Seetharaman S. Fundamentals of metallurgy
Подождите немного. Документ загружается.

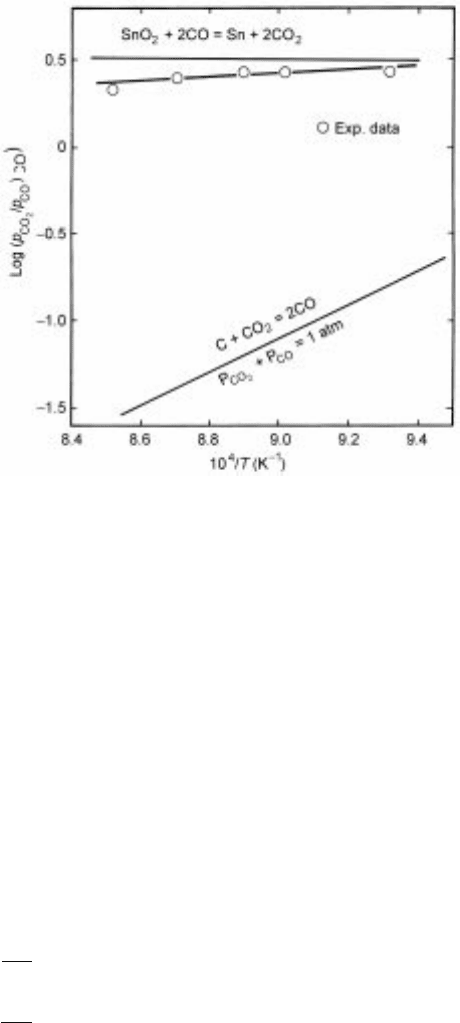
thus the overall rate of reaction is controlled by the C±CO
2
reaction taking place
at the observed p
CO
2
and p
CO
.
Sohn and Szekely (1973) developed a theoretical basis for ana1yzing the
general case in which both reactions aff ect the overall rate and for establishing a
criterion for the controlling step. Let us consider a uniform mixture of solids B
and D. They may be in the form of small particles or pellets that undergo
reactions of the types discussed in Sections 7.3.1 and 7.3.2. We shall here
consider isothermal systems and the case of uniform gas concentrations between
the particles or pellets of solids B and D. This latter condition is applicable when
the overall reaction, and thus the overall gas production, is reasonably fast. This
condition is also valid if the mixture is placed in a container with only a small
opening that allows the gas product to exit but does not allow the back diffusion
of any gas from the surroundings. From the mass balances of the gaseous
species, the rates of net generation of the gaseous species are given as follows:
dn
A
dt
ÿv
1
av
2
7:110a
dn
C
dt
cv
1
v
2
7:110b
where v
1
and v
2
are the net forward rates of reactions 7.109a and 7.109b,
respectively, per unit volume of the solid mixture. These terms can be expressed,
7.14 Observed value s of p
CO
2
/p
CO
ratios compared with the comp uted
equilibrium values for the component reactions in the carbothermic reduction
of stannic oxide.
306 Fundamentals of metallurgy

using equations 7.85 and 7.85b, as follows:
v
1
B
B
b
dX
B
dx
7:11a
v
2
D
D
d
dX
D
dt
7:11b
The reaction rate terms, dX
i
=dt, depend on the reaction mechanism of each solid.
It can be given by any of the solutions given earlier for the reaction of a single
solid such as the differential form of equations 7.71, 7.83, 7.91 or 7.94b. It must
be noted that the bulk concentration terms in these equations are now the
interstitial concentrations, which vary with time as the reaction progresses.
If the total pressure of the system is maintained constant, the following
relationships hold:
dn
A
dt
C
A
V
p
dV
dt
7:112a
dn
C
dt
C
C
V
p
dV
dt
7:112b
where dV =dt is the rate of volume generation of the gas mixture, and V
p
is the
volume of the mixture. We have made a pseudo-steady-state assumption that
the gas-phase concentrations at any time are at the steady-state values
corresponding to the amounts and sizes of the solids at that time, namely,
CdV=dt VdC=dt.
