Seetharaman S. Fundamentals of metallurgy
Подождите немного. Документ загружается.


Table 12.1
Typical operating conditions of the different mills
Vibrating
Planetary Vertical
Horizontal
Horizontal
mill
ball mill attritor
attritor
ball mill
Cycle time (h)
0.01±0.5
1±10
0.1±10
0.1±10
>
10
Powder amount
> 1 kg (industry) 10
±100 g 100 kg (industry) 100 kg (industry)
> 1 ton
1 g (laboratory)
100 g (laboratory) 100 g (laboratory)
Mill speed (rpm) 1000 (industry) 80
±400 60 (industry) 1800
10±50
3300 (laboratory)
300 (laboratory)
Ball diameter (mm) 1
±10
6±30
6±10
6±10
6±25
Ball filling level
50±70% 70%
30%
50%
Powder filling level
1:10 powder 1:10 powder 1:10 powder
100% of ball interstices
to ball weight to ball weight to ball weight
ratio
ratio
ratio
Powder size (
m) 1
±10
5±100
5
±100
5±100
10±50
On a larger scale, the economic point of view prevails and the efficient use of
the equipment and raw materials, as well as energy consumption, become
important.
Coldstream process
In this process, a high-pressure air-stream (e.g. 7 MPa) containing the particulate
material, enters a vacuum chamber through a venturi nozzle where it impacts a
tungsten carbide target.
9
The pressure drop at the exit of the nozzle induces a
temperature drop thus chilling and embrittling the material inducing its fracture
when it impacts the tungsten carbide target. The mater ial is then transpor ted to a
first classifier, which allows oversized products to drop into a storage vessel for
subsequent impact against the target. This process applies to many body-centred
cubic metals that go through a ductile to brittle transition at low temperatures. It
is used for hard, abrasive, relatively expensive materials such as tungsten
carbide, tungsten alloys, molybdenum, tool steels, beryllium, and other alloys
(Inconel, nickel and cobalt high temperature alloys). This process allows a rapid
production (1 t/day) of irregular micron-sized particles.
12.2.2 Atomisation route
Atomisation is often used to produce metal powders and especially pre-alloyed
powders (brasses, stainless steels, superalloys, NiAl . . .). Moreover its high
cooling rates (10
2
to 10
7
K/s) allows producing alloys that cannot be obtained by
casting. The flexibility of this method, coupled to its applicability to many
alloys, easy process control and high production rate makes it a very interesting
route.
10,12
The world capacity of production is estimated to be higher than one
million tons per year.
11
Atomisation occurs when a jet of liquid is converted to very small droplets.
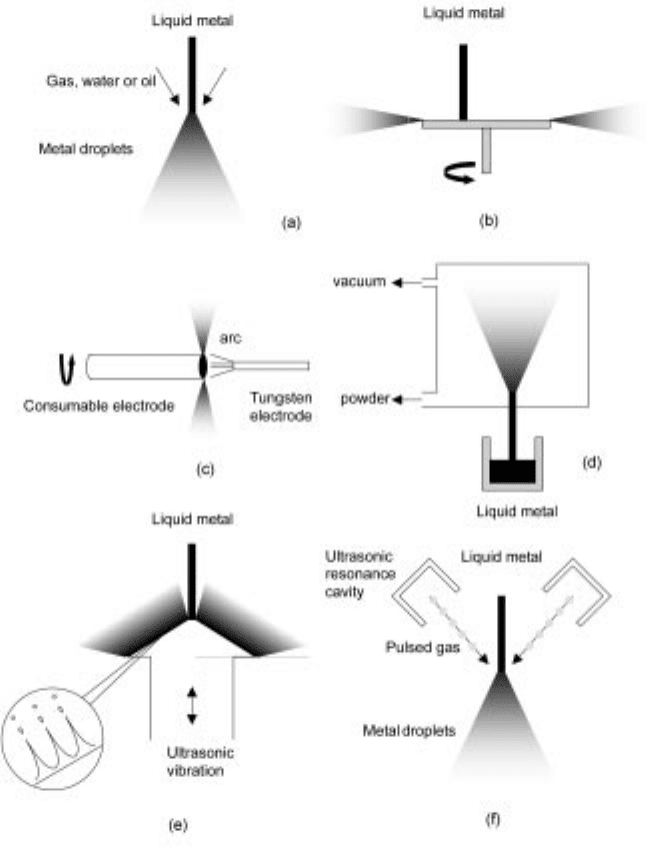
The different atomisation processes are (see Fig. 12.2):
· Two-fluid atomisation, where a molten metal is broken up into droplets by
impingement of high-pressure jets of water, oil or gas (a).
