Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

286
Photochemistry
of
Planetary
Atmospheres
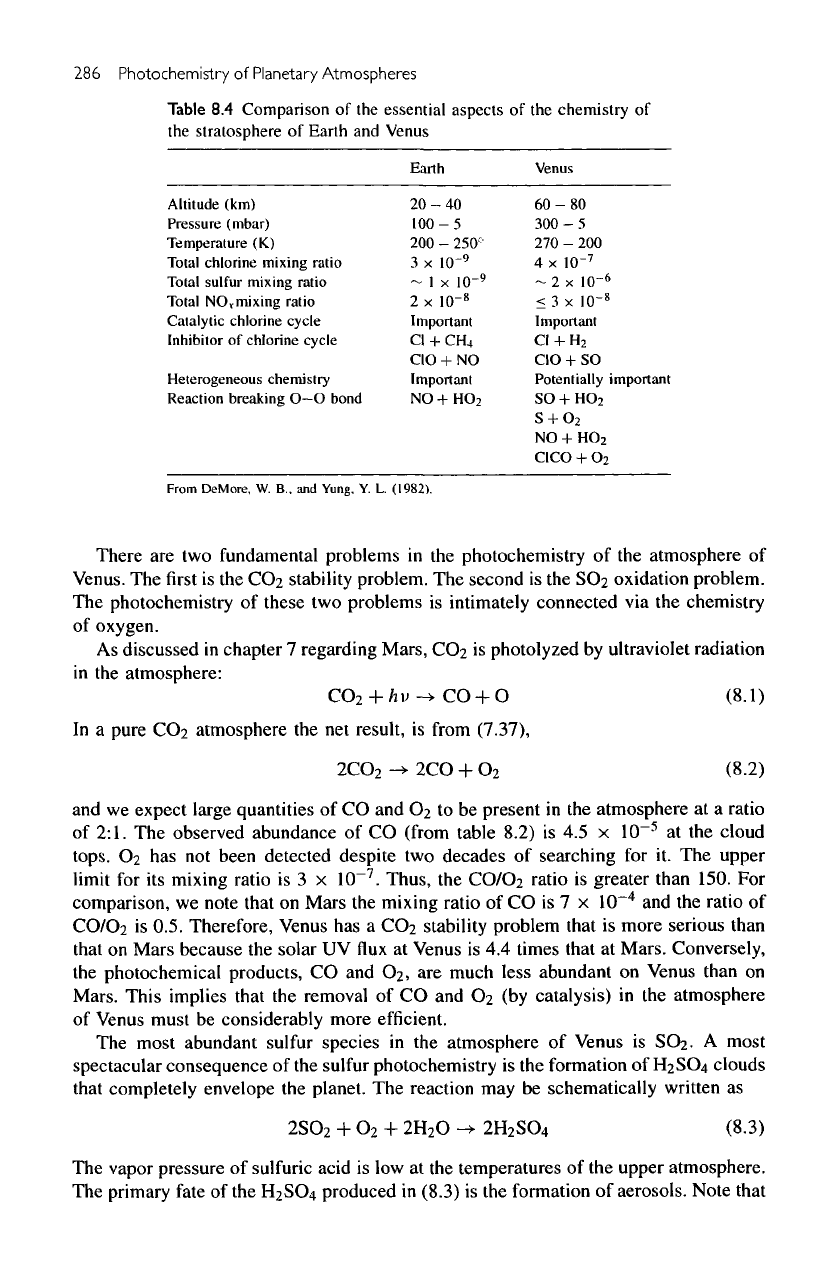
Table
8.4
Comparison
of the
essential aspects
of the
chemistry
of
the
stratosphere
of
Earth
and
Venus
Earth Venus
Altitude
(km)
Pressure (mbar)
Temperature
(K)
Total
chlorine
mixing
ratio
Total
sulfur
mixing
ratio
Total
NO,
mixing ratio
Catalytic
chlorine cycle
Inhibitor
of
chlorine cycle
Heterogeneous chemistry
Reaction
breaking
O—
O
bond
20-40
100
- 5
200
-
250'
71
3 x
10~
9
~ 1 x
10~
9
2 x
10~
8
Important
Cl
+
CH
4
CIO
+ NO
Important
NO
+
HO
2
60-80
300
- 5
270
-
200
4 x
10-
7
-2
x
10"
6
<
3 x
ID'
8
Important
CI
+
H
2
CIO
+ SO
Potentially
important
SO
+
HO
2
S
+
0
2
NO
+
HO
2
CICO
+
O
2
From
DeMore,
W. B.. and
Yung,
Y. L.
(1982).
