Roll Forming Handbook / Edited by George T. Halmos
Подождите немного. Документ загружается.

“White rust”isthe destruction of the surface of galvanized steel or zinc by oxygen or other chemical
elements. The reaction is accelerated by the presence of moistureacting as acatalyst.
The zinc coating may be applied by hot dip galvanizing or electrodeposition and is primarily intended
to serve as aprotective layer for the main metal substrate.
However,this protective film has its own limitations when subjected to environments antagonistic to
its protection properties. The rate of attack is related to temperature,phand composition of moisture
and the concentration of dissolved gases within that moisture. The higher the oxygen and carbon dioxide
content, and the softer the water,the greater the rate of surface breakdown is. When the water is present,
the corrosion cycle starts with the formation of zinc oxide, which, in turn, is converted to zinc hydroxide
and then to basic zinc carbonate in the presence of carbon dioxide. The final product prevents the onset
of further corrosion at that particular area of attack providing no highly acidic or alkaline contaminant
becomesinvolved later.
Under enclosed conditions such as blind angles and boxsections, rapid attack by the available oxygen
gives rise to apitting condition as the exhausted gas is not replaced as fast as it is used. The corrosion
pattern, therefore, is nonuniform and typical of “white rust”conditions.
It is essential that roll formed sections and tubes be drained of as much coolant as possible. Weep holes
should be pierced or drilled in blind sections whenever feasible. Storage should be in selected areas where
freecirculation of air is not aproblem in ordertoavoid stagnation of anyresidual fluid.
Zinc is rapidly attackedinacidic and alkaline aqueous conditions. Storage and manufacturewithin the
vicinityoffumes fromplating or pickling shops will accelerate metal surface breakdown.
The accumulation of metal fines in acoolant reservoir is another sourceofpotential problems. Should
the particulate contaminants not be filtered out or removed, therewill be aconstant deposition on the
metal as it is formed. The particles of fines will create isolated areas of chemical and electrochemical
corrosion. Zinc is sacrificial to steel (iron).
The chemical and physical properties of the lubricant maycontribute to “white rust.”Some of these
properties are unstable water-soluble materials, activechemical agents such as chlorine or sulfur (which
are not properly inhibits). In general, stable emulsions of properly selected synthetic solubles are best for
galvanized surfaces. Evaporativecompounds can work well on these surfacesalso.
Another precaution that should be takeninlubricating galvanized material is to prevent excessive
amounts of water-based lubricant from being allowedtoremainonrollformed sections. This trapped
water can definitely contribute to a“white rust” condition, especially when parts are nested or stacked.
Some operators will blow offthe excessive water-soluble lubricant beforethe cutoffinorder to
minimize the amount of residual left on the part. As noted above, stacking parts so that the configuration
provides for air circulation is extremely helpful.
Conditions of highrelativehumidityabove 70 to 75% aggravate corrosion. When the metal surface
temperature dropsinthe presence of highhumidity, the condensed moistureonthe section is rich in
oxygen because the thin water film is exposed to alarge volume of air.Conditions are then ideal for
extensive uniform corrosion. Closely packed, tightly nested, products stored outside the plant cool down
during the night. During the day,the large amount of water condenses on the surface of the cold metal.
Because thereare so manydifferent precoated materials being rollformed, extra caremust be takento
check first with both your lubricant and material supplier beforeattempting to use anyforming lubricant
on coated stock. This is especially true not only on galvanized material but on spray coated, painted,
vinyl, paper clad, and electroplated material.
Further Reading
[137] Ivaska, J. 1991. The three keysegments of roll forming lubrication. SME Te chnical Report,
MFR91-14.
[401] Harmon,J.J.1990. Laws Involved in Governing the Use, Management and Disposal of Metal-
working Lubricants. Cors &Bassett, Environmental Group,Cincinnati, OH.
Roll Forming Handbook7 -22
[402] Heidenreich, E.E. 1990. HowtoSet-UpaWaste Minimization Program for Coolants and
Lubricants. Edjetech Services, Edjean Technical Services, Kipton,OH.
[403] Ivaska, J.,Jr. 1983. Howmetal forming lubricants affect the finishing process. SME Technical
Paper,FC83-690.
[404] Ivaska, J.,Jr. 1987. Lubricant implications for integrated pressworkers. SME Technical Paper,
MF87-003.
[405] Nachtman, E.S., Kalpakjian, S. 1985. Lubricants and Lubrication in Metal Working Operations.
Marcel Dekker,New York.
[406] Waste Minimization Opportunity Assessment Manual USEPA ,July 1988, Publication No.EPA/
625/7-88/003. Hazardous Waste Engineering Research Laboratory, Cincinnati, OH.
[407] Serious Reduction of Hazardous Waste, for Pollution Prevention and Industrial Efficiency.
OTA, September 1986, D.C. Publication No.OTA-ITE-317. U.S. Government PrintingOffice,
Washington.
[411] Strategic Waste Minimization InitiativeUSEPA, February, 1989, Version 1.1, Center for
Environmental Research Information, Cincinnati, OH.
[412] Economic Assessment of Industrial Waste Minimization Strategies, USEPA, April, 1989, Center
for EnvironmentalResearch Information, Cincinnati, OH.
Lubrication 7 -23