· Centrifugal atomisation, where a liquid-stream is dispersed into droplets by
the centrifugal force of a rotating electrode (c), disc or cup (b).
· Vacuum or soluble-gas atomisation, where a molten metal is supersaturated
with a gas that causes atomisation of the metal in a vacuum chamber (d).
· Ultrasonic atomisation, where a film of liquid metal (e) or the atomising fluid
(f) is agitated by ultrasonic vibration.
Atomised metal powders are generally found to follow a log normal distribution.
The particles size distributions are generally from few m up to 500 m and the
mean particle size from 10 to few 100 m. The geometric standard deviation
g
measures the spread of particle size around the median mass diameter d
m
and
Understanding and improving powder metallurgical processes 477
typically varies from about 1.7 to 2.3 for water- and gas-atomised metal powders.
Compared to other types of powders, the atomised powders are relatively compact
(apparent density up to 65% of the theoretical density), with a high packing density
and a low specific surface area. This induces good flow characteristics, good
compressibility but low sintering activity. Particles shape varies from irregular to
12.2 Atomisation processes. (a) gas, water or oil atomisation, (b and c)
centrifugal atomisation, (d) vacuum atomisation, (e and f) ultrasonic
atomisation.
478 Fundamentals of metallurgy
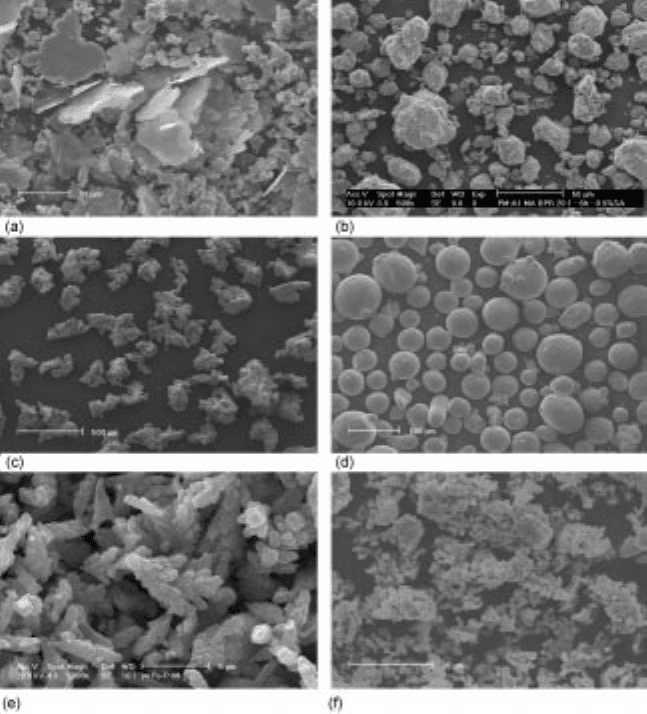
spherical for respectively water and gas atomisation (Fig. 12.3c, d). The required
particle shape depends on the following process used to obtain the final product.
Spherical powders are us ed for therma l s prayi ng loose sint ering or hot
consolidation (e.g. extrusion, isostatic pressing). But irregular powders are needed
to guarantee a high enough green strength after cold pressing. The cleanliness of
the powder is also a very important parameter. The possible contaminants are bulk
dissolved impurities, surface impurities and inclusions (mainly from melt practice).
