Seetharaman S. Fundamentals of metallurgy
Подождите немного. Документ загружается.

There are two types of plasma, thermal plasma and cold plasma, depending
on the respective temperature of the electrons (T
e
) and the heavy particles
(neutral particles and ions (T
h
)). In thermal plasmas (pressure > 10 kPa), the
temperatures of the gas and the electrons are comparable and are of many
thousands of degrees. Starting materia ls are typically ato mised at these
temperatures and the powder synthesis occurs during condensation outside the
plasma when the gasses cool.
30
In contrast, in a low-pressure (p < 200 Pa) low-
temperature plasma (cold plasma) the electron temperature is much higher than
the gas or ion temperature, which is close to room temperature because of the
poor collisional coupling betwee n electrons and heavy particles at reduced
pressures.
31
As the growth of particles may be completely determined by
chemical kinetic factors, thermal and low-temperature plasmas may produce
materials with different structures and properties.
Plasma methods are very popular as they enable the preparation of two-
component compounds as well as multicomponent powders with a high purity.
Besides ceramic powders (SiC, SiN, BN), metallic nanoparticles are produc ed.
Nano-powders are used for their light emission properties (Si) or magnetic
recording tapes. Nanostructured thin fil ms, consisting of nanoparticles
embedded in a metal matrix have also been produced.
12.3 Forming processes towards near-net shape
Near-net-shape components can be obtained by a conventional two-step process
consisting in first giving the shape of the component (compaction) followed by
its consolidation (si ntering). Other processes allow the shaping and the
consolidation of the component in one step (hot isostatic pressing, hot extrusion,
hot forging, etc.). Because of its economical importance infiltration is also
shortly described in this part, as well as the fast developing rapid prototyping
processes.
12.3.1 Conventional route
Compaction
Compaction is a widely used method to form semi-finished or near-net-shape
components from a powder. The quality of compaction (density gradients,
micro-cracks) has a great influence on distortion of the product shape after
sintering step as well as on the properties (defect-free components) of the final
product.
Powder compacts can be obtained by unia xial pressing, isostatic pressing,
metal injection moulding (MIM) or by less used processes such as powder
rolling, extrusion and dynamic or explosive compaction.
486 Fundamentals of metallurgy
Uniaxial pressing
Uniaxial pressing (dry or die pressing) is the most common method of
compaction to form PM components. This low-cost process is adapted to high-
volume (up to few hundreds of parts per minute) production of `relativel y
simple' geometry powder compacts.
32
It consists in compacting a dry powder (i.e. < 2 wt% water) in a die at a
pressure ranging from 20 to 700 MPa by operating one or more rigid punches
(higher numbers of punches are needed with increasing complexity of the
component). A sufficiently high pressure is required to guarantee a sufficient
strength of the green compact for subsequent handling and processing.
Compaction consists in the following three steps: filling the die with the
powder, compacting the powder and ejecting the powder compact from the die.
Die filling and compaction control the uniformity of the green density in the
powder compact, which is a crucial parameter. Green density gradients have to
be minimi sed in order to reduce differential shrinkage during sintering and shape
distortion, as well as to avoid induced defects that limit the properties and
reliability of the part. Ejection of the compact is also a critical step because
macroscopic defects can be created.
Uniform and high packing density of a powder during die filling favours high
green density with low green density gradients after compaction. This implies
the use of good-flowing powders, which are also necessary to guarantee
reproducible die filling. High-flowability powders have a relatively large
particle size distribution lying between 40 and 400 m and are usually spherical
or equiaxial. Powders with a low flowability (fine (1±10 m) irregular powders)
have to be granulated before dry pressing (e.g. by spray drying) with 1 to 5 wt%
organic additives such as binders, plasticisers and lubricants, which also
improve the handling of the compact and/or the compactibility of the powder.
Organic binders (wax, polyethylene glycol) allow the increase the green strength
of the compact. Plasticisers (water, ethylene glycol) are used in combination
with binders to improve the deformability of the powder during compaction.
