Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


72
ZORLL
16.
H.
D. Conway and
J.
P.
R.
Thomson, “The determination of bond strength of polymeric films
by indentation debonding,”
J.
Adhes.
Sci.
Techno/.,
2, 227-236 (1988).
17.
U.
Zorll, “Testing of the impact resistance of coatings under aspects of deformation energy,”
presented at the 15th FATIPEC Congress, Amsterdam, 1980.
Congress
Book,
Vol.
111,
pp.
18.
R.
D. Adams, P. Cawley, and C. C.
H.
Guyott, “Non-destructive inspection of adhesively-
bonded joints,”
J.
Adkes.,
21, 279-290 (1987).
19. P. A. Meyer and
J.
L.
Rose, “Modeling concepts for studying ultrasonic wave interaction with
adhesive bonds,”
J.
Adhes.,
8, 107-120 (1976).
20.
H.
G. MoslC and
B.
Wellenkoetter, “Acoustic emission on painted steel sheets,” presented at
the European Federation of Corrosion Conference
on
Surface Protection by Organic Coatings,
Budapest, 1979.
Proceedings,
5,
pp.
17-33.
21. W. Mielke, “Application of acoustic emission analysis for evaluation of the adhesion of
coatings,” presented at the 19th FATIPEC Congress, Aachen, 1988.
Congress
Book,
pp.
22.
B.
E. Dom,
H.
E. Evens, and D.
M.
Torres, “Thermographic detection of polymerhetal
adhesion failures,” in
Adhesion Aspecfs
qfPolyeric
Coatings,
K.
L.
Mittal, Ed. New York:
Plenum Press, 1983, pp. 597-621.
94- 109.
47 1-486.

Coating Calculations
Arthur
A.
Tracton
Consultant. Bridgewater, New Jersey
1
.O
INTRODUCTION
Coatings are defined as mixtures of various materials. The questions arise as to how much
of which materials, and how do these things relate. The materials fall into four general
categories:
1.
Resins
2.
Pigments
3.
Solvents
4.
Additives
1.1
Resins
These are the generally solid, sticky stuff that holds the system together. They are also
called binders, and in a solvent, they are the vehicle for the system. They may be “single
package”
or
“two package.” Single package is just the liquid resin, or the resin in solvent.
Two package means blending an “A” part with a
“B”
part to cause a chemical reaction.
In both systems we need to know the amount
of
solid resin present. This dry material
divided by the total of the dry plus the solvent, is frequently called “resin solids.” With
the two-package systems, we need to know not only the solids but also the ratio of these
solids to form the desired film. This ratio may be designated as a simple ratio as
1
to
1.
Or
it may be based on
1
or
100,
as
0.3
to
1,
or
30
parts per hundred, or a total of
100
as
43
to
57.
These ratios determine the film properties.
We will also need to know the density (weight per unit volume, usually as pounds
per gallon) of the resin
or
vehicle to help calculate volume.
1.2 Pigments
These are the dry materials added to the coating
to
give it color or resistance properties
or hiding, etc. Pigments can be divided into many different categories for different pur-
73

74
TRACTON
poses. Some materials are the primary pigments versus filler pigments. Some materials
are organic in nature and others inorganic. Properties such
as
light fastness, particle size,
specific color, etc. are reasons for choice. For calculation purposes, dry density and evapo-
rative material are needed for each pigment.
1.3
Solvents
The material which permits
you
to apply the coating
in
a
liquid state is called the solvent.
These may be organic solvents or even water, which is a solvent. They are in the formula-
tion to aid in mixing, defining viscosity, and applying the coating. After application, they
evaporate and leave
a
dry film. For calculation purposes, the density needs to be known.
1.4
Additives
These are ingredients added in small
(<5%)
amounts to alter some properties of the
coating. They must be included in the calculation
as
they have both solids and density
factors that must be accounted for.
1.5
Conventions
The relationships among the various ingredients are looked at in the two aspects of weight
and volume. Each aspect not only tells the relationship but
also
implies some property
of
the
formulation. Experienced formulators may use many of the relationships interchangeably
because
of
a
deep understanding of the processes. Common practice, many years
ago,
was
to manufacture paint in
100-gallon
batches. Ingredients were then spoken
of
as “pounds
per
100
gallons.” However, if the batch was not
100
gallons, then the ingredients were
spoken
of
as “pounds per
gallon.”
Consequently, conversations such
as
“It has
100
pounds of pigment already.” “Well, let’s take
it
up to
2
pounds” make sense when one
understands the practice of interchanging the basis for the number.