Surface oxidation is often considered as the main purity index.
The process parameters controlling the powder characteristics (size, distribu-
tion, shape and oxygen content) are related to the atomiser design (nozzle definin g
12.3 Morphology of powders obtained by (a) milling, (b) mechanical alloying,
(c) water atomisation, (d) gas atomisation, (e) electrolysis (courtesy of Ye
Xingpu) and (f) precipitation (courtesy of Rudy de Vos).
Understanding and improving powder metallurgical processes 479
the geometry of the atomisation), the atomiser operating conditions (nature of the
fluid, pressure, temperature, atmosphere) and the material (melting temperature,
viscosity, surface tension, superheat). Some of these parameters have a great
influence on the powder characteristics. For example, the production of fine
powders is favoured by low metal viscosity, low metal surface tension, high
superheat of the metal (difference between the temperature of the molten metal
and its melting temperature), small nozzle diameter, high atomising pressure, high
flow rate and high velocity of the atomising fluid, short metal stream and short jet
length.
13
Spherical atomised powders are obtained by increasing the superheating
of the liquid metal (reduction of viscosity), increasing its surface tension by
addition of deoxidisers, such as B, P and Si and increasing the solidification time
of the particle (atomising with gas instead of water). More details about the
atomising parameters can be found in references 1 and 3.
In the following, the different atomisation methods will be described in more
detail.
Water atomisation ± oil atomisation
Water atomisation, with its capacity of at least 700 000 tons per year and
production rates up to 500 kg/min (8 kg/s), is the process mostly used for the
commercial production of irregular (Fig. 12.3c) metal powders (iron, stainless
steels, tool steels, soft magnetic alloys, nickel alloys and copper).
11
The
production costs are lower than the other atomisation methods. However,
limitations exist in relation to powder purity (relatively high oxygen content
(1 wt%)), particularly with reactive metals and alloys. The median particle size
decreases with increasing water pressure. Most industrial plants use a water
pressure in the range of 5 to 20 MPa, that results in mass median particle sizes of
30 to 150 m. Finer powders (median particle size from 5 to 20 m) can be
produced by using much higher water pressures (50 to 150 MPa).
Oil atomisation is similar to water atomisation. Oil is used as atomising
medium in order to decrease the irregular character of the particle shape and to
reduce oxidation, especially for molten metal containing elements such as Cr,
Mn or Si, which are readily oxidised.
12,14
Gas atomisation
The production of gas atomised powders is lower than water-atomised ones
because of its higher cost. It is about 300 000 tons per year for air atomisation
and around 50 000 tons per year for inert gas (nitrogen, argon and helium)
atomisation, the later ensuring a low oxygen content (100 ppm) of the spherical
powders (ferrous and non-ferrous alloys) (Fig. 12.3d).
11,12
In gas atomisation the powder size distribution strongly depend s on the
nozzle design (`free-fall' configuration or `confined'). Confined nozzle designs
480 Fundamentals of metallurgy
increase the amount of fine powder particles (<10 m) because of a higher
velocity and density of the gas on contact with the metal.
10
In practice, the mean
particle size decreases with increasing gas pressure and decreasing melt flow
rate. The pressures used in gas atomisation are generally in the range of 0.7 to
3 MPa. High-pressure gas atomisation (HPGA) allows the production of very
fine particles, e.g. Sn-5Pb with a mean size of 5 m was obtained at 12.5 MPa.
15
New trends in gas atomisation concern the nozzle design.
14
In conventional
nozzles, the atomising medium impacts the molten stream in the turbulent region
instead of the laminar region as for a Laval nozzle. The use of such a Laval
nozzle reduces the particle size and spread. A moderate gas pressure (e.g.
2 MPa) is sufficient to produce powders with d
50
10 m with Ar or N
2
as
atomising gas or 5 m with He.
