Tarek Ahmed. Reservoir engineering handbook
Подождите немного. Документ загружается.


where p = system pressure, psia
p
pr
= pseudo-reduced pressure, dimensionless
T = system temperature, °R
T
pr
= pseudo-reduced temperature, dimensionless
p
pc
, T
pc
= pseudo-critical pressure and temperature, respectively, and
defined by the following relationships:
It should be pointed out that these pseudo-critical properties, i.e., p
pc
and T
pc
, do not represent the actual critical properties of the gas mixture.
These pseudo properties are used as correlating parameters in generating
gas properties.
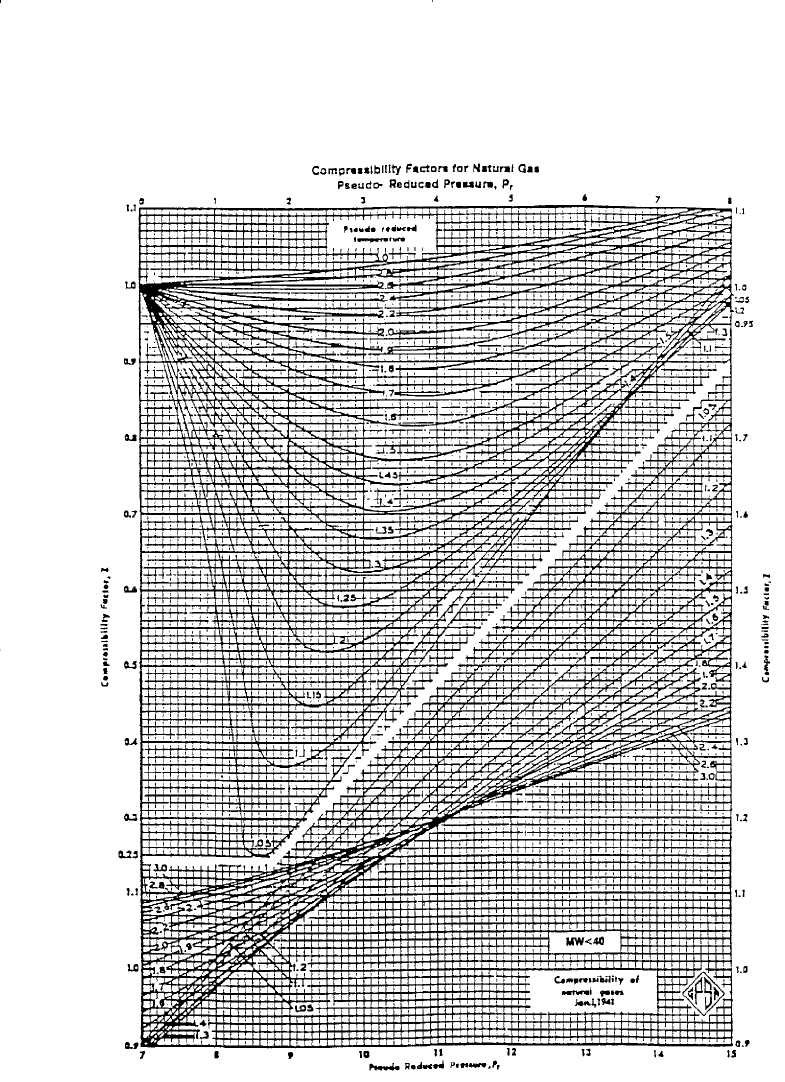
Based on the concept of pseudo-reduced properties, Standing and Katz
(1942) presented a generalized gas compressibility factor chart as shown
in Figure 2-1. The chart represents compressibility factors of sweet natur-
al gas as a function of p
pr
and T
pr
. This chart is generally reliable for nat-
ural gas with minor amount of nonhydrocarbons. It is one of the most
widely accepted correlations in the oil and gas industry.
Example 2-5
A gas reservoir has the following gas composition: the initial reservoir
pressure and temperature are 3000 psia and 180°F, respectively.
Component y
i
CO
2
0.02
N
2
0.01
C
1
0.85
C
2
0.04
C
3
0.03
i - C
4
0.03
n - C
4
0.02
Calculate the gas compressibility factor under initial reservoir condi-
tions.
TyT
pc i ci
i
=
=
Â
1
(2 -15)
pyp
pc i ci
i
=
=
Â
1
(2 -14)
38 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 38
Reservoir-Fluid Properties 39
Figure 2-1. Standing and Katz compressibility factors chart. (Courtesy of GPSA
and GPA Engineering Data Book, EO Edition, 1987.)
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 39