The density (weight per unit volume)
of
material is usually expressed
as
pounds per
gallon.
To convert numbers to
a
volume measurement (volume per unit weight) or ‘bulking
factor,” divide the density into
1.
1
Density
as
pounds/gallon
Bulking factor
=
2.0
CALCULATIONS
To start with, list the ingredients of the formulation. From the supplier
of
the material,
get the density, the nonvolatile level, and the price of each ingredient. Make
a
list
as
shown in Table
1.
Calculate the bulking factor (gallons/pound). Based
on
your knowledge,
experience, and laboratory testing, you will estimate
a
rough relationship of ingredients,
probably by weight.
2.1
Formulation Weight
Add up
all
the ingredients you have considered or used in the formulation
(530
pounds).
2.2
Formulation Volume
Multiply the weight of each ingredient by its bulking factor. This will give the volume
of the weight used. Total this column
(50.97
gallons).
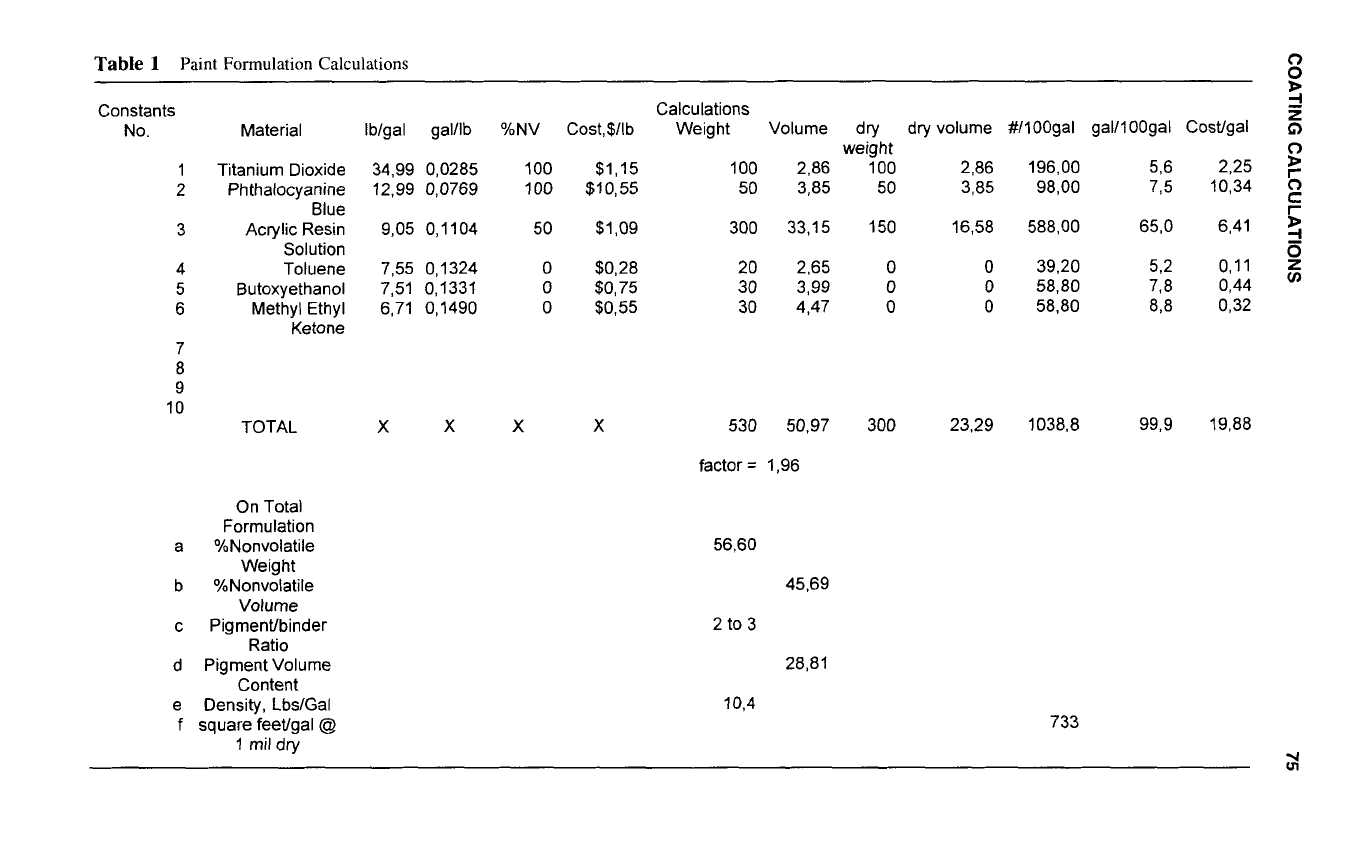
Table
1
Paint Formulation Calculations
Constants
No. Material
1
Titanium Dioxide
2
Phthalocyanine
Blue
3
Acrylic Resin
Solution
4
Toluene
5
Butoxyethanol
6
Methyl Ethyl
Ketone
7
8
9
10
TOTAL
On
Total
Formulation
a %Nonvolatile
Weight
b %Nonvolatile
Volume
c Pigmenubinder
Ratio
d
Pigment Volume
Content
e
Density, Lbs/Gal
f
square feetlgal
@
1
mildry
lblgal galllb
34,99 0,0285
12,99 0,0769
9,05 0,1104
7,55 0,1324
7,51 0,1331
6,71 0,1490
X
X
Calculations
%NV Cost,$llb Weight Volume dry dry volume
weiaht
100
100
50
0
0
0
X
$1,15 100 2.86
$10,55 50 3,85
$1,09 300 33,15
$028 20 2,65
$0,75 30 3,99
$0,55
30 4,47
X
530 50,97
factor
=
1,96
loo
2,86
50 3,85
150 16,58
0 0
0
0
0 0
300 23,29
~ ~~~~~~
#/100gal gall1 OOgal Cosugal
196,OO 5,6
98,00 7,5
588,OO
65,0
39,20 52
58,80 7,8
58.80 83
1038,8 99,9
56,60
45,69
2
to
3
28,81
10,4
733
2,25
10,34
6,41
0,11
0,44
0,32
19,88

76 TRACTON
2.3
Formulation Density
Dividing the formulation weight by the formulation volume gives the density of the formu-
lation (10.4 pounds/gallon).
2.4
Formulation Nonvolatile by Weight
Multiply the weight of each ingredient by its nonvolatile content, and total the column
(300 pounds). Divide this total by the formulation weight (530 pounds) to get 0.5660 and
multiply by 100 to get 56.6% NV-WT.
2.5
Formulation Nonvolatile by Volume
Multiply the volume of each ingredient by its nonvolatile content, and total the column
(23.29 gallons). Divide this total by the formulation volume (50.97 gallons) to get 0.4569