There
are two
fundamental problems
in the
photochemistry
of the
atmosphere
of
Venus.
The first is the
CO
2
stability
problem.
The
second
is the SO2
oxidation problem.
The
photochemistry
of
these
two
problems
is
intimately connected
via the
chemistry
of
oxygen.
As
discussed
in
chapter
7
regarding Mars,
CO2 is
photolyzed
by
ultraviolet radiation
in
the
atmosphere:
In
a
pure
CC>2
atmosphere
the net
result,
is
from
(7.37),
and
we
expect large quantities
of CO and
O
2
to be
present
in the
atmosphere
at a
ratio
of
2:1.
The
observed abundance
of CO
(from table
8.2)
is 4.5 x
10~
5
at the
cloud
tops.
O
2
has not
been detected despite
two
decades
of
searching
for it. The
upper
limit
for its
mixing ratio
is 3 x
10~
7
.
Thus,
the
CO/O2 ratio
is
greater than
150.
For
comparison,
we
note that
on
Mars
the
mixing
ratio
of CO is 7 x
10~
4
and the
ratio
of
CO/O2
is
0.5.
Therefore, Venus
has a CO2
stability problem that
is
more
serious than
that
on
Mars because
the
solar
UV flux at
Venus
is 4.4
times that
at
Mars. Conversely,
the
photochemical products,
CO and
©2,
are
much less abundant
on
Venus than
on
Mars. This implies
that
the
removal
of CO and O2 (by
catalysis)
in the
atmosphere
of
Venus must
be
considerably more
efficient.
The
most abundant
sulfur
species
in the
atmosphere
of
Venus
is
SO2.
A
most
spectacular consequence
of the
sulfur
photochemistry
is the
formation
of
H2SO4 clouds
that
completely envelope
the
planet.
The
reaction
may be
schematically written
as
The
vapor pressure
of
sulfuric
acid
is low at the
temperatures
of the
upper atmosphere.
The
primary
fate
of the
H
2
SO4
produced
in
(8.3)
is the
formation
of
aerosols.
Note that

Venus
287
the
consumption
of
Oz
by
SC>2
in
(8.3) accounts
for the
unusually
low
concentration
of
02 in the
atmosphere
of
Venus. Reaction (8.3) scavenges
H
2
O
in the
upper atmosphere.
In
addition, H2SO4
aerosols
are
highly
hygroscopic
and
serve
to
desiccate
the
upper
atmosphere even
further.
The
oxidation
of CO to
CO
2
(the
stability
problem)
and the
oxidation
of SO2 to
sulfate
on
Venus require
the
assistance
of
catalytic chemistry.
It was
recognized
in
1971
that
chlorine could play
a
fundamental role
in the
photochemistry
of
Venus, prior
to the
recognition
of its
importance
in the
terrestrial stratosphere.
The
purpose
of
this
chapter
is to
develop
the
chemistry
of
chlorine
in
some
detail.
However,
our
overall knowledge
of the
chemistry
of
Venus
is far
from complete.
The
major barriers
to
further
understanding
are the
lack
of
reliable observational data
on
atmospheric composition below
the
clouds
and the
lack
of an
adequate kinetic
database
for
sulfur
chemistry.
8.2
Photochemistry
Since
CO
2
is the
dominant
species
in the
atmospheres
of
Venus
and
Mars,
the
moti-
vations
for the
stability
problem
are the
same
(see section 7.2.1).
8.2.1
HO.,
Catalytic Chemistry
Since water vapor
had
been detected
on
Venus,
the
early models attempted
to
solve
the
CO2
stability
problem
by
invoking
the
HO,
chemistry that
had
been
success-
fully
applied
to
Mars.
The
chemistry involves catalytic cycles that
are
described
in
section
7.2.2.
An
obvious
source
of
HO.,
radicals
is
photolysis
of
H
2
O:
However,
due to the
drying
of the
upper atmosphere
by
H2SO4
aerosols,
the H2O
mixing
ratio
is of the
order
of
10~
6
,
even though
the
maximum mixing ratio below
the
clouds
is
greater
than
1 x
10~
4
.
A
larger source
of
HO
V
is the
photolysis
of
HCI:
The
Cl
atom produced
in
(8.5) could result
in the
production
of
another
HO
V
radical
by
The
source
of H2 in
(8.6)
is
postulated
to be at the
surface
of the
planet, where
the
equilibrium
reaction
turns
H2O
into
H
2
.
Hence,
the net
result
is
288
Photochemistry
of
Planetary
Atmospheres
Note that
in
chemical scheme (I), chlorine plays
the
role
of a
catalyst
in the
dissociation
of
H
2
O
into
H and O
atoms.