16
Another possibility is hot gas atomisation. As the hot gas exit velocity in the
supersonic gas nozzle increases subst antially with the temperature of the
atomisation die, a higher kinetic energy is available to disrupt the melt stream
into finer droplets and hence lower powder median size. But for each atomising
temperature, the nozzle design has to be optimised in the area of expansion of
the gas at supersonic velocity.
Vacuum atomisation ± tandem atomisation
In the vacuum atomisation, a molten metal supersaturated with gas (noble gas or
hydrogen) under pressure (1 to 3 MPa) is exposed to vacuum as a thin stream,
resulting in melt disintegration into fine droplets, as the dissolved gas bursts out
of the molten metal.
11,14
This method is mainly used to produce spherical
superalloy powders.
12
The combin ation of vacuum and ga s atomisation, known as tandem
atomisation, allows the production of fine super alloy powder.
17
After gas
atomisation, the droplets begin to cool down and the retained dissolved gas is
rejected from the solidifying metal dendrites. So the remaining liquid metal
becomes more and more supersaturated until a gas bubble nucleates that grows
explosively to produce a secondary atomisation event. The partially solidified
droplets shatter into much finer droplets that subsequently solidify into ultra-fine
powder particles.
Centrifugal atomisation (rotating elect rode, rotating disc)
In centrifugal atomisation, centrifugal forces break up the molten metal as
droplets that then solidify as powder particles.
10
In the rotating electrode process (REP), a consumable electrode is rotated
(1000 up to 50 000 rpm) while it is melted by an electric arc. The molten metal is
ejected at the edge of the electrode under the influence of centrifugal forces in
the form of droplets that solidify as spherical particles in an inert gas filled
Understanding and improving powder metallurgical processes 481
chamber. There is no liquid metal±crucible contact, which prevents the
contamination by ceramic particles. This is also an advantage for molten
titanium alloys that are corrosive to nearly all container materials.
The median particle size (about 250 m) decreases with decreasing melt
rates, increasing rotational speed and electrode diameter. Usually the spreading
of the particle size is much less by centrifugal than by gas atomisation.
Special methods ± ultrasonic atomisation
There are several ultrasonic atomisation techniq ues such as capillary-wave
atomisation (no commercial application) (Fig. 12 .2e), ultrasonic gas atomisation
(USGA) (Fig. 12.2f) and double ultrasonic atomisation (still in development).
In the capillary-wave atomisation, the liquid metal meets a vibrating surface
at ultrasonic frequencies and droplets (< 100 m) are ejected from the surface. In
ultrasonic gas atomisation (USGA) the molten metal stream is disintegrated by
impact with multiple high velocity gas pulses. The high-pressure gas (1.4 to
8.2 MPa) is accelerated by a shock wave tube (resonance cavities) to speeds of
up to Mach 2 at frequencies ranging from 60 to 120 kHz. The particle size is
usually less than 30 m. It is used commercially for production of low-melting
alloys such as aluminium alloys and on a lab-scale for stainless steels, Cu-, Ni-
and Co-based alloys.
10
In double ultrasonic atomisation, the metal stream is guided towards the inner
wall of a tubular resonator excited at ultrasonic frequencies. The molten metal
wets this vibrating wall and disintegrates according to the capillary-wave
atomisation process. Meanwhile, non-stationary shock waves are generated in an
inert gas flowing into the same tube. The pressure pulses, like in ultrasonic gas
atomisation (USGA), theref ore further disintegrate the capillary-wave-atomised
droplets. As the break-up of a molten metal occurs in two steps, the problem of
stream diameter in USGA is cancelled.
12.2.3 Aerosol routes
In aerosol-based processing techniques a liquid precursor (nitrates, acetates,
chlorides, alkoxides) is atomised into finely divided submicrometer liquid droplets
(aerosol) that are distributed in a gas medium. This aerosol enters a heated reaction
zone, where the solvent (methanol, ethanol, acetylacetone) is rapidly evaporated or
combusted and the intimately mixed chemical precursors are decomposed and/or
undergo chemical reaction to yield the desired products. The temperature, the
composition of the chemical precursors, the flow rate of the aerosol and the
atmosphere are the main parameters. The temperature should be high enough to
allow the complete reaction, but low enough to prevent excessive grain growth.