We also have the condition that
C
A
C
C
C
T
7:113
Equations 7.110 to 7.113 may be solved for C
A
and C
C
. Using these values, the
rates of reaction of the solids may then be obtained from the appropriate
expressions used for dX
B
=dt and dX
D
=dt. Integration of these rates over dt will
yield incremental values of X
B
and X
D
. Using the new values of X
B
and X
D
,
equations 7.110 to 7.113 are solved again to obtain C
A
and C
C
at the next time
step. The procedure is repeated to give conversions as functions of time. In
certain simple cases, analytical solutions are possible (Sohn and Szekely,
1973).
Solid±solid reactions proceeding through gaseous intermediates with no net
production gas
This situation arises when ac 1 in reactions represented by equations 7.109a
and 7.109b. An example is the oxidation of metal sulfides with lime in the
presence of water vapor to produce the corresponding oxides (Sohn, 1983; Sohn
and Kim, 1984b, 1987, 1988; Soepriyanto et al., 1989). This reaction can in
general be expressed by the following:
The kinetics of metallurgical reactions 307

Me
x
S H
2
O Me
x
O H
2
S (7.114a)
CaO H
2
S CaS H
2
O (7.114b)
Overall: Me
x
S CaO Me
x
O CaS (7.114c)
Using this scheme, selected metal sulfides such as molybdenum disulfide and
zinc sulfide can be transformed into the corresponding oxides without producing
a sulfur-containing gas. The role of lime in this process is twofold: (1) it improves
the thermodynamics of the reaction between sulfide and steam, which has a very
low equilibrium constant due to the positive Gibbs free energy change, by
eliminating hydrogen sulfide, and (2) the reaction between hydrogen sulfide and
lime fixes sulfur as cal cium sulfide which can be further treated to recover sulfur.
Reactions 7.114a, b, c involve no net consumption or generation of the
gaseous intermediates, and the gaseo us species do not appear in the overall
stoichiometry. The gaseous species simply act as carriers of oxygen and sulfur
atoms. In this respect, the mechanism for this type of reaction might be termed
`catalysis by gases' (Sohn, 1991).
The kinetics of this reaction can be described by equation 7.110. The
difference between this case and the reactions with net generation of gases is
that, since no gases are formed or consumed,
v
1
av
2
7:115
Equations 7.111 and 7.113 are also valid in this case. The solution of these
equations gives the conversion as a function of time. Using this approach, the
detailed kinetics of reactions of various sulfide minerals with lime has been
described (Sohn and Kim, 1987, 1988; Soepriyanto et al., 1989; Sohn, 1991).
Successive gas±solid reactions in which the reactant gas reacts with the first
solid, producing an intermediate gas which in turn reacts with the second solid
Two notable examples, in which successive gas±solid reactions play a major
role, utilize lime as a scavenger of sulfur-containing gases . The first is the
hydrogen reduction of metal sulfides in the presence of lime (Kay, 1968;
Habashi and Yos tos, 1977; Sohn and Rajamani, 1977; Shah and Ruzzi, 1978;
Rajamani and Sohn, 1983; Sohn and Won, 1985; Won and Sohn, 1985), which
can be represented by the following general scheme:
H
2
Me
x
S H
2
S xMe (7.116a)
H
2
S CaO H
2
O CaS (7.116b)
H
2
Me
x
S CaO H
2
O xMe CaS (7.116c)
In this reaction scheme, lime serves two purposes: (1) it fixes sulfur in the solid,
thereby reducing the emission of hydrogen sulfide into the atmosphere, and (2)
308 Fundamentals of metallurgy
the presence of lime improves the otherwise unfavorable thermodynamics of
reaction 7.116a by removing hydrogen sulfide from the gas phase. Reaction
7.116b is highly favorable thermodynamically. The second example is the
roasting of sulfide minerals in the presence of lime (Haver and Wong, 1972;
Bartlett and Haung, 1973; Haung and Bartlett, 1976).
Sohn and co-workers (Sohn and Rajamani, 1977; Rajamani and Sohn, 1983;
Sohn and Won, 1985) developed a model for successive gas±solid reactions in a
porous pellet. The model takes into consider ation the effects of the relative
amounts of the solids, grain sizes, the pellet size and porosity, and the diffusion
of gaseous species, as well as the effect of improved thermodynamics in systems
represented by the first example. The model describes not only the rates of
reaction of the solid reacta nts but also the fraction of the intermediate gas (the
sulfur-containing gases in the above examples) that is captured by the second
solid (lime in the above examples).