The
reason that scheme
(I) is
more
effective than
the
direct
photolysis
of
H
2
O
(8.4)
is
that
HC1
absorbs
at
longer wavelengths
in the UV
range,
where
the
solar
flux is
larger.
The
validity
of the
HO.
V
chemistry
for
explaining
the
stability
of
CO
2
on
Venus
was
questioned after
the
reaction
was
shown
to be
extremely fast, with rate coefficient equal
to 3.2 x
10~"
cm
3
s~'.
This reaction
effectively
removes
the
HO
r
radicals before they
can
catalytically
oxidize
CO to
CO
2
.
However,
in
view
of the
importance
of
heterogeneous chemistry
for
converting
HC1 and
C1ONO2 into labile forms
of
chlorine
(see
section
8.2.5
and
chapter
10),
the
HO.
t
chemistry
on
Venus should
be
reexamined.
8.2.2 Chlorine Catalytic
Chemistry
Laboratory studies have shown that chlorine
is
capable
of
oxidizing
CO to
CO
2
.
There
are two
crucial steps.
The first
step
is the
formation
of the
chloroformyl
radical
by
the
three-body reaction
The
second
step
is the
formation
of the
peroxychloroformyl
radical
by
The
structure
of
this radical
is
known
and may be
represented
as
The
complex
is not
very stable
and may be
decomposed
by
collision:
The
empirical rate coefficient
for
(8.10),
taking
(8.11)
into account,
is
where
M is the
number density
of the
atmosphere.
The
peroxychloroformyl radical
is
removed
by
reactions
with
O and Cl
atoms, resulting
in the
production
of
CO
2
:
The net
results
may be
summarized
in two
catalytic cycles
Venus
289
Note
that
schemes
(Ha)
and
(lib)
are
analogous
to
HO
A
schemes
(la)
and
(lib)
de-
scribed
in
chapter
7.
Reactive chlorine species, derived
from
(8.5), serve
as
catalysts
for
these chemical schemes.
8.2.3
Sulfur
Chemistry
Four
sulfur
species have been
firmly
identified
in the
atmosphere
of
Venus:
SO
2
,
SO,
COS,
and
H
2
SO4
(in
aerosols). Their abundances
are
summarized
in
table 8.2.
The
presence
of
thiozone
(83)
and
polysulfur
(S.
v
)
in the
clouds
has
been inferred
but
has not
been proved. There
are two
parts
to the
chemistry
of
sulfur
species
in the
atmosphere
of
Venus.
In the
deep atmosphere
and on the
surface,
the
chemistry
is
dominated
by
equilibrium
chemistry. Above
the
cloud tops,
the
chemistry
is
driven
by
photochemistry.
Thus,
the
partitioning
of
sulfur
among
the
different
species represents
a
chemical
tug of war
between
equilibrium
chemistry
in the
lower atmosphere
and
photochemistry
of the
upper atmosphere.
We first
discuss
the
photochemistry
in the
atmosphere above
the
clouds.
The
most reducing species
of
sulfur
that
has
been observed
is
COS.
In the
upper
atmosphere
it
readily undergoes photolysis:
where
S('D)
is the first
excited state
of the S
atom.
The
most
likely
fate
of
S('D)
is
quenching:
The S
atom gets oxidized
to SO by
reacting
with
O:
Further
oxidation
to
SO
2
can
proceed
via the
three-body reaction
or the
self-reaction
Note that
the net
result
of
(8.18)-(8.20)
is the
oxidation
of S to
SO
2
using
O
2
.
290
Photochemistry
of
Planetary
Atmospheres
Both
SO and
SO
2
are
readily photolyzed
in the
upper
atmosphere
of
Venus:
but
because
of the
fast reactions
(8.18)
and
(8.20),
the net
result
is the
breaking
of
the
O—
O
bond,
as
shown
in the
following:
Once
the
strong
O—
O
bond
is
broken,
the
oxidation
of
SO
2
to
SO^
readily takes
place
by
the
three-body reaction
followed
by the
formation
of
H
2
SO4:
The net
result
may be
summarized
by
where
the
O
2
in
scheme
(IV)
is
turned into
O by
schemes
(Ilia)
and
(IHb).
The
efficiency
of
scheme
(IV)
for
consuming
O
2
counterbalances
the
result
of an
important
catalytic cycle that forms
the
O—
O
bond:
Note
that
this scheme
is
primarily responsible
for the
destruction
of odd
oxygen
in
the
terrestrial stratosphere
by
chlorine.