and multiply by
100
to get 45.69% NV-VOL.
2.6
PigmentlBinder Ratio (Weight)
The pigmenthinder ratio is a weight relationship, and depending on the formulator’s
background can be expressed
in
several mathematical ways.
1. Direct ratio: Pigment to binder
=
1/1
2. Percentage: Pigment to binder
=
pigment/(pigment
+
binder)
=
1/(1
+
1)
3. Parts: Parts of pigment per
1
or 100 parts of resin
=
100/100
=
0.50
2.7
Pigment Volume Content (Volume)
The standard abbreviation is
“PVC,”
which should not be interpreted
as
polyvinyl chloride
or any other item. This is a volume relationship expressed as a percentage.
Pigment volume content (PVC,
%)
=
volume of the pigments
volume of the pigments
+
volume of the binder
x
100
PVC
=
([2.86
+
3.85]/23.29)
X
100
=
28.81%
The volume relationship of the pigment to the binder appears to be a more critical number.
Studies have shown that there is a point where the level of pigment
in
a given system is
high enough to cause deterioration of the film produced. This point is termed the critical
pigment volume content (CPVC).
2.8
Converting to a 100-Gallon Formulation
To
convert the initial formulation to a 100-gallon basis divide the initial volume (50.97
gallons) into
100
gallons to get a factor (1.96). Multiply this factor by the weight of each
ingredient in the initial formulation to get pounds in 100 gallons. Multiply the factor by
the volume of each ingredient
in
the initial formulation to get gallons per 100 gallons.
If
the amount to be manufactured is other then
100
gallons, divide the desired volume by
the initial volume to get a factor, and multiply
as
above.

COATING CALCULATIONS
77
2.9
Cost
Multiply the cost ($/pound)
of
each ingredient by the weight in the pounds/100 gallon
formulation and divide by 100 to get the cost of the ingredient. Total the column for cost
per gallon ($/gallon).
2.1
0
Coverage
Frequently, the question is raised of how much a gallon will cover.
A
1-gallon volume
will cover 1604 square feet at one wet mil (0.001 inches), assuming no
loss
of any type.
If the paint were
SO%
volume solids, this would give a film thickness of
0.5
mil. On the
other hand,
if
I
wanted
a
2-mil dry film from a
SO%
volume solids paint,
I
would have
to apply the paint at 4 wet mils per gallon, or
400
square feet per gallon. The standard is
1
mil dry. In the example, 1604
X
0.4569
=
733
square feet per gallon.