Lubricants (wax, magnesium stearate, stearic acid) reduce the interfacial
frictional forces between individual particles favourable to powder compaction
and/or between particles and die surfaces. They also reduc e the required ejection
pressure of the compact, thus avoiding macro-defect formation. Those additives
will be eliminated by thermal decomposition before sintering or in a special
zone of a continuous sintering furnace.
During powder compaction, the applied pressure induces particle
rearrangement, deformation, in som e cases fracturing, and finally consolidation
of the particulate assembly. Green density and compact strength increase with
increasing compaction pressure.
12
Two scales of green density gradients can
occur during powder compaction: (i) macros copic density gradient induced by
non-uniform die filling and/or pressure gradients during compaction and (ii)
Understanding and improving powder metallurgical processes 487
microscopic density gradient due to packing defects, hollow particles (granules),
and/or insufficient particle (granule) deformation during compaction. Non-
uniform pressure/stress gradient is due to the non-uniform pressure/stress
transmission at particle±partic le and particle±die (die wall friction) contacts.
Green density gradients are minimised by using lubricants, smooth surf ace die,
low compaction ratio, double-action or floating die pressing instead of single-
action pressing and by adapting the numbers of punches to the number of
different thickness areas in the part.
During ejection, the compact integrity is favoured by a sufficiently high green
strength to withstand the applied forces on the compact required to eject it from
the die, a low ejection pressure, favoured by the use of lubricant to reduce die
wall friction and a low elastic springback (expansion of the powder compact
after ejection from the forming die). The negative effect of a differential
springback (the axial springback being higher than the radial one) can be
reduced by maintaining a small axial pressure on the compact during ejection
(punch hold-down ejection or withdrawal of the die).
14
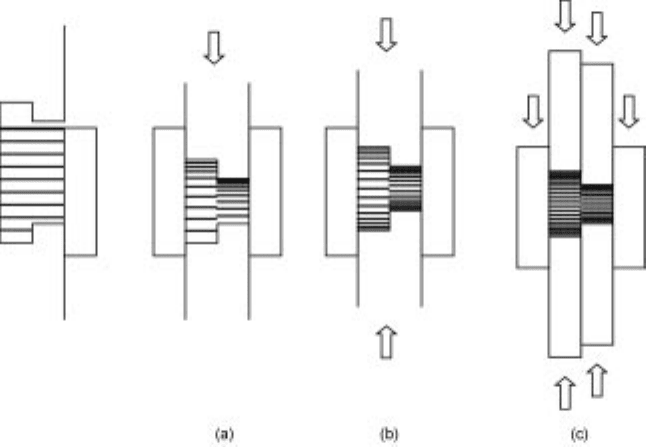
Uniaxial pressing can be performed with a single-action, double-action or
floating die press (Fig. 12.4).
A single-action press uses only one moving punch. As it induces high green
density gradients in the axial directions, it may be used for parts with a very low
height to diameter aspect ratio and simple shape. A double action press has at
least two independently moving punches that induce a more uniform compaction
12.4 Uniaxial pressing: (a) single action, (b) double action, (c) floating die and
double action multiple punches.
488 Fundamentals of metallurgy
especially for simple large and thick parts. In pressing of multi-level compacts,
the several punches have to be operated independently so that the different areas
in the die are equally compacted. In a floating die press, a similar effect can be
obtained, the movement (floating) of the die corresponding to an upwards
movement of the lower punch.
14
In more sophisticated floating die press, the
punches and the die can move independently in order to reduce the green density
gradients. This allows the production of complex parts with tapers, holes and
multiple steps. If higher working speeds and precise control of the movements
are required, the die is directly moved by the press.
Isostatic pressing
Isostatic pressing (cold isostatic pressing) consists in compacting a powder in an
elastomeric contain er submersed in a fluid at a pressure of 20 to 400 MPa. Cold
isostatic pressing allows the production of simple-shaped small or large powder
compacts (up to 2000 kg) with a uniform green density even for large height/
diameter ratio part (impossible by uniaxial pressing), but with the sacrifice in
pressing speed and dimensional control, requiring subsequent machining in the
green compact. Cold isostatic pressing is used for powders that are difficult to
press such as hard metals.