It was a
puzzle
why the
atmosphere
of
Venus
contains
so
little
O
2
in
view
of the
catalytic power
of
scheme
(V).
The
reason
is
that
cycles
(Ilia)
and
(Hlb)
are
powerful enough
to
reverse
the
action
of
cycle
(V),
with
the
net
result that
O
2
is
consumed
by
scheme
(IV).
There
is a
chemical cycle
that
results
in the
simultaneous oxidation
of CO to
CO
2
and
SO
2
to
H
2
SO
4
:

Venus
29
1
Note that
in the
above cycle
a
crucial reaction
is
(8.27),
the
oxidation
of SO to
SO
2
by
reacting
with
CIO
.
This chemical scheme consumes
O
2
,
by
breaking
the
strong
O-O
bond
in
reaction (8.14).
In
addition
to
oxidation
to
H2SO4, there
is
another possible fate
for
sulfur
com-
pounds
in the
atmosphere
of
Venus: formation
of
polysulfur.
S
atoms
are
generated
by
the
reactions (8.16)
and
(8.17), (8.20),
and
(8.22).
S
2
may now be
formed
via
Production
of
S,,
is
possible through successive addition reactions such
as
83
is the
sulfur
analog
of
ozone, known
as
thiozone.
As the
number
of
sulfur
atoms
increases,
the
polyatomic
sulfur
compounds tend
to
have lower saturation vapor
pres-
sures.
It is
convenient
to
name
all
sulfur
species
beyond
Sg
"polysulfur"
or
S
x
.
In
the
UV
region
S
x
absorbs strongly,
and it is
believed
to be the
principal constituent
of
the
unidentified
UV
absorber
in the
upper atmosphere
of
Venus. Note that reactions
(8.28)-(8.32)
leading
to the
formation
of
S
x
compete with reactions
(8.18)-(8.20)
that
lead
to the
formation
of
SO
2
.
The key
reactions
that
determine
the
rates
of
forma-
tion
of
oxidized versus reduced
sulfur
are
(8.18)
and
(8.28). Therefore, production
of
oxidized
sulfur
is
favored when
&|g[O
2
]
>
£
2
g[COS],
that
is,
The
order
of
magnitude
of
A:
2
s/^ig
is
10~
3
,
and the
mixing
ratio
of COS in the
lower
atmosphere
is 2.5 x
10~
7
.
This suggests that
the
critical mixing ratio
of of
O
2
given
by
(8.33)
is
around
10~
10
.
When
O
2
abundance
exceeds
this
value, production
of
oxidized
sulfur
species
is
favored,
and
ultimately
H
2
SO4
is
produced. When
O
2
abundance
is
below this value,
production
of
polysulfur
becomes
possible. Since
the
source
of
O
2
is
photolysis
of
CO
2
in
the
upper atmosphere
and the
source
of COS is
equilibrium chemistry
in the
lower
atmosphere
and the
surface,
we can
imagine
that
the
chemistry
of the
downwelling
air
parcel
and
that
of the
upwelling
air
parcel could
be
quite
different.
This
may
indeed
be the
explanation
for the
patchiness
and the
transience
of the UV
markers
in the
cloud
tops
of
Venus.

292
Photochemistry
of
Planetary
Atmospheres
8.2.4 Equilibrium Chemistry
The
bulk
of
photochemistry described
in the
sections
8.2.1-8.2.3
takes place above
the
cloud tops (about
60
km),
in the
region
of the
atmosphere known
as the
stratome-
sosphere.
As
shown
in figure
8.1,
the
troposphere
of
Venus extends from
the
surface
to
about
60 km. The
troposphere
is
shielded from
UV
radiation
by
CO
2
,
clouds,
and
aerosols. Photochemistry
is not
expected
to be
important. However,
the
high
temper-
ature
and
pressure make
the
lower atmosphere
and the
surface
a
favorable region
for
equilibrium
chemistry.
Our
knowledge
of the
chemistry
in the
deep atmosphere
and
on
the
surface
of
Venus
is
less secure than that above
the
cloud tops.
The
greatest
uncertainty
is in the
kinetic
rate coefficients
of
reactions that drive
the
composition
toward
thermodynamic
equilibrium.
The
consequences
of
photochemistry
in the
upper atmosphere
of
Venus
may be
summarized
by the
following
stoichiometric reactions:
where
we
assume that
the
"parent
species"
coming
up
from
the
lower atmosphere
are
CO
2
,
SO
2
,
H
2
O,
and
COS,
and the final
photochemical products
are CO,
H
2
SO4,
and
polysulfur
(S.
v
,
with
x > 8).