REFERENCES
1.
Paul Nylen and Edward Sunderland,
Modern Surface Coatings.
Interscience.
2.
Charles R. Martens,
Technology
of
Painrs,
Varnishes and
Lucquers.
Robert Krieger.
3.
Charles R. Martens,
Waterborne Coatings.
Van Nostrand Reinhold.
4.
Dean
H.
Parker,
Principles
of
Surface Coatings Technology.
Interscience.
5.
Norman I. Gaynes,
Fortnulation
of
Organic Coatings.
Van Nostrand Reinhold.
6.
H.
P. Payne,
Organic Coatings Technology.
New York: John Wiley.
This Page Intentionally Left Blank
Infrared Spectroscopy
of
Coatings
1
.O
INTRODUCTION
Infrared
(IR)
spectroscopy is the single most useful technique for characterizing coatings.
IR
spectroscopy is
a
cost effective and efficient means of gathering information about
coatings. Often the information from the
IR
studies can point the way
to
what other
information or techniques are needed
to
solve
a
problem. Ease of sample preparation is
one advantage
of
IR.
There are numerous ways of presenting the coating sample to the
infrared spectrometer. This wide variety
of
sampling accessories enables the study
of
liquids and solids under
a
wide range
of
conditions. There is large body of literature
on
infrared methodology,'.' and there are extensive collections of reference spectra available.
Almost all components of coatings can be identified by infrared.
IR
spectroscopy can
monitor changes that occur to coatings, such
as
drying, curing, and degradation. Quality
control of raw materials and process monitoring during coating synthesis and formulation
can be done by
IR
spectroscopy.
Most important to the identification of coatings and the study of their properties is
the skill of the analytical scientist. This factor
is
often overlooked because the trend
in analytical instrumentation in recent years has been increasing computer control and
automation. Even when these systems are at hand, they have little value without
a
well-
trained and experienced analytical scientist behind them. The individual with
a
coatings
problem or application is well advised to seek the services
of
an experienced spectroscopist.
This chapter starts with an introduction to the principles and instrumentation of
infrared spectroscopy. It then covers sample preparation and sampling accessories. Spectral
interpretation is then briefly described, followed by a section on applications.
2.0
PRINCIPLES AND INSTRUMENTATION
The atoms of any molecule are continuously undergoing vibrations and rotations of various
kinds. The frequencies of these molecular motions are of the same order
of
magnitude
79

80
KENDALL
(
10'3-10'' cycles per second) as those of
IR
radiation. When the frequency
of
molecular
motion is the same as that
of
IR
radiation impinging on that molecule, and when there is
a change
in
dipole moment during that motion, the molecule can absorb the radiation
incident upon it. A plot or graph
of
these absorptions, known as absorption bands, as a
function of wavelength or frequency, comprises an infrared spectrum.
The mid-infrared extends from about
2
to
25
pm
(5,000
to
400
cm-'), the most
useful range for chemical analysis. The most convenient unit for infrared wavelengths is
microns or micrometers (pm,
lo-('
meters). It is more common now
to
express the infrared
spectrum in terms of wave numbers, for which the units are reciprocal centimeters (cm-').
Wave numbers are proportional to frequency, and are calculated (in cm-') from the wave-
length
in
pm by dividing 10,000 by the wavelength. The near-infrared region of the
spectrum, occurring at higher frequency (wave numbers) and shorter wavelengths than
the mid-infrared, has found considerable use in recent years, particularly in process control
and in monitoring relatively well defined materials.' It is not very useful for identifying
complete unknowns and will not be discussed in this chapter.
No
two substances that absorb
IR
radiation absorb it at the same frequencies to the
same extent. Therefore an
IR
absorption spectrum is a "fingerprint" of a substance useful
in differentiating one molecule from another. Moreover, the spectrum of a mixture, unless
certain hydrogen bonding situations or chemical reactions take place, is simply the sum
of the spectra of the individual components in the mixture.
Infrared spectrophotometers, the instruments used for chemical analysis by
IR,
in-
clude three essentials: a source
of
IR
radiation, a dispersing means to separate the radiation
by frequency (wavelength), and a detector that changes the received radiation into an
electrical signal. The two most common detectors are deuterated triglycine sulfate (DTGS),
operating at room temperature, and mercury cadmium telluride (MCT). The latter is more
sensitive but must be operated at liquid nitrogen temperatures. Most spectrometers are
controlled by a computer, which is also used to process the data and perform library
searches.