Metal injection moulding
Metal injection moulding (MIM) is adapted to the production of small complex
near-net-shape compounds (wall thickness down to 0.3 mm) with very good
tolerances (0.3 to 0.5%), especially for medium (thousands of parts/year) to high
volume (millions of parts/year) production.
33
Because of the high raw-material
costs, MIM is usually limited to the production of parts lighter than 100 g.
This process consists in filling a die cavity with a viscous mixture of powder
and binder at around 130 to 200ëC under a pressure up to 150 MPa.
12
Metal
injection moulding parts are produced in four different steps: feedstock
formulation, moulding, debinding and sintering.
The feedstock is a homogeneous pelletised (granulation to a special shape)
mixture of fine spher ical metal powder with a mean size ranging from 5 to
15 m and 30 to 45 vol.% organic binder.
33
A binder system usually has three
components, a backbone that provides green strength and that will stay after
debinding, a filler phase that is easily extracted during initial stages of debinding
and surfactant to control feedstock rheology. During moulding, the feedstock is
introduced to the heated injection unit by, for example, a rotating screw, the
mixture is then injected in the die by axial movemen t of the screw and finally the
part is ejected from the die after quick cooling. The main parameters are
injection temperature, pressure and speed, as well as thermal conductivity and
viscosity of the feedstock and the mould temperature.
Understanding and improving powder metallurgical processes 489
The debinding can be done by thermal decomposition, solvent extraction or a
combination (also called catalytic decomposition). A typical binder removal rate
is 2 to 3 mm wall thickness per hour. Finally, the part undergoes a densification
up to 95% to 99% of the theoretical density as well as a shrinkage of 14% to
20% during the sintering step (see below).
The MIM process is applied to produce parts in stainless steels (304L, 316L),
tool steels (M2), soft magnetic alloys (Fe-50 %Ni, Fe-3 %Si, 430L), alloys for
glass-to-metal sealing applications (kovar), cobalt-, nickel- and titanium-based
alloys used for wear medical, automotive and aerospace applications.
Powder rolling ± extrusion ± dynamic and explosive compaction
Powder rolling (roll compacting) consists in compacting a powder continuously
passing between two turning rolls.
14
A binder is usually added to the powder to
favour densi fication. For a given thickness of the strip (defined by the distance
between the two rolls), the density of the compacted strip can be increased by
the increasing the diameter of the roll and reducing the rolling speed (maximum
speed lying about 0.5 m/s). The main advantage of powder rolling compared to
conventional casting and rolling process is a low amount of rolling passes
necessary to produce a thin strip. The main disadvantages are a high powder
price and a low production rate.
Extrusion consists in forcing a viscous mixture of powder and binder through
a die. The obtained, shaped product is sintered with a slow heating rate in order
to remove the binder.
During dynamic and explosive compaction (high-energy rate compaction),
that is only applied at lab-scale, powders are compacted at very high velocities
(200 m/s) by the propagation of a high-pressure wave. One set-up uses the
conventional die compaction with an upper punch moving at high velocity
through the action of an explosive charge or compressed gas. Another one
consists in encapsulating a powder in a mild steel tube and subjecting this tube
to the action of sheet explosives taped to it.
The advant ages are a high green density, a higher green and sinter ed strength
and lower density gradient.
13,14
Green density of 99% of theoretical density has
been achieved for aluminium, stainless ste el, amorphous powders,
12
even for
tungsten compacts a relative density of 97.6% has been obtained.
Sintering
Sintering originally used to produce clay pots
35
is nowadays involved in the
fabrication of net-shape components in ceramics, cermets, metals and com-
posites. Th e application fields are automobile and aeronautic industries (valves,
bearings, aircraft wings weight), electrical and electronic industries (tungsten
wires, ultrasonic transducers), medical industries (dental or hip implants).