These simple and inexpensive processes can be adapted for the production of
ultrafine multicomponent powders with a well-controlled stoichiometry (ultrasonic
482 Fundamentals of metallurgy
droplet generators and electrostatic atomisation).
18,19
These powders have a narrow
size distribution and tend to be spherical and unagglomerated. This technology is
mainly applied to the fabrication of oxides. However, dense spherical palladium
particles
20
or 70 wt% Ni±30 wt% Fe alloy powders of 10±80 nm
21
were produced
by spray pyrolyse.
12.2.4 Physical routes
The main physical route to produce powder is physical vapour deposition
(PVD). Even if the PVD process is usually applied for coatings, some set-up
(inert gas condensation, electrical explosion wire, laser ablation) allows the
production of very fine (10 nm) powders with high purity (vacuum environment
process). However, the production rate is very low and the costs are very high.
During th e PVD process vapour phase species can be produced by
evaporation of the bulk material, sputtering, or ion plating. These vapour phase
species will collide with the inert-gas molecule and undergo homogeneous phase
nucleation to form powder particles that will be then collected.
24
When the
vapour phase species are produced by evaporation, this process is also called
inert gas condensation (IGC). It is used to produce Zn
22
and Ni
23
nano-powders.
However, the synthesis of multicomponent powder is difficult because the
different elements have different evaporation temperatures or sputtering rates.
This can be solved by using the laser ablation method.
12.2.5 Chemical routes
The numerous chemical processes can be classified as a function of the type of
chemical decomposition involved in the metallic powder production. These are
decomposition of a solid by a gas (oxide reduction), precipitation from a
solution (electrolysis, wet reduction, precipitation), condensation and thermal
decomposition (carbonyl process, hydrid-dehydird process).
Decomposition of a solid by a gas (oxide reduction)
This process consists of the reduction of the oxides by gasses such as hydrogen,
carbon monoxide.
12,14,25
A typical reaction is: MO (s) + H
2
(g) ! M (s) + H
2
O (g).
The process parameters are composition and flow rate of the reducing gas,
reduction temperature (main parameter), temperature profile in the furnace and
bed depth of the oxide if reduction is performed in a stationary system. Non-
stationary reduction, such as in rotary kilns or fluidised bed reactors favours
access of the reducing gas to the metal oxide particles and so improves the kinetics
of reduction.
Oxide-reduced powders are very porous and thus are called sponge powders.
They contain most of their residual oxides within the particles, instead of a
Understanding and improving powder metallurgical processes 483
surface enrichment as for atomised powders. This process is commercially used
for the production of iron, copper, tungsten and molybdenum powders, and on a
smaller scale, for cobalt and nickel powders.
Precipitation from a solution (electrolysis, wet reduction, precipitation)
Metal precipitation from a solution (obtained by leaching an ore) can be
accomplished directly by electrolysis or wet reduction (Sherritt Gor don process).
Indirect precipitation from a salt solution is done by first precipitating a
compound of the metal (hydroxide, carbonate, or oxalate) followed by heating,
decomposition, and reduction.
Electrolysis or electrodeposition from an aqueous solution consists in
depositing a pure metal on an electrode. The deposit can be a loose adhering
powder or sponge that can be disintegrated mechanically into fine particles (Cu,
Ag) or a coherent dense brittle layer of metal that can be ground into powder
(Fe, Mn).
25
Powder-like deposits are favoured by low cation concentration
(limits particle growth), low pH (favours conductivity), high current density and
frequent removal of the deposit at the cathode (brush-down interval). This
technique is mostly used to produce irregular shape (dendritic) powders such as
Fe, Cu and Ag, but it has also been employed for Sn, Cr, Be, Sb, Cd, Pb, Pd and
Zn. The main advantage of electrolytic powder is its high purity level after
removal of i mpu rities coming from the electrolyte. However, the high
production cost of, for example, electrolytic iron powders, limits their use to
niche applications such as catalyst or food additives (Fig. 12.3e).