Staged reaction of a solid with a gas in which the solid forms a series of
thermodynamically stable intermediate phases
A notab le example of such a system is the gaseous reduction of hematite (Fe
2
O
3
)
to iron through the successive formation of magnetite (Fe
3
O
4
) and wustite (FeO)
(Sohn and Chauba1, 1984; Sohn, 1981).
Simultaneous reactions between solid reactants and gases
This type of system can be describ ed by writing the expressions for the
consumption of the gaseous reactants by the different solids as well as the
conversion of different solids by reac tion with different gases in equations 7.82
and 7.83 together with equat ions 7.2 and 7.3 and solving the resulting equation
by a numerical method. The reader is referred to the literature for further detail
(Sohn and Braun, 1980, 1984; Lin and Sohn, 1987; Paul et al., 1992).
Multiparticle systems
In the above sections, the discussion mainly involved the analysis of reactions
taking place in a single particle or pellet of solids. The eventual objective for
studying single particle systems is, of course, to apply the results to the analysis
and design of multiparticle systems of industrial importance. Examples of
multiparticle gas±solid contacting equipment include packed beds, moving beds,
fluidized beds, and rotary kilns. The extension of single particle studies to
multiparticle systems will depend on the nature of the particulate asse mblages,
the mode of gas±solid contacting, and the spatial variation of the gas properties
within the system. Therefore, general analyses of these processes will not be
presented here, and the reader is referred to the literature for developments in
this area (Zhou and Sohn, 1996; Paul et al., 1992; Sohn, 1991; Rhee and Sohn,
The kinetics of metallurgical reactions 309
1990; Sohn and Lin, 1990; Gao et al., 1983; Herbst, 1979; Szekely et al., 1976;
Evans and Song, 1974).
7.5 Liquid±liquid reactions
There are instances during treatment of molten metals where reactions between
immiscible molten phases need to be considered. Most often this involves the
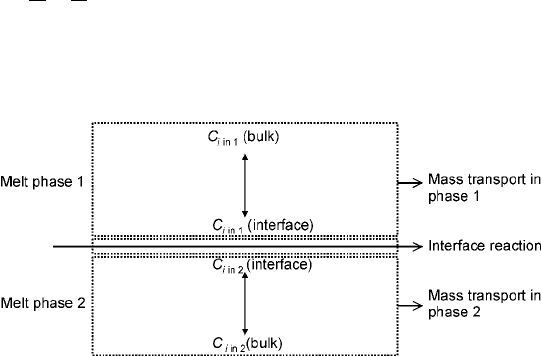
partition of a solute or impurity element between the two phases. As a general
picture, imagine the interface between two phases as shown in Fig. 7.15 and the
partition of an element i.
From a kinetic point of view, the rate controlling step is one or several of the
following:
(i) Reactant species has to move between the bulk of phase 1 to the interface.
(ii) Reaction has to take place at the interface between phases 1 and 2.
(iii) Reactant has to move between the interface and the bulk of phase 2.
At high temperatures, in the absence of surface active elements that might
sterically hinder the reaction, the chemical reaction is often rapid enough to
establish local equilibrium and avoid concentration build up at the interface. The
flux of the solute can then be written,
J
i
k
1
C
i
1
ÿ C
b
1
k
2
C
b
2
ÿ C
i
2
7:117
Here the ks are mass transfer coefficients. It should be noted that if the species i
could be in different chemical forms depending on the phase, i.e. Ca and Ca
+
.
The partition coefficient is defined as: m C
i
1
=C
i
2
. The interfacial concentra-
tions can then be eliminated from the equation 7.117 to yield:
J
i
1
k
1
m
k
2
mC
b
2
ÿ mC
b
1
7:118
For the case where the interface is not in equilibrium, a similar expression can be
derived, assuming that forward and back reactions are simple:
7.15 Schematic of a liquid±liquid interface.
310 Fundamentals of metallurgy

J
i
1
k
1
1
k
rxn
m
k
2
mC
b
2
ÿ mC
b
1
7:119
Here an additional resistance term containing the chemical reaction rate constant
has been added.