Thus,
the net
result
of
photochemistry
in the
upper
atmosphere
is the
production
of
species that
are far out of
equilibrium. Note that
the
production
of CO in
reactions (8.34)
and
(8.35)
represents only
a
small
fraction
(about 20%)
of the
photolysis
of
CO
2
.
The
photolysis
of
CO
2
(8.3)
is a
source
of
oxidant
only
when
CO
remains unoxidized.
The
bulk
of
CO
2
photolysis
is
reversed
by
chemical schemes
(Ha)
and
(lib),
and
would
not
contribute
to the
oxidation
of
SO
2
and
COS.
The
rates
of the
reactions
(8.34)-(8.36)
are
such that
all the
SO
2
and COS
in
the
bulk atmosphere
of
Venus would
be
exhausted
in
less than
10,000
yr. In
this
time
the
buildup
of CO
would exceed
the
observed abundance. Therefore,
for a
steady
state composition
to be
maintained, reactions
(8.34)-(8.36)
must
be
reversed
in the
lower atmosphere
or the
surface.
The
backward reaction
of
(8.34)
is
quite simple.
In the
lower atmosphere,
at
high
temperature,
H2SO4
is
unstable
and
will
decompose into
H
2
O
and
sulfur
trioxide:
SO}
is a
potent oxidant
and
readily attacks
CO:
The net
result
is the
reverse
of
(8.34):
The
reversal
of
(8.35)
is
more complex, involving pyrite formation
on the
surface
of
the
planet. First,
we
have
the
reaction
of
SO
2
with carbonate rock
to
form anhydrite
(CaSO
4
):
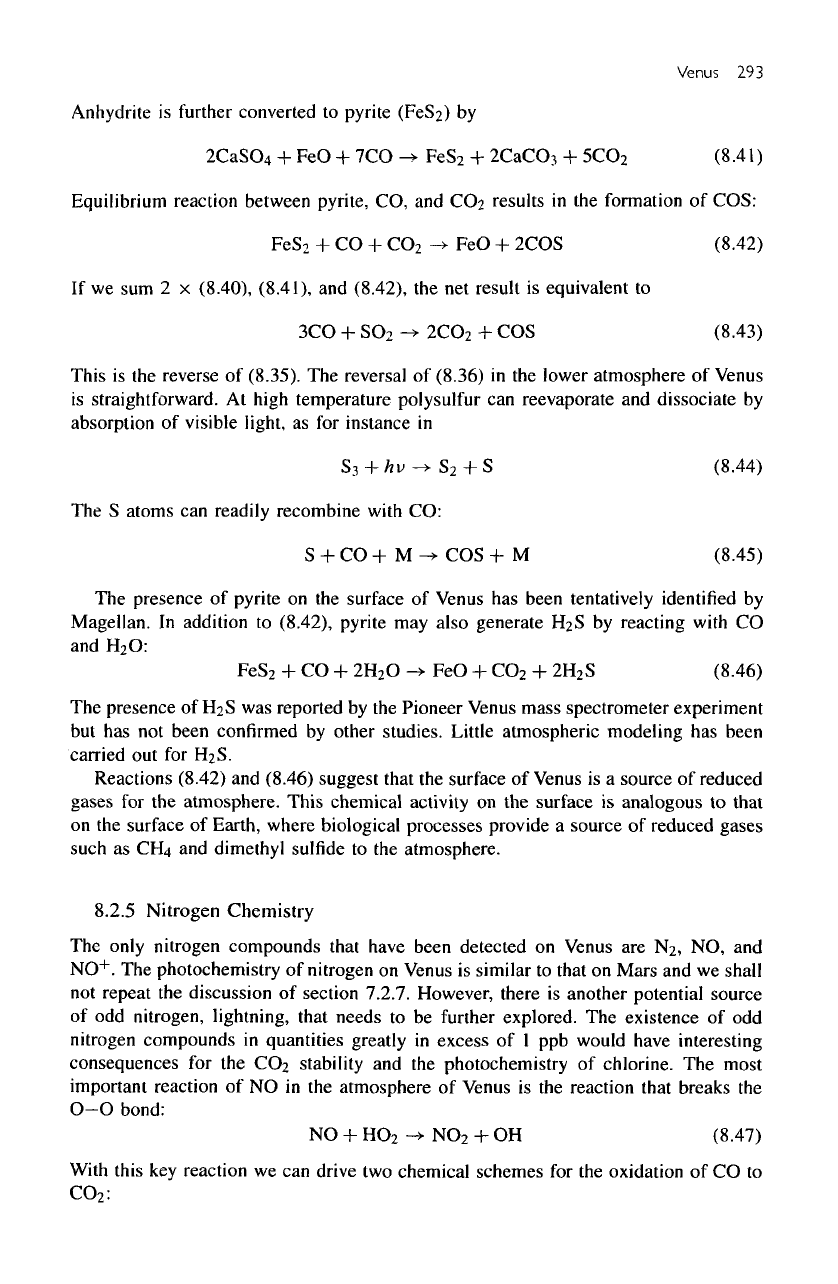
Venus
293
Anhydrite
is
further
converted
to
pyrite (FeS2)
by
Equilibrium
reaction between pyrite,
CO, and CO2
results
in the
formation
of
COS:
If
we sum 2 x
(8.40),
(8.41),
and
(8.42),
the net
result
is
equivalent
to
This
is the
reverse
of
(8.35).