490 Fundamentals of metallurgy
During sintering porosity and the microstructure change irreversibly from
contacting particles to almost dense material. This induces improvement of
many properties such as strength, ductility, conductivity, magnetic permeability,
and corrosion resistance.
Sintering can be defined as a thermal treatment for bonding particles into a
coherent, predominantly solid structure via mass transport events that often
occur on the atomic scale.
34
The sintering induces the consolidation (increase of
strength) of a loose or compacted powder and is usually accompanied by
densification (shrinkage). During sintering the reduction of total surface energy
(usual driving force for mass flow during sintering) is due to the decrease of
surface area by formation of inter-particle bonds and the reduction of surface
curvature. The path of the atomic motion occurring during the mass flow in
response to the driving force is called the sintering mechanism.
12
For metal
powders, the mechanisms are usually diffusion processes with surface, grain
boundary or lattice paths. Sintering progr esses in different stages; for each stage
(i.e. for each driving force type corresponding to a particular particle-pore
geometry), different ways of mass flo w (i.e. sintering mechanism) are possible.
The knowledge of the relations between the different sintering parameters and
each sintering mechanism during the different sta ges allows modelling and thus
optimising the sintering parameters.
The main sintering parameters are:
· particle size (reduction of particle size increases the surface energy per unit
volume of the powder and so the driving force associated with sintering, thus
the sintering rate);
· particle size distribution (large difference in curvature of the grains, due to
grain size difference, will promote coalescence, i.e. the growth of the large
grain at the expense of the smaller one);
· temperature (exponent ial influence on sintering because it is involved in the
activation energy of the sintering mechanisms (e.g. diffusion processes));
· time (influences diffusion);
· green density (density gradients occurring during compaction will induce
differential shrinkage during sintering and maybe distortion of the part
because higher green density induces lower shrinkage);
· applied external pressure (to obtain fully dense material, HP or HIP
processes);
· amount of liquid, if any;
· sintering aids (favouring diffusion in the solid or liquid state);
· atmosphere.
A particular sintering atmosphere can be used to protect the metal powder from
oxidation (argon, vacuum), to remove the oxidati on layer on the powder
(reducing atmosphere such as hydrogen, carbon monoxide, dissociated ammonia
or natural gas), to control the carbon content of the powder, to remove the
Understanding and improving powder metallurgical processes 491
lubricants and binders introduced duri ng compaction or even to react with the
powder (form ation of nitride). Sintering can be classified as solid state, liquid
phase, reaction sintering and microwave sintering.
Solid state sintering
In solid state sintering the microstructure changes are divided into three different
stages. The first stage corresponds to the growth of the bond, called neck, between
two particles, independently of the growth of the neighbouring necks. The pores are
interconnected with an irregular shape. The intermediate stage occurs as the
merging necks shrink the pores to form interconnected pores with a more smooth
usually cylindrical shape. Most of the densification and change in properties occurs
in this intermediate stage. The final stage corresponds to pore closure, where the
pores become spherical and isolated. By definition the driving force changes for
each sintering stage. During the initial stage the driving force is the curvature
gradient between the particle and the neck, during the intermediate stage it is the
curvature around the cylindrical pore and during the final stage the curvature
around the spherical pore. For every sintering stage, the mass transport process
(sintering mechanism) can be described by a characteristic equation.
There are sintering mechanisms that induce shrinkage (i.e. densification) such
as volume diffusion, grain boundary diffusion, plastic flow and viscous flow (for
the amorphous solids) and some that do not such as surface diffusion,
evaporation±condensation and volum e diffusion from a surface source to a
surface sink. Different sintering mechanisms are involved at different moments
during the sintering process. For example, a finer particle size usually favours
sintering by surface or grain boundary diffusion compared to volume diffusion.
During the initial stage, the different sintering mechanisms can be diffusion
(surface-, volume- or grain boundary-diffusion), evaporation or disloca tion
motion. The sintering mechanism is usually described by the size of the growing
neck between the particles but shrinkage or relative change in surface area can
also be used.
