to the average
time between collisions. Replacing p
2
c
in (7.10) by means of (7.11) we obtain
immediately
k
ν,L
=
α
2
L
k
0
α
2
L
+ (ν − ν
0
)
2
(7.12)
Finally, we introduce the line intensity or the line strength S from the definition
S =
∞
−∞
k
ν
dν
(7.13)
Substitution of (7.12) into this equation yields the intensity of the Lorentz line
S = πα
L
k
0
(7.14)
so that the absorption coefficient of the Lorentz line can be written as
k
ν,L
=
1
π
α
L
S
α
2
L
+ (ν − ν
0
)
2
(7.15)
7.1 The shape of single spectral lines 209
This is the form of the absorption coefficient which is normally used in radiative
transfer calculations.
For any line shape the absorption coefficient can be formulated as
k
ν
= Sf(ν − ν
0
)
(7.16)
where f (ν − ν
0
) is the so-called line-shape factor. According to (7.13) the
line-shape factor is normalized, that is
∞
−∞
f (ν − ν
0
)d(ν − ν
0
) = 1
(7.17)
From (7.15) we see that the line-shape factor of the Lorentz line is given by
f
L
(ν − ν
0
) =
1
π
α
L
α
2
L
+ (ν − ν
0
)
2
(7.18)
Integrating this equation over all frequencies according to (7.17) shows that the
Lorentz line-shape factor is normalized.
It is customary in spectroscopy to introduce the wave number ˜ν instead of the
frequency ν.Ifλ represents the wavelength then ˜ν is defined as ˜ν = 1/λ (cm
−1
).
Using the basic relation ν = c/λ = c ˜ν, where c is the speed of light in a vacuum,
we may replace k
ν,L
by k
˜ν,L
. However, since c cancels out in (7.12), the form of the
absorption coefficient remains the same. As is common usage, we will not replace
k
ν,L
by k
˜ν,L
but simply continue to write k
ν,L
.Ifν represents the wave number
expressed in units of cm
−1
then α
L
must also be expressed in units of cm
−1
and
S in cm
−2
so that the absorption coefficient k
ν
has units of cm
−1
. Otherwise, if
ν represents the frequency then α
L
must be expressed in s
−1
and S in units of
cm
−1
s
−1
. Whenever a question arises about the set of units used, it is usually
not difficult to decide if we work with the frequency or the wave number system.
Unfortunately, some writers even call the wave number simply the frequency. The
problem associated with the introduction of the wave number is discussed in more
detail by Goody (1964a).
The shape of the Lorentz line is shown in Figure 7.2. Note that per definition the
area under the line profile is S.
Since the collision of molecules depends on their number density and on their
velocity, it is expected that the Lorentz half-width is a function of pressure and
temperature. In the following we will discuss a simplified collision model by assum-
ing the existence of preferably elastic spheres. Let us consider identical molecules
with radius r which are frozen in position with the exception of one individual
molecule that is moving along an irregular zigzag path with an average velocity ¯v.
At the instant of collision the center-to-center distance of the colliding molecules
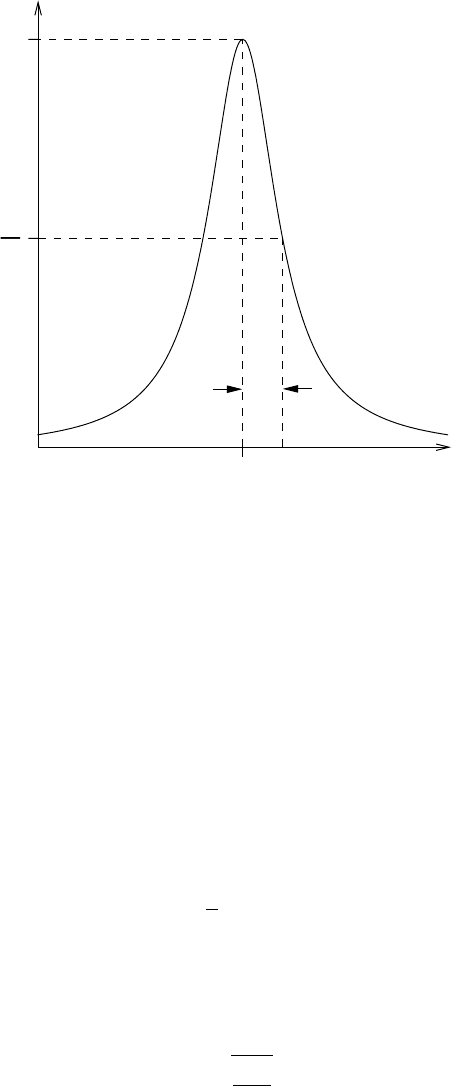
210 Transmission in spectral lines and bands of lines
k
ν,L
k
0
k
0
2
α
L
ν
0
ν
Fig. 7.2 Shape of the Lorentz form of the spectral line.