The
reversal
of
(8.36)
in the
lower atmosphere
of
Venus
is
straightforward.
At
high temperature polysulfur
can
reevaporate
and
dissociate
by
absorption
of
visible
light,
as for
instance
in
The S
atoms
can
readily recombine
with
CO:
The
presence
of
pyrite
on the
surface
of
Venus
has
been tentatively
identified
by
Magellan.
In
addition
to
(8.42),
pyrite
may
also
generate
H
2
S
by
reacting
with
CO
and
H
2
O:
The
presence
of
H
2
S
was
reported
by the
Pioneer Venus mass spectrometer experiment
but
has not
been confirmed
by
other studies. Little atmospheric modeling
has
been
carried
out for
H
2
S.
Reactions
(8.42)
and
(8.46)
suggest that
the
surface
of
Venus
is a
source
of
reduced
gases
for the
atmosphere.
This
chemical
activity
on the
surface
is
analogous
to
that
on
the
surface
of
Earth, where biological
processes
provide
a
source
of
reduced
gases
such
as
CHa
and
dimethyl
sulfide
to the
atmosphere.
8.2.5 Nitrogen Chemistry
The
only nitrogen compounds that have been detected
on
Venus
are
N
2
,
NO, and
NO
+
.
The
photochemistry
of
nitrogen
on
Venus
is
similar
to
that
on
Mars
and we
shall
not
repeat
the
discussion
of
section
7.2.7.
However, there
is
another potential source
of
odd
nitrogen, lightning, that
needs
to be
further
explored.
The
existence
of odd
nitrogen
compounds
in
quantities greatly
in
excess
of 1 ppb
would have interesting
consequences
for the
CO
2
stability
and the
photochemistry
of
chlorine.
The
most
important
reaction
of NO in the
atmosphere
of
Venus
is the
reaction that breaks
the
O-O
bond:
With
this
key
reaction
we can
drive
two
chemical schemes
for the