The intermediate stage is characterised by densification usually coupled with
grain growth during the latter phase of the intermediate stage. The smaller
grains, having a higher curvatur e, are progressively incorporated to the
neighbour grain by grain boundary motion. This grain boundary motion induces
drag forces on the pores, which can move by volume, surface diffusion or
evaporation±condensation mec hanism across the pore. When the moving rate of
the grain boundary is too high (e.g. favoured by high temperature), the pores
cannot impinge the grain boundary any more and become isolated inside the
grain (beginning of the third stage). In this case, the rate of densification is much
smaller because volume diffusion is less fast than grain boundary diffusion.
35
Consequently, it is important to minimise pore±grain boundary separation by
careful temperature control, the incorporation of second-phase inclusion such as
492 Fundamentals of metallurgy
oxide particles into the microstructure to impinge the grain boundary, or the use
of narrow initial particle size distribution.
37,38
The usual parameter to follow the
sintering is the rate of densification. The two sintering mechanisms involved in
densification are grain boundary and volume diffusion. Surface diffusion or
evaporation±con densation mechanisms, inducing no shrinkage, are also
expected to be active in smoothing the pore structure and in pore migration
with grain boundaries during grain growth. Long sintering times (compared to
the first stage) are required to achieve significant property or density change s.
Temperature has a complex effect on the sintering because diffusion, grain
growth and pore motion are all thermally activated.
The third stage is characterised by the presence of isolated spherical pores. If
the closed pores are mobile enough to stay coupled to the grain boundary, then
continued shrinkage is expected. This is favoured by a homogeneous grain size,
which lowers the curvature of the grain boundary and so decreases their motion
rate. If not, after separation from the grain boundary, the pore must emit
vacancies that move by volume diffusion, which is a slow process, towards the
distant grain boundary. This leads to a drop of the densification rate. With
prolonged sintering, the larger pores grow at the expense of the small er ones
(that emit more vacancies in the grain because of higher curvature). This is
called pore coarsening or Ostwald ripening. In addition to pore coarsening, the
pore size can increase by coalescence, due to grain growth by grain boundary
motion dragging pores towards each other. If the pore has trapped gas, an
internal pressure is induced inside the pore, which limits densification. If this gas
is soluble in the matrix, the densification rate is controlled by the internal gas
pressure and not by the limit of solubility.
The usual parameter to follow the sintering during the final stage is the rate of
densification, but the rate of shrinkage, surface area change, or neck growth
could also be used. The rate of densification depends on the pore amount, pore
radius, volume diffusion, grain size distribution and stress effects (compressed
trapped gas workin g against pore shrinkage).
39
Liquid phase sintering (HSS)
Liquid phase sintering is involved when powders of different composition are
mixed. Usually the constituent, that remains solid during sintering, should have
a relatively high solubility in the formed liquid and inversely the solubility of the
liquid in the solid should be low to ensure that this liquid phase is not transient.
Common systems are: WC-Co, Fe-Cu, Cu-Sn, etc.
36
The main advantage of liquid phase sintering is the lower sintering time
required compared to solid state sintering. During heating, the mixture of
powders first undergoes solid state sintering, which can induce significant
densification, before the formation of the liquid at the sintering temperature.
After the liquid is formed, the sintering depends on the amount of liquid and is
Understanding and improving powder metallurgical processes 493
usually divided into three stages: rearrangement, solution±r eprecipitation, and
the third stage. If the amount of liquid is sufficient to fill all the interparticle
spaces, the theoretical density can be obtained during the rearrangement stage.
For lower liquid contents, the solid skeleton slows down the densification and the
contribution of the last two sintering stages becomes significant. In fact, less than
15 vol.% of liquid is usually used to avoid distortion of the part during sintering.