is 2r. Thus the collision cross section σ
c
of this molecule is given by
σ
c
= π (r + r )
2
= 4πr
2
(7.19)
During the time t the molecule has moved the distance ¯vt.Ifn is the number of
molecules at rest per unit volume, during t the individual molecule experiences a
total number of N
c
collisions with
N
c
= σ
c
n ¯vt (7.20)
The collision frequency p
c
, that is the number of collisions per unit time, is given
by
p
c
=
1
¯τ
= σ
c
n ¯v (7.21)
where, as before, ¯τ is the average time between two successive collisions. From
the kinetic gas theory it is known that ¯v depends on the temperature T and on the
mass m of the molecules as expressed by
¯v =
%
8kT
πm
(7.22)
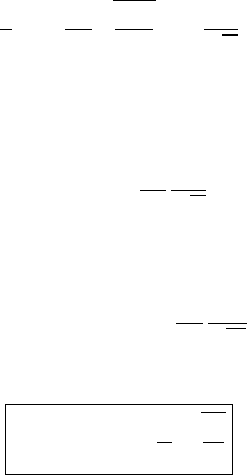
7.1 The shape of single spectral lines 211
where k is the Boltzmann constant. For a brief derivation of ¯v see Appendix 7.6.1.
The pressure p of an ideal gas is given by
p = nk T (7.23)
Substituting (7.22) and (7.23) into (7.21) we obtain for the reciprocal of the average
collision time
1
¯τ
= σ
c
p
kT
%
8kT
πm
= C
p
√
T
(7.24)
The constant C depends on the gas.
We are now ready to give an expression for the pressure and temperature
dependence of the half-width of the Lorentz line. Since α
L
= 1/2π ¯τ we find
α
L
(p, T ) =
C
2π
p
√
T
(7.25)
For reference values of the pressure and temperature (p
0
, T
0
) we denote the
half-width by
α
L,0
= α
L
(p
0
, T
0
) =
C
2π
p
0
√
T
0
(7.26)
Utilizing this equation in (7.25) gives
α
L
(p, T ) = α
L,0
p
p
0
%
T
0
T
(7.27)
Thus it is seen that the Lorentz half-width depends linearly on pressure and thus
decreases with height. Furthermore, the temperature dependence of α
L
is relatively
weak as compared to the pressure dependence so that it is sometimes completely
ignored. The standard half-width α
L,0
can be determined by experiment or from
quantum theory. Values of α
L,0
for atmospheric gases can be found in tabulated
form in the literature.
As we have seen, the average time ¯τ can be determined from classical gas
kinetic theory enabling an estimate of α
L
. However, such an estimate is several
times too small. Although the absolute magnitude of α
L
cannot be found accurately,
the pressure dependence given in (7.27) is accurately followed. A more detailed
discussion on this subject is given, for example, in Goody (1964a).
7.1.2 The thermal Doppler line
At high altitudes in the atmosphere, that is at low pressure, the collision broadening
becomes less important while at the same time the Doppler shift in frequency
212 Transmission in spectral lines and bands of lines
Direction of observer
Molecule
v
v
s
Fig. 7.3 Molecule moving with velocity component v towards the observer.