oxidation
of CO to
CO
2
:
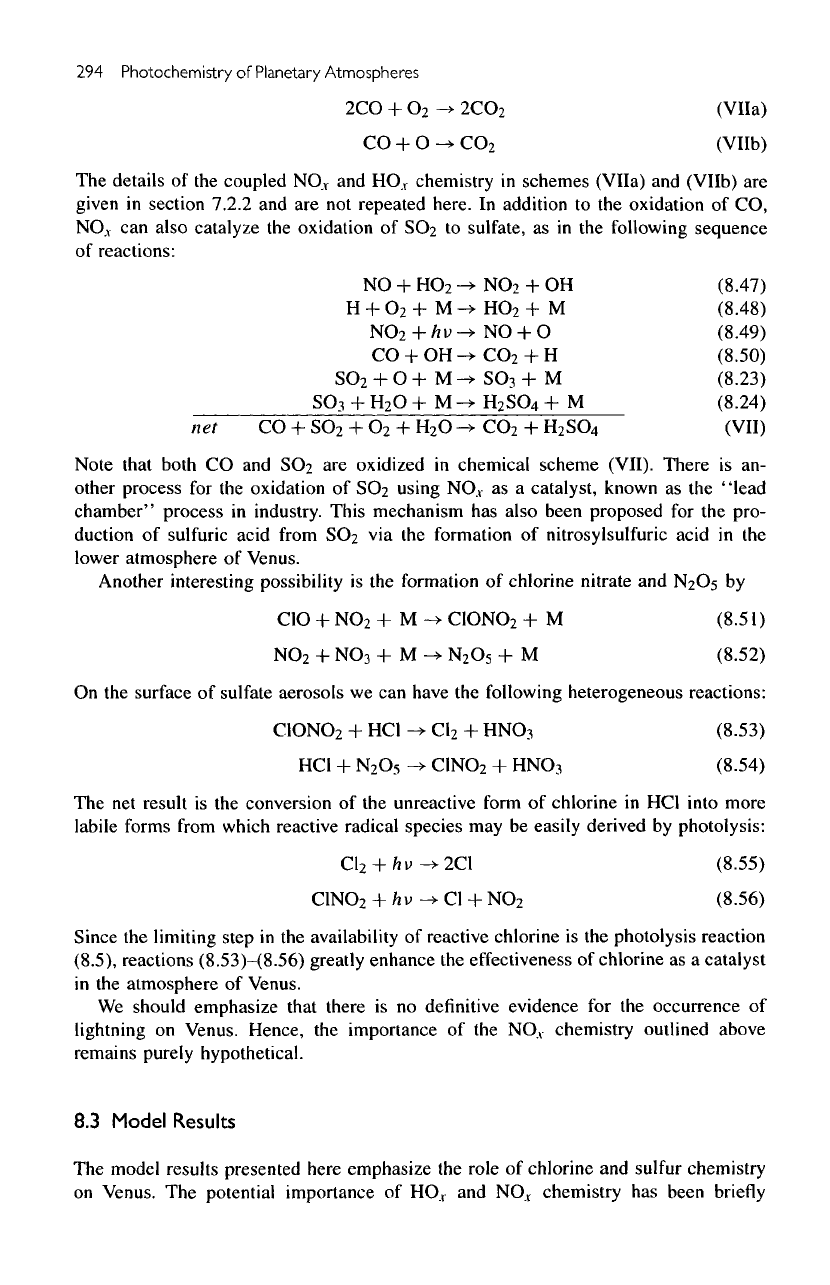
294
Photochemistry
of
Planetary
Atmospheres
The
details
of the
coupled
NO_
V
and
HO
V
chemistry
in
schemes
(Vila)
and
(VHb)
are
given
in
section 7.2.2
and are not
repeated here.
In
addition
to the
oxidation
of CO,
NO.,
can
also
catalyze
the
oxidation
of
SO
2
to
sulfate,
as in the
following sequence
of
reactions:
Note that both
CO and SO2 are
oxidized
in
chemical
scheme
(VII).
There
is an-
other
process
for the
oxidation
of
SO
2
using
NO.,
as a
catalyst, known
as the
"lead
chamber"
process
in
industry. This mechanism
has
also
been
proposed
for the
pro-
duction
of
sulfuric acid from
SO2 via the
formation
of
nitrosylsulfuric acid
in the
lower atmosphere
of
Venus.
Another interesting possibility
is the
formation
of
chlorine nitrate
and
N
2
Os
by
On the
surface
of
sulfate
aerosols
we can
have
the
following heterogeneous reactions:
The net
result
is the
conversion
of the
unreactive form
of
chlorine
in HC1
into more
labile
forms from which reactive radical
species
may be
easily derived
by
photolysis:
Since
the
limiting step
in the
availability
of
reactive chlorine
is the
photolysis reaction
(8.5),
reactions
(8.53)-(8.56)
greatly enhance
the
effectiveness
of
chlorine
as a
catalyst
in
the
atmosphere
of
Venus.
We
should emphasize that there
is no
definitive
evidence
for the
occurrence
of
lightning
on
Venus. Hence,
the
importance
of the
NO.,
chemistry outlined above
remains purely hypothetical.
8.3
Model
Results
The
model results presented here emphasize
the
role
of
chlorine
and
sulfur
chemistry
on
Venus.