During the first stage (rearrangement) and in case of a wetting liquid, the
liquid spreads as soon as the liquid is formed between the solid particles under
the influence of capillary forces. The rate of densification controlled by viscous
flow is very high at the begi nning and then continuously slows down. As the
densification rate governed by rearrangement decreases, another mechanism
called dissolution±diffusion±reprecipitation, characteristic for the second stage
prevails. The solubility of a grain in its surrounding liquid increases with the
curvature of the grain, i.e. with a decrease of the grain size. The difference of
solubility as a function of grain size induces a concentration gradient of solute
species in the liquid, that diffuse from the small grains to the large ones, on
which the solute species precipitate (reprecipitation) when the solubility limit in
the liquid is reached. So during this stage, grain coarsening occurs (Ostwald
ripening). Simultaneously the elimination of the high-energy vapour interface is
ob tained by grain shape accommodation (fl attening) during solution±
reprecipitation events inducing densification by higher packing of the grains.
The third stage corresponds to the densification of a rigid solid skeleton with a
rate similar to the one obtained in solid state sintering.
Reaction sintering
Solid state reaction sintering of metal powder mixtures is a way to produce
alloys such as carbon steel, Fe-Ni, Fe-Mn, Fe-Si, Fe-Cr, Fe-Mo and Cu-Ni. The
principal phenomenon occurring during sintering is the solid state interdiffusion
between the different compounds. The driving force is the chemical potential
gradient due to concentration differences. This phenomenon superimposes the
metal powder self-diffusion caused by surface and interfacial tension forces,
occurring in solid state sintering of pure or pre-alloyed powders. Reaction
sintering is favoured by fine particle size (smalle r the diffusion distance) and
high temperature (higher coefficient of diffusion).
Reaction sintering can also occur during liquid phase sintering (Mo + 2Si !
MoSi
2
) or by reaction of the sintering atmosphere with the powder (3Si + 2N
2
!
Si
3
N
4
).
Microwave sintering
Sintering of metal powders is a surprising recent development in microwave
applications because bulk metals reflect microwaves. However, compacted
494 Fundamentals of metallurgy
metal powders at room temperature will absorb microwaves and will be heated
very effectively and rapidly (above 100ëC/min), inducing sintering. Metals such
as iron, steel, copper, aluminium, tin, nickel, cobalt, tungsten have been sintered
to high density by microwaves. Cylinders, rods, gears, and other automotive
components with until now a maximum size of 10 cm have been produced in 30
to 90 min.
40
Contrary to the conventional heating where the transfer of thermal energy is
done by conduction to the inside of the part, microwave heating is a volumet ric
heating consisting in an instantaneous conversion of electromagnetic energy into
thermal energy. The mechanisms involved in microwave sintering are not
completely understood at the moment. However, the sample size and shape, the
distribution of microwave energy and the mag netic and electromagnetic field
radiation are important parameters.
12.3.2 Other routes
This part is mainly dedicated to full density sintering processes such as hot
pressing, hot isostatic pressing, hot extrusion, hot forging and field assisted
sintering. However, other techniques such as infiltration and rapid prototyping
techniques will also be presented.
Full density is required to improve product properties such as rupture and
fatigue strength, toughness, thermal or electrical conductivity. This can only be
achieved when stress and temperature are simultaneously applied during den-
sification to close the pores.
13
Because the powder usually has to be protected
from reaction with air, this makes the hot compaction processes complex and
costly. So these techniques are reserved for expensive materials with special
properties such as beryllium and magnesium alloys (finer grain size), super-
all oys (elimination of segregation), high speed steels and dispersion
strengthened alloys (homogeneous distribution of the second phase). The main
parameters are temperature, applied stress, strain rate and grain size.
`Near-net-shape' components, implying material saving, combined with
higher properties can make these processes competitive compared to the
conventional casting, forging, machining route.
Hot pressing
Hot pressing consists in applying pressure with a hot punch on the metal powder
placed in a heated die usually under a protective atmosphere.
14
The main
problem with hot pressing is to find a suitable die material, which has to
withstand the applied pressure without reaction with the metal powder. Although
the total amount of deformation of the compact is relatively limited compared to
hot extrusion or hot forging, complete densification is generally achieved.
Understanding and improving powder metallurgical processes 495