The Fourier transform infrared spectrometer
(FTIR)
became the dominant type
of
laboratory infrared spectrometer during the
1980s."'
The
FTIR
spectrometer uses an inter-
ferometer to produce an interference pattern (the interferogram). A digital computer
quickly performs a mathematical operation, a Fourier transform, to produce the familiar
transmission or absorbance spectrum.
FTIR
spectrometers have a high energy throughput
and can produce useful spectra in a matter of seconds. Their wavelength or frequency
calibration is quite accurate. All references in this chapter to
IR
spectrometers are to
FTIR
spectrometers.
3.0
DATA COLLECTION
3.1
Separation
Often it is possible to analyze a coating with minimal preparation. At other times it is
worth the extra effort to separate a coating into its components." The polymers and resins
can be separated from the inorganic components, pigments, and extenders,
on
the basis
of
solubility. The proper solvent must be chosen that can dissolve the organic components.
Heating usually increases the solubility of the organic components. After dissolution,
filtration or centrifugation can be used to separate the inorganic pigments and fillers from
the dissolved polymers or resins. The dissolved components can be prepared for infrared
INFRARED SPECTROSCOPY OF COATINGS
81
analysis by casting
a
film on
a
salt plate. Since the solvents can be retained in the cast
film, it is recommended that the cast film be dried in an oven before the 1R spectrum is
scanned. The spectrum of the cast film should be checked for the presence
of
residual
solvents. The inorganic components can be scanned
as
potassium bromide (KBr) pellets
or Nujol mulls. It may be possible selectively to remove additives from the principal
polymers by extraction with the proper solvent.
3.2
Transmission Spectra
A wide variety of methods exist for the investigation of samples by IR spectrometry.'
The choice depends on the nature of the sample, the kind of information desired, and the
time available. Transmission is the classic sampling method. It is highly reproducible,
so
it is accurately quantitative. Transmission often requires laborious preparation
of
solid
samples, but it is still the principal sampling method for gases and liquids. In
a
transmission
spectrum the infrared beam passes through the sample, and the transmission or absorbance
of IR radiation
as
a
function of wave number is measured. The interaction of the infrared
beam with the sample is ratioed to
a
suitable reference to give the transmission spectrum.
The absorbance is the log of the transmission. Most of the available reference spectra
were scanned
as
transmission spectra. Liquids can be scanned
as
a
thin layer between two
salt plates or in special liquid cells. Powders should have
a
particle size less than the
wavelength of the shortest wavelength used, typically less than
2
Fm. Powders can be
mixed with potassium bromide, cesium iodide, or another salt and pressed into
a
transparent
pellet. Alternatively, they can be mulled with Nujol (a brand of mineral
oil)
and spread
between salt plates. Such transmission spectra are useful for identifying inorganic pigments
and extenders.',' Dissolved materials can be made into
a
cast film by placing the solution
on
a
salt plate and evaporating the solvent.
3.3
Attenuated Total Reflectance
Attenuated total reflectance (ATR) is one of the most widely used sampling techniques,
an easily installed accessory to most IR spectrometers. ATR equipment requires more
energy throughput than simple transmission measurements, but this is no problem for
modern Fourier transform spectrometers. In ATR the infrared beam is directed
so
that it
is inside
a
crystal, and the sample is placed on the outer surface of the ATR crystal.
Zinc selenide, germanium, and thallous bromide-iodide
(KRS-5)
are common ATR crystal
materials. Recently, diamond has become available for use
as
an ATR crystal.'0 It is
almost indestructible. Good contact between the sample and the crystal is vital, otherwise
a
useful ATR spectrum is not attainable. The ATR spectrum is very similar, but not
identical, to the transmission spectrum and can be searched against libraries of transmission
spectra. ATR is especially useful for surface analyses. Since the impinging IR radiation
beam penetrates the surface of the sample up against the ATR crystal roughly to the depth
of
the wavelength
of
the IR beam, ATR is essentially a technique for examining surfaces.
It penetrates surfaces to
a
depth on the order of several
to
tens
of
micrometers.
Liquids, including viscous liquids, can be scanned by ATR. If volatility is
a
concern,
then ATR accessories with
a
cover can be used. Solids can be examined by ATR
if
good
contact can be made with the crystal. Usually there is provision
to
apply
a
moderate
amount
of
pressure
to
the sample to improve contact. ATR offers many possibilities for
coatings applications, and these have been reviewed." It may be possible to examine
a
coating without removing it from the substrate by placing it directly on the ATR crystal.