The
potential importance
of
HO,
and
NO
V
chemistry
has
been
briefly
Venus
295
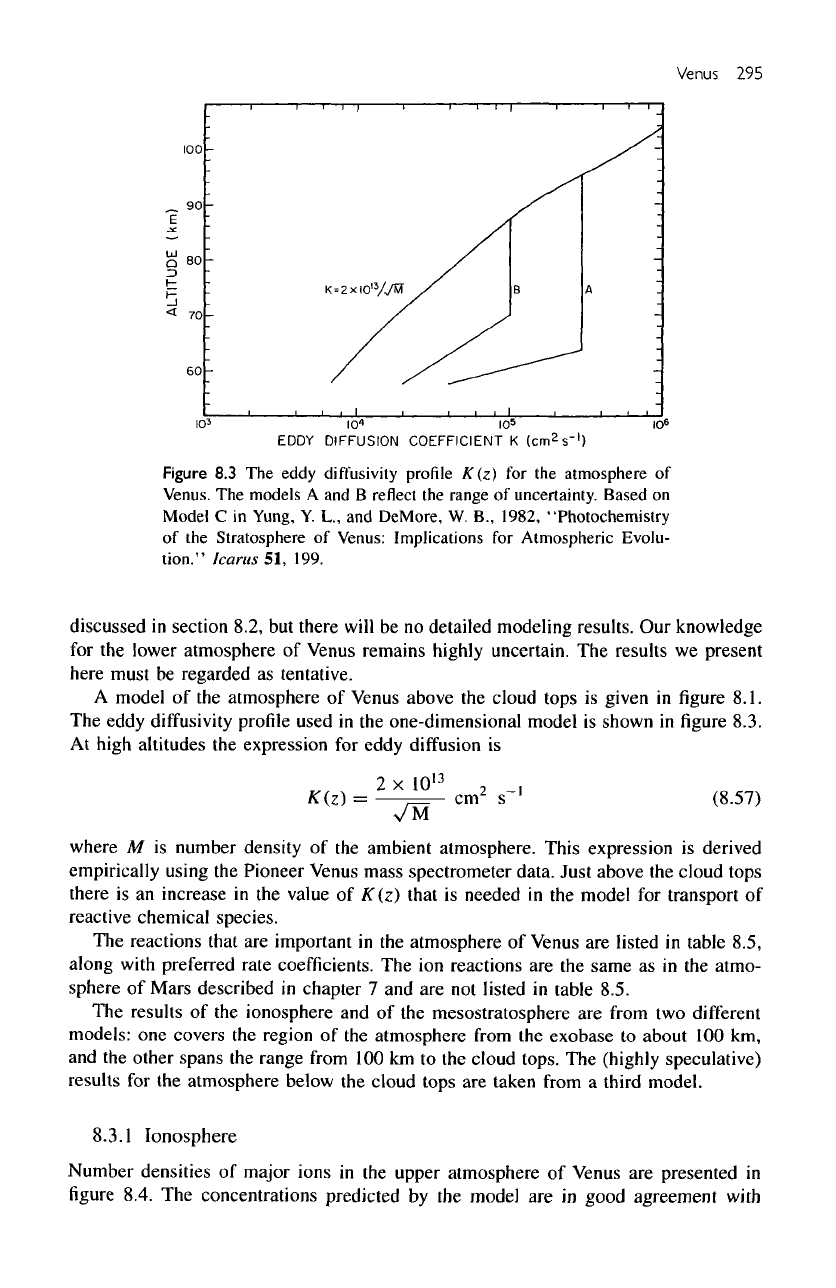
Figure
8.3 The
eddy
diffusivity
profile K(z)
for the
atmosphere
of
Venus.
The
models
A and B
reflect
the
range
of
uncertainty.
Based
on
Model
C in
Yung,
Y. L., and
DeMore,
W.
B.,
1982,
"Photochemistry
of the
Stratosphere
of
Venus: Implications
for
Atmospheric
Evolu-
tion."
Icarus
51,
199.
discussed
in
section 8.2,
but
there
will
be no
detailed modeling results.
Our
knowledge
for
the
lower atmosphere
of
Venus remains highly uncertain.
The
results
we
present
here must
be
regarded
as
tentative.
A
model
of the
atmosphere
of
Venus above
the
cloud tops
is
given
in
figure
8.1.
The
eddy
diffusivity
profile
used
in the
one-dimensional model
is
shown
in figure
8.3.
At
high altitudes
the
expression
for
eddy
diffusion
is
where
M
is
number density
of the
ambient atmosphere. This expression
is
derived
empirically
using
the
Pioneer Venus mass spectrometer data. Just above
the
cloud tops
there
is an
increase
in the
value
of
K(z)
that
is
needed
in the
model
for
transport
of
reactive chemical
species.
The
reactions
that
are
important
in the
atmosphere
of
Venus
are
listed
in
table 8.5,
along
with
preferred rate coefficients.
The ion
reactions
are the
same
as in the
atmo-
sphere
of
Mars
described
in
chapter
7 and are not
listed
in
table 8.5.
The
results
of the
ionosphere
and of the
mesostratosphere
are
from
two
different
models:
one
covers
the
region
of the
atmosphere from
the
exobase
to
about
100 km,
and
the
other spans
the
range from
100 km to the
cloud
tops.
The
(highly
speculative)
results
for the
atmosphere below
the
cloud
tops
are
taken from
a
third
model.
8.3.1
Ionosphere
Number densities
of
major ions
in the
upper atmosphere
of
Venus
are
presented
in
figure
8.4.
The
concentrations
predicted
by the
model
are in
good
agreement
with