Reactions between molten metals and slags are an example of liquid±liquid
reactions. During metal extraction and liquid state processing slags are often
used to achieve a multitude of purposes such as thermal insulation, contami-
nation prevention, lubrication, and heat transfer control. Generally, industrial
slags and fluxes contain SiO
2
, Me
x
O (metal oxides) and, depending on the slag,
additional compounds like Al
2
O
3
, CaF
2
and P
2
O
5
. The ratio SiO
2
/Me
x
O is an
indication of the degree of polymerization. In this section some of the
fundamental aspects of chemical reactions between slags and metals are
discussed with the case of FeO/Fe as an example. The activation energy for the
interfacial reaction is 120 kcal per mole (Richardson, 1974) for a reaction
between molten iron and slag:
FeO Fe
O (7.120)
Due to the relatively low activation energy this reaction is expected to be mass
transport cont rolled. Slags have in general higher viscosities and due to their
ionic/polymeric nature the transport of Fe
2+
±O
2ÿ
in the slag phase is likely the
rate determining step. In general, the activation energy for the chemical reaction
step is expected to be related to the enthalpy change. When transferring species
from a slag to a metal, more bonds have to be broken than formed, since the
solutes share ionic bonds in the slag phase. Thus, the activation energies would
be expected to be high for those elements that share many bonds in the slag
structure such as Si. The activation energy for the reaction,
SiO
2
Si 2O (7.121)
is 270 kcal per mole.
One of the noteworthy aspects of melt±melt reactions is the ability to achieve
large interfacial areas to enhance reaction rates. Kozakevitch (Kozakevitch et
al., 1955 and Kozakevitch, 1969) followed the desulfurization of liquid steel by
molten slag by using X-rays to observe the change in the shape of a sessile drop
of the steel in liquid slag (held in a crucible). It was noted that there was a
marked change in the shape of the drop from non-wetting to wetting and then
back to non-wetting conditions. These changes are equivalent to the following
changes in the metal±slag interfacial tension (
ms
):
(i) a high initial
ms
;
(ii) a reduction to a very low
ms
value while there is rapid desulfurization;
(iii) an increase in
ms
to its initial value when desulfurization is complete.
Studies on the dephosphorization of steel have yielded similar behavior
(Jakobsson et al., 1998).
The kinetics of metallurgical reactions 311

These dramatic changes in
m
associated with rapi d mass transfer are usually
referred to as dynamic interfaci al tension. When
ms
is very low, any disturbance
or turbulence can cause droplets of one phase to move into the other phase. This
is usually referred to as emulsification and the formation of a metal emulsion in
the slag phase (or vice versa) leads to fast kinetics for the refining reactions
because of the huge surface area/mass ratio. Thus from a refining process
viewpoint a low interfac ial tens ion is advantageous and mo der n metal
production processes make use of this.
7.6 Solid±liquid reactions
7.6.1 Solidification
The transformation of an element of liquid into solid will follow a heat
balance:
c
p
@T
@t
L
@f
s
@t
ÿ Q
S
V
7:122
where , c
p
and L are the density, specific heat capacity and latent heat of the
metal respectively. S and V are the surface area and volume of the element and f
s
is the fraction solid. Q is the net heat flux from the volume element. This
equation can be closed by specifying an f
s
-t-T relationship if Q and f
s
can be
identified. Q is dependent upon the thermal field caused by the stru cture around
the element, the mold characteristics and fluid flow in the melt. The fraction
solid, f
s
, depends on the solidification kineti cs, which is governed by the rates of
nucleation and growth which are also influenced by fluid flow. The strong
interdependence of melt fluid flow, heat transfer and crystal growth complicates
the theoretical treatment of solidification. Recently, studies have presented
numerical modeling techniqu es that can simulate solidification structures
ranging from dendrite morphology (as reviewed in Boettinger et al., 2000) to
microporosity (as reviewed in Lee et al., 2001) and particle pushing (Stefanescu
et al., 1998). The dendrite mor phology models range from phase field
simulations which accurately track the development of a dendritic grain in
simplified thermal conditions to cellular automata models which track only the
dendrite tip locations in complex geometries for the prediction of grain
structures (Gandi n et al., 1993). Microporosity models range from analytic
solutions to complex simulations of the interactions of pores and the developing
microstructure (Lee et al., 2001). Particle pushing models have also spanned a
range from analytic solutions (Stefanescu et al., 1998) to the use of phase field
(Ode et al., 2000). One common feature of many of these models is their
highlighting of the importance of the kinetics of solidification processes upon
the final structure. Information on high temperature kinetics is, however,
difficult to obtain, especially on nucleation rates.