Solution
Component y
i
T
ci
,°R y
i
T
ci
p
ci
y
i
p
ci
CO
2
0.02 547.91 10.96 1071 21.42
N
2
0.01 227.49 2.27 493.1 4.93
C
1
0.85 343.33 291.83 666.4 566.44
C
2
0.04 549.92 22.00 706.5 28.26
C
3
0.03 666.06 19.98 616.4 18.48
i - C
4
0.03 734.46 22.03 527.9 15.84
n - C
4
0.02 765.62 15.31 550.6 11.01
T
pc
= 383.38 p
pc
= 666.38
Step 1. Determine the pseudo-critical pressure from Equation 2-14:
p
pc
= 666.18
Step 2. Calculate the pseudo-critical temperature from Equation 2-15:
T
pc
= 383.38
Step 3. Calculate the pseudo-reduced pressure and temperature by apply-
ing Equations 2-12 and 2-13, respectively:
Step 4. Determine the z-factor from Figure 2-1, to give:
z = 0.85
Equation 2-11 can be written in terms of the apparent molecular
weight M
a
and the weight of the gas m:
Solving the above relationship for the gas specific volume and density,
give:
pV z
m
M
RT
a
=
Ê
Ë
Á
ˆ
¯
˜
p
T
pr
pr
==
==
3000
666 38
450
640
383 38
167
.
.
.
.
40 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 40

where v = specific volume, ft
3
/lb
r
g
= density, lb/ft
3
Example 2-6
Using the data in Example 2-5 and assuming real gas behavior, calcu-
late the density of the gas phase under initial reservoir conditions. Com-
pare the results with that of ideal gas behavior.
Solution
Component y
i
M
i
y
i
• M
i
T
ci
,°R y
i
T
ci
p
ci
y
i
p
ci
CO
2
0.02 44.01 0.88 547.91 10.96 1071 21.42
N
2
0.01 28.01 0.28 227.49 2.27 493.1 4.93
C
1
0.85 16.04 13.63 343.33 291.83 666.4 566.44
C
2
0.04 30.1 1.20 549.92 22.00 706.5 28.26
C
3
0.03 44.1 1.32 666.06 19.98 616.40 18.48
i - C
4
0.03 58.1 1.74 734.46 22.03 527.9 15.84
n - C
4
0.02 58.1 1.16 765.62 15.31 550.6 11.01
M
a
= 20.23 T
pc
= 383.38 P
pc
= 666.38
Step 1. Calculate the apparent molecular weight from Equation 2-5:
M
a
= 20.23
Step 2. Determine the pseudo-critical pressure from Equation 2-14:
p
pc
= 666.18
Step 3. Calculate the pseudo-critical temperature from Equation 2-15:
T
pc
= 383.38
Step 4. Calculate the pseudo-reduced pressure and temperature by apply-
ing Equations 2-12 and 2-13, respectively:
r
g
a
v
pM
zRT
==
1
(2 -17)
v
V
m
zRT
pM
a
== (2 -16)
Reservoir-Fluid Properties 41
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 41