The wet reduction (Sherritt Gordon process) is based on the separation and
precipitation of Cu, Ni and Co from a salt solution, by reduction with hydrogen.
High temperature and high hydrogen pressure are used to increase the reaction
rates. The pH value is increased by adding ammoni a in order to guarantee a
complete reduction. Powders with a good compactibility and different particle
shapes can be produced (e.g. Co with d
50
60 m). Moreover, co-precipitation
or successive precipitation of different metals allows the production of alloyed
or composite powders.
Many metal powders are produced by the precipitation of their soluble salts
as insoluble hydroxide, carbonate or oxalate (indirect precipitation) (Fig. 12.3f).
Subsequent heating decomposes these compounds into the respective metals or
metal oxides and gaseous products.
18
Different precipitation methods can be
used: direct strike precipitation (addition of a precipitating agent), solvent
removal (sol-gel), hydrophilic non-solvent addition or precipitation from
homogeneous solution (PFHS) (use of a precursor whose decomposition
kinetics controls the rate of release of precipitating agent).
26
The parameters
governing the powder characteristics (morphology and agglomeration) are
concentration, pH, temperature, anion associated to the soluble metal cation,
choice of the precipitating reaction and aging conditions. This process is
484 Fundamentals of metallurgy
employed for platinum, selenium, tellurium, silver, nickel and silver-cadmium
oxide compounds through co-precipitation.
25
Condensation (precipitation from a gas)
The main processes are inert gas condensation (IGC) and chemical vapour
decomposition (CVD). CVD implies thermal dissociation and/or chemical
reaction of the gaseous reactants (halides, hydrides and halohydrides of metal s
and metalloids) on or near a heated surface to form stable solid products. The
decomposition of the gaseous reactants can be homogeneous and/or
heterogeneous reaction forming respectively powders or films. The different
heating methods lead to a variety of CVD methods such as plasma-assisted CVD
(PACVD), as well as different types of precursors, e.g. metalorganic CVD
(MOCVD) etc. . . . The CVD process parameters are deposition temperature,
pressure, input gas ratio and flow rate.
The main drawbacks of CVD are the toxic, corrosive, flammable and/or
explosive precursor gasses and the difficulty to deposit multicomponent
materials with a well-controlled stoichiometry because the different precursors
have different vaporisation rates. Ultra-fine powders (few nm up to few 10 nm)
can be produced.
27
Thermal decomposition (carbonyl process, hydride-dehydride process)
The carbonyl process was first used for the production of nickel powders (in
1889). Other possible metal carbonyls include iron, cobalt and, in fact, all of the
metals of the first, second and third transition metal series.
25,28
Thermal decomposition of gaseous cobalt, iron and nickel carbonyls occurs at
temperatures about 200ëC to have favourable reaction kinetics for acceptable
powder production rates. Fine spherical iron powder (less than 10 m) while fine
irregular porous nickel powder is obtained with this process. However, the
carbonyls are dangerous for the health even at very low concentrations (ppb-level).
Hydride decomposition (hydride/dehydride process) is used for metals that
are too ductile to be milled into very fine particles. The metal is embrittled by
hydrogenation during heating in hydrogen atmosphere at elevated temperatures.
Absorption of only a few per cent of hydrogen makes most transition metals so
brittle that they readily can be comminuted. After milling, the hydrogen can
simply be removed by heating the powder in a dynamic vacuum. This process is
used for Ti, Ta and Nb.
25,28
12.2.6 Plasma techniques
Gaseous plasma consists of a mixture of electrons, ions and neutral particles and
is electrically neutral (negative and positive charges balances each other) even if
it is electrically conducting due to the presence of free charge carriers.
29
Understanding and improving powder metallurgical processes 485