312 Fundamentals of metallurgy

7.6.2 Metal±melt refractory reactions
Refractory vessels that contain molten metals and slags are subjected to
degradation due to chemical or mechanical attack by the molten phases.
Chemical attacks occur through the reduction of oxide compounds in the
refractory by more reactive elements in the melt.
For example, at high Al levels, there can be a significant reaction with the
MgO in the refractories according to:
3hMgOi 2
Al ) 3Mg (v) hAl
2
O
3
i (7.123)
This reaction rate can be controlled by the mass transport of Al to the reaction
site or by the gas phase mass transport of Mg vapor away from the reaction site
since its partial pressure is equal to or less than 1 atmosphere. It is also possible
that the Mg (v) can react to produce undesired spin el inclusions in the metal
melt.
Erosion at the slag±gas or slag±metal interfaces is usually referred to as `slag
line attack'. It is caused through the establishment of Marangoni flows at the
interfaces. Mukai (1998) investigated this form of erosion for oxide refractories
and MgO/C refractories. For oxide materials (e.g. SiO
2
) and systems where the
dissolution of SiO
2
leads to an increase in surface tension, the region of the slag
film in longer contact with the refractory will have higher SiO
2
contents. These
regions will have higher surface tension and Marangoni flow will occur towards
these regions. Since these regions occur at the top of the slag film there will be a
flow of slag up the refractory until the Marangoni forces are balanced by
buoyancy forces. However, if SiO
2
dissolution causes a decrease in surface
tension of the slag the erosion pattern is different with rotational and cyclical `up
and down' motions of the slag.
The erosion of MgO /C and Al
2
O
3
/C refractories occurs by a two-stage
process. When MgO particles protrude from the surface the refractory will be
wetted by the slag and the slag will dissolve the MgO leaving C particles
protruding from the surface. Under these conditions the metal phase will wet the
refractory better than the slag. The metal thereupon dissolves the carbon leaving
MgO protruding from the surface and the whole process is then repeated.
Tsotridis and Hondros (1998) developed a mathematical model to predict
erosion wear in vessels. This accounted for interfacial wear due to both thermo-
and diffuso-capillary flows and erosion in the lower part of the vessel du e to
buoyancy flows.
7.6.3 Electrochemical reactions
Electrochemical reactions are a type of chemical reaction where electric energy
is consumed or created. An electrochemical reaction could be e.g.:
A ne
ÿ
! A
nÿ
7:124
The kinetics of metallurgical reactions 313
This is a reduction reaction. The quantitative link between electrical energy and
chemical reactions was established by Faraday (see, for example, Uhlig and
Revie, 1985) who found that, for a reaction such as the one above, the loss of
product A (in moles = m) is related to a current at A according to: m n F it.
The chemical free energy of the reaction is G
A
and a corresponding reversible
potential can be computed, using the Nernst equation, G
A
ÿnFE
A
(where F
is Faraday's constant).
The reaction above cannot occur by itself since the electrons consumed need
to be supplied by a different reaction, called an oxidation reaction.