Step 5. Determine the z-factor from Figure 2-1:
z = 0.85
Step 6. Calculate the density from Equation 2-17:
Step 7. Calculate the density of the gas assuming an ideal gas behavior
from Equation 2-7:
The results of the above example show that the ideal gas equation esti-
mated the gas density with an absolute error of 15% when compared with
the density value as predicted with the real gas equation.
In cases where the composition of a natural gas is not available, the
pseudo-critical properties, i.e., p
pc
and T
pc
, can be predicted solely from
the specific gravity of the gas. Brown et al. (1948) presented a graphical
method for a convenient approximation of the pseudo-critical pressure
and pseudo-critical temperature of gases when only the specific gravity
of the gas is available. The correlation is presented in Figure 2-2. Stand-
ing (1977) expressed this graphical correlation in the following mathe-
matical forms:
Case 1: Natural Gas Systems
T
pc
= 168 + 325 g
g
- 12.5 g
g
2
(2-18)
p
pc
= 677 + 15.0 g
g
- 37.5 g
g
2
(2-19)
Case 2: Gas-Condensate Systems
T
pc
= 187 + 330 g
g
- 71.5 g
g
2
(2-20)
r
g
lb ft==
()(.)
(.)( )
./
3000 20 23
10 73 640
884
3
r
g
lb ft==
()(.)
(. )( . )( )
./
3000 20 23
085 1073 640
10 4
3
p
T
pr
pr
==
==
3000
666 38
450
640
383 38
167
.
.
.
.
42 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 42

p
pc
= 706 - 51.7 g
g
- 11.1 g
g
2
(2-21)
where T
pc
= pseudo-critical temperature, °R
p
pc
= pseudo-critical pressure, psia
g
g
= specific gravity of the gas mixture
Example 2-7
Rework Example 2-5 by calculating the pseudo-critical properties
from Equations 2-18 and 2-19.
Reservoir-Fluid Properties 43
300
0.5
350
400
450
500
550
600
650
700
0.6 0.7 0.8 0.9
Specific Gravity of the Gas
Pseudo-critical Properties of Natural Gases
Pseudo-Critical Temperature,°R Pseudo-Critical Pressure, psia
1.0 1.1 1.2
Miscellaneous gases
Miscellaneous gases
Condensate well fluids
Condensate well fluids
Limitations:
Max. 5% N
2
2% CO2
2% H
2
S
Figure 2-2. Pseudo-critical properties of natural gases. (Courtesy of GPSA and
GPA Engineering Data Book, 10th Edition, 1987.)
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 43

Solution
Step 1. Calculate the specific gravity of the gas:
Step 2. Solve for the pseudo-critical properties by applying Equations
2-18 and 2-19:
T
pc
= 168 + 325 (0.699) - 12.5 (0.699)
2
= 389.1°R
p
pc
= 677 + 15 (0.699) - 37.5 (0.699)
2
= 669.2 psia
Step 3. Calculate p
pr
and T
pr
.
Step 4. Determine the gas compressibility factor from Figure 2-1:
z = 0.824
Step 5. Calculate the density from Equation 2-17:
EFFECT OF NONHYDROCARBON COMPONENTS
ON THE Z-FACTOR
Natural gases frequently contain materials other than hydrocarbon
components, such as nitrogen, carbon dioxide, and hydrogen sulfide.
Hydrocarbon gases are classified as sweet or sour depending on the
hydrogen sulfide content. Both sweet and sour gases may contain nitro-
gen, carbon dioxide, or both. A hydrocarbon gas is termed a sour gas if it
contains one grain of H
2
S per 100 cubic feet.
The common occurrence of small percentages of nitrogen and carbon
dioxide is, in part, considered in the correlations previously cited. Con-
r
g
lb ft==
()(.)
(. )( . )( )
./
3000 20 23
0 845 10 73 640
10 46
3
p
T
pr
pr
==
==
3000
669 2
448
640
389 1
164
.
.
.
.
g
g
a
M
===
28 96
20 23
28 96
0 699
.
.
.
.
44 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 44