becomes the main line broadening effect. Let us assume that a molecule which is
moving with velocity v along a path s has the velocity component v towards the
observer, see Figure 7.3. If the frequency of the radiation emitted by the stationary
molecule is ν
0
, then the observed frequency ν
0
is given by
ν
0
= ν
0
1 +
v
c
, |v|c (7.28)
where c is the speed of light. Hence, the Doppler shift in frequency is
ν
0
= ν
0
− ν
0
= ν
0
v
c
(7.29)
The number concentration of molecules, dn, belonging to the velocity interval
(v, v + dv) can be obtained from Maxwell’s one-dimensional velocity distribution,
see also Appendix 7.5.1
dn = n
%
m
2πkT
exp
−
mv
2
2kT
dv
=
n
√
πv
0
exp
−
v
v
0
2
dv with v
0
=
%
2kT
m
(7.30)
Due to the square of the velocity in the exponent the component v can either be
positive (towards the observer) or negative (away from the observer). In order to
account for the Doppler effect of arbitrary v we have to integrate over the Maxwell
distribution as illustrated in Figure 7.4. Then the average of the velocity squared is
given by
v
2
=
1
n
∞
−∞
v
2
dn =
1
√
πv
0
∞
−∞
v
2
exp
−
v
v
0
2
dv =
v
2
0
2
(7.31)
First we consider the case of pure Doppler broadening, that is we neglect the
effect of both natural as well as pressure broadening. In the following section
we will discuss the combined effect of pressure and Doppler broadening. If the

7.1 The shape of single spectral lines 213
dn
dv
dv
v
Fig. 7.4 Maxwell’s one-dimensional velocity distribution for the component v.
molecule is at rest, then the absorption coefficient is proportional to the Dirac
δ-function
k
ν
= Sδ(ν − ν
0
) (7.32)
Thus, according to (7.16) the line-shape factor for monochromatic emission is given
by δ(ν − ν
0
). If we take the Doppler effect due to a particular velocity v into account
we obtain
k
ν
= Sδ
ν − ν
0
(7.33)
Note that this equation corresponds to the emission or absorption by a non-
broadened monochromatic line with frequency ν
0
. From (7.28) we see that instead
of integrating over all possible velocities v it is equivalent to integrate over all cor-
responding frequencies ν
0
. The right-hand side of (7.33) corresponds to a situation
where only a single frequency shift occurs. Therefore, the absorption coefficient
for all possible frequency shifts can be obtained from
k
ν
= S
∞
−∞
P
ν
0
δ
ν − ν
0
dν
0
(7.34)
where the probability distribution function P(ν
0
) follows directly from the Maxwell
distribution
P
ν
0
dν
0
=
dn
n
=
1
√
πv
0
exp
−
v
v
0
2
dv (7.35)
214 Transmission in spectral lines and bands of lines
From (7.28) we have dν
0
= (ν
0
/c)dv so that
P
ν
0
=
c
√
πν
0
v
0
exp
−
v
v
0
2
(7.36)
Substituting this expression into (7.34) and evaluating the integral results in the
absorption coefficient of the Doppler line
k
ν,D
=
Sc
√
πν
0
v
0
exp
−
(ν − ν
0
)c
ν
0
v
0
2
(7.37)
The maximum of the absorption coefficient occurs at the line center where ν = ν
0
and is given by
k
0
=
Sc
√
πν
0
v
0
(7.38)
Finally, we determine the particular frequency ν = ν
0
± α
D
where k
ν,D
= k
0
/2.
This leads to the half-width of the Doppler line which has the form
α
D
=
√
ln 2
ν
0
v
0
c
=
√
ln 2
ν
0
c
%
2kT
m
(7.39)
It is noteworthy that the Doppler half-width depends on temperature only but not
on pressure.
By comparing (7.16) with (7.37) one may easily see that the line-shape factor
of the Doppler line is given by
f
D
(ν − ν
0
) =
√
ln 2
√
πα
D
exp
−
(ν − ν
0
)
√
ln 2
α
D
2
(7.40)
where use was made of (7.39). Finally, it is not difficult to verify that, in accordance
with (7.17), the Doppler line-shape factor is also normalized.
7.1.3 The Voigt profile
A comparison of broadening effects due to molecular collisions and the Doppler
effect reveals that pressure broadening dominates in the troposphere and lower
stratosphere while Doppler broadening is most important in atmospheric layers
above 50 km. At sea level the Doppler half-width α
D
is about two orders of mag-
nitude smaller than α
L
. In an altitude range from about 30 to 50 km, however, both