B
nÿ
! ne
ÿ
B 7:125
The two reactions have to occur at a location called electrodes, where electrons,
ions and reactants (A and B are usually solids or gases) have to be present. The
electrode where oxidation occurs is called the anode and the one where
reduction occur s is called the cathode. The electrons given off at the anode are
supplied to the cathode through an electronic conducting phase. To close the
circuit, an ionic current is passed between the electrodes through an ionic
pathway, called the electrolyte. The entire electrochemical system, consisting of
electrodes, electrolyte and electron lead, is called an electrochemical cell. The
overall cell reaction is the sum of anode and cathode reactions and the cell free
energy and reversible cell potential can be calculated accordingly. There are two
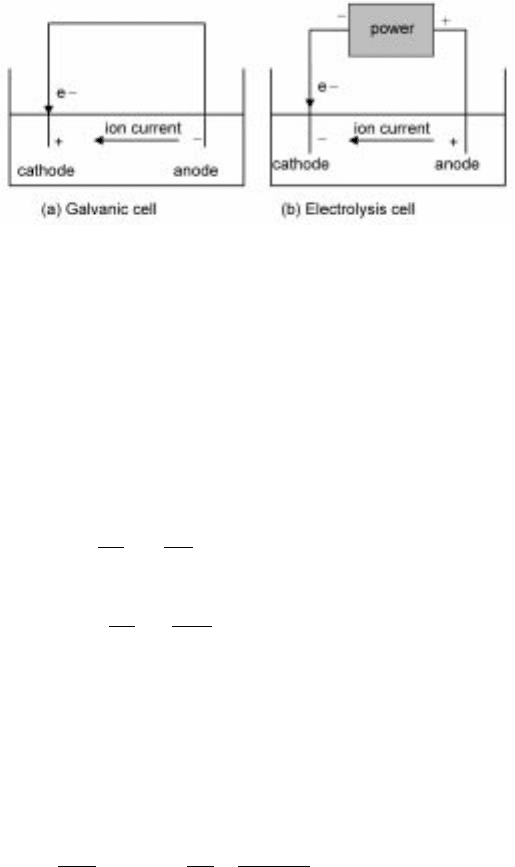
basic types of electrochemical cells. In galvanic cells (see Fig. 7.16a), the cell
free energy is negative and thus the reversible cell potential is positive. In these
types of cells, to which most corrosion cells, fuel cells and batteries belong,
electrical energy is released. The second type is electrolysis cells (Fig. 7.16b),
which have a negative cell potential and thus are not spontaneous. Electrolytic
metal extraction processes are examples of this latter type.
For an in-depth understanding of electrochemical kinetics there are several
excellent textbooks available (e.g. Bard and Faulkner, 2001). In this chapter, the
topic is introduced in two cases, corrosion in an aqueous solution and
electrolytic Al extraction.
Corrosion of Zn in a de-aerated environment
Consider Zn metal reacting to form ions in the presence of water according to:
Zn ! Zn
2+
2e
ÿ
(7.126)
The standard half cell potential of this anodic oxidation reaction is: E
0
Zn
0.763 V (Uhlig and Revie, 1985) vs the standard hydrogen potential at room
temperature. The Zn ions dissolve in the water that serves as an electrolyte. The
electrons are supplied through the Zn metal itself and delivered to a cathodic
reduction reaction:
2H
+
+ 2e
ÿ
H
2
(g) (7.127)
314 Fundamentals of metallurgy
The standard half cell potential vs the standard hydrogen electrode is 0 V for this
reaction. This reaction will have to occur at the Zn surface too and therefore the
exact location of the anode and cathod e cannot be specified. This cell can be
viewed as the case in Fig. 7.16a, with the Zn dissolution reaction occurring at the
anode and the hydrogen reduction reaction at the cathode. Applying Kirchoff's
law to the cell, one obtains:
E
H
ÿ j
H
j E
Zn
ÿ j
Zn
j ÿ i
X
R
ohm
0 7:128
In this equation, E
H
and E
Zn
are the reversible cell potentials:
E
H
E
0
H
ÿ
RT
2F
ln
P
H
2
a
H
7:129
and
E
Zn
E
0
Zn
ÿ
RT
2F
ln
a
Zn
2
1
7:130
The terms
H
and
Zn
are called over-potentials and account for the kinetic
resistances at the electrodes. In general they include the effects of (i) charge
transfer reaction resistance, (ii) mass transport resistance and (iii) intermediate
chemical reaction and adsorption effects. As a result, they are non-linear
functions of current, which can be derived similarly to what was done for
reaction rate theory but adding the effect of electrical potential on stabilizing or
de-stabilizing the presence of electrons:
j
H
j
RT
H
F
2:3 log
i
i
0;H
C
H
0; t
C
H
7:131
Here, is the transference number (between 0 and 1), usually close to 0.5. i
0,H
is
the exchange current of the charge transfer reaction and is a measure of the
electrocatalytic nature of the electrode. Roughly it corresponds to the chemical
reaction rate constant. The second term in the parenthesis is the ratio between
reactant concentration at the electrode and in the bulk that depends on the mass
transport of reactants to the reaction site. It should be mentioned that the mass
transport is carried out through migration, diffusion and fluid flow. The
combined flux equation being:
7.16 (a) Galvanic cell and (b) electrolysis cell.
The kinetics of metallurgical reactions 315