centrations of up to 5 percent of these nonhydrocarbon components will
not seriously affect accuracy. Errors in compressibility factor calculations
as large as 10 percent may occur in higher concentrations of nonhydro-
carbon components in gas mixtures.
Nonhydrocarbon Adjustment Methods
There are two methods that were developed to adjust the pseudo-criti-
cal properties of the gases to account for the presence of the nonhydro-
carbon components. These two methods are the:
• Wichert-Aziz correction method
• Carr-Kobayashi-Burrows correction method
The Wichert-Aziz Correction Method
Natural gases that contain H
2
S and or CO
2
frequently exhibit different
compressibility-factors behavior than do sweet gases. Wichert and Aziz
(1972) developed a simple, easy-to-use calculation procedure to account
for these differences. This method permits the use of the Standing-Katz
chart, i.e., Figure 2-1, by using a pseudo-critical temperature adjustment
factor, which is a function of the concentration of CO
2
and H
2
S in the
sour gas. This correction factor is then used to adjust the pseudo-critical
temperature and pressure according to the following expressions:
T¢
pc
= T
pc
-e (2 - 22)
where T
pc
= pseudo-critical temperature, °R
p
pc
= pseudo-critical pressure, psia
T¢
pc
= corrected pseudo-critical temperature, °R
p¢
pc
= corrected pseudo-critical pressure, psia
B = mole fraction of H
2
S in the gas mixture
e=pseudo-critical temperature adjustment factor and is defined
mathematically by the following expression
e=120 [A
0.9
- A
1.6
] + 15 (B
0.5
- B
4.0
) (2 - 24)
where the coefficient A is the sum of the mole fraction H
2
S and CO
2
in
the gas mixture, or:
¢
=
¢
+-
p
pT
TBB
pc
pc pc
pc
()1 e
(2 - 23)
Reservoir-Fluid Properties 45
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 45

A = y
H
2
S
+ y
CO
2
The computational steps of incorporating the adjustment factor e into
the z-factor calculations are summarized below:
Step 1. Calculate the pseudo-critical properties of the whole gas mixture
by applying Equations 2-18 and 2-19 or Equations 2-20 and 2-21.
Step 2. Calculate the adjustment factor e from Equation 2-24.
Step 3. Adjust the calculated p
pc
and T
pc
(as computed in Step 1) by
applying Equations 2-22 and 2-23.
Step 4. Calculate the pseudo-reduced properties, i.e., p
pr
and T
pr
, from
Equations 2-11 and 2-12.
Step 5. Read the compressibility factor from Figure 2-1.
Example 2-8
A sour natural gas has a specific gravity of 0.7. The compositional
analysis of the gas shows that it contains 5 percent CO
2
and 10 percent
H
2
S. Calculate the density of the gas at 3500 psia and 160°F.
Solution
Step 1. Calculate the uncorrected pseudo-critical properties of the gas
from Equations 2-18 and 2-19:
T
pc
= 168 + 325 (0.7) - 12.5 (0.7)
2
= 389.38°R
p
pc
= 677 + 15 (0.7) - 37.5 (0.7)
2
= 669.1 psia
Step 2. Calculate the pseudo-critical temperature adjustment factor from
Equation 2-24:
e=120 (0.15
0.9
- 0.15
1.6
) + 15 (0.1
0.5
- 0.1
4
) = 20.735
Step 3. Calculate the corrected pseudo-critical temperature by applying
Equation 2-22:
46 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 46
T¢
pc
= 389.38 - 20.735 = 368.64
Step 4. Adjust the pseudo-critical pressure p
pc
by applying Equation 2-23:
Step 5. Calculate p
pr
and T
pr
:
Step 6. Determine the z-factor from Figure 2-1:
z = 0.89
Step 7. Calculate the apparent molecular weight of the gas from Equa-
tion 2-10:
M
a
= (28.96) (0.7) = 20.27
Step 8. Solve for gas density:
The Carr-Kobayashi-Burrows Correction Method
Carr, Kobayashi, and Burrows (1954) proposed a simplified procedure
to adjust the pseudo-critical properties of natural gases when nonhydro-
carbon components are present. The method can be used when the com-
position of the natural gas is not available. The proposed procedure is
summarized in the following steps:
Step 1. Knowing the specific gravity of the natural gas, calculate the
pseudo-critical temperature and pressure by applying Equations
2-18 and 2-19.
r
g
lb ft==
()(.)
(. )( . )( )
./
3500 20 27
089 1073 620
11 98
3
p
T
pr
pr
==
=
+
=
3500
630 44
555
160 460
368 64
168
.
.
.
.
¢
=
+-
p
pc
(.)(.)
..(.)(.)
669 1 368 64
389 38 0 1 1 0 1 20 635
Reservoir-Fluid Properties 47
Reservoir Eng Hndbk Ch 02a 2001-10-24 09:23 Page 47