8
Coil Processing,
Material Handling,
and Plant Layout
GeorgeT.Halmos and
Joseph Horvath Retired
Delta Engineering Inc.
8.1 Flow of Material ................................................................. 8 -2
8.2 Coil Handling and Storage ............................................... 8 -3
Receiving Coils
†
Coil Storage
†
Storage Racks
†
Storing Coils with Horizontal Axis on the Floor
†
Storing Coils with Vertical Axis on the Floor
†
Coil Upender
8.3 Sheet Handling and Storage ............................................. 8 -6
Placing Bundles into Feeding Position
†
Separating
and Grabbing Sheets
8.4 In-Line Coil Handling ....................................................... 8 -9
Purpose of In-Line Coil Storage
†
Coil Ramp
†
Coil Cradle
†
Coil Rack
†
Coil Conveyor
†
Turnstile
†
Coil Car
8.5 Coil End Welding .............................................................. 8 -14
Seldom used Coil End Welding Methods
†
Recommended
Coil End Joining Methods
†
Nonrecommended Coil End
Joining Methods
8.6 Strip (Coil) Accumulators ................................................ 8 -16
Overhead Loop and Vertical Loop
†
Horizontal Coil
Accumulator
†
Vertical Rotary Accumulator
†
Continuous
Coil Stack Feed and Pallet Decoilers
8.7 Flattening and Leveling ..................................................... 8 -19
When is aFlattener Required?
†
Flattening
†
Levelers and Shape Correctors
8.8 In-Line Sheet Handling ..................................................... 8 -22
Sheet Grabs
†
Sheet Conveyors
8.9 Finished Product Handling ............................................... 8 -23
Handling Individual Product
†
Separating and Protecting
Finished Products
8.10 Finished Product Storage .................................................. 8 -31
In-Plant Storage
†
Outdoor Storage
†
Marking, Counting,
and Weighing
†
Truck Loading
†
Railway Cars
†
Containers
8.11 Material Handling Equipment ......................................... 8 -34
Forklift Trucks and Side Loaders
†
Cranes
8.12 Material Handling Accessories ......................................... 8 -38
Crane Attachments
†
“C” Hook
†
Coil Grabs
†
8 -1

Fork Attachment
†
Below the Hook Coil Upenders
†
Slings and Chains
†
Other Crane Attachments
8.13 Crane Controls ................................................................... 8 -40
Pendulum Control
†
Control in Cab
†
Radio Control
8.14 Plant Layout ....................................................................... 8 -41
Flow of Material
References ....................................................................................... 8 -43
8.1 Flow of Material
As aresult of the highproductivityofrollforming lines, large quantities of material are received,
processed through,and shipped out of the manufacturing plants. At manyplants, the operators and
material handling personnel spend moretime with handling material than with producing goods.
The handling and storing of the material is concentrated in the following areas:
1. Incoming material receiving,unloading,inspecting,and marking
2. Material (coil or sheet) storage
3. Moving coils (or occasionally bundles of sheets) to the processing line
4. Passing material through the line
5. Removal of rollformed products fromthe line
6. Stacking,bundling,marking,and packaging products
7. Storage of rollformed products
8. Transporting finished products to the next operating station or shipping them out of the plant.
The selection of equipment and accessories for incoming material handling depends on local
conditions and should be assessed separately in each instance. The following conditions may influence
the selection:
*
In-plant or outdoor receiving
*
Available building and layout of plant
*
Method of transportation used for incomingmaterial (trucks: open bed or van; railwaycars: open
beds or boxcars)
*
Floor-ordepressed-level receiving area
*
Quantityofmaterial received per day and per year
*
Orientation of coils (center line, or “eye”ofthe coil horizontal or vertical) when received and
stored
*
Requirements to weighcoils
*
Method of coil storage
*
Method of loading coils onto roll forming line
*
Availabilityofin-line coil storage
*
Amount of equipment to be serviced
*
Special requirements (e.g., sensitivityofthe material to handling or environment)
*
Available funds.
Primarymetal manufacturers, coil coaters, or service centers usually deliverthe wider,hot rolled, cold
rolled, galvanized, or sometimes prepainted steel coils with their axes in horizontal position. Narrow
coils, most prepainted steel coils, aluminum, and other metals are shipped with vertical axes (“eyein
the sky”) on pallets.
Unless specific instruction are given to the supplier,the direction of coil axes will be determined by the
convenience of handling,loading,and shipping,and by the sensitivityofthe material to the horizontal
and vertical accelerations and decelerations during transportation.
Roll Forming Handbook8 -2

At most plants, the cranes, forklift trucks, and other material handling equipment haveadditional
functions to loading and unloading the roll forming line. They are also used to unload incoming
trucks, load material for storage, remove finished products from the line, and load them on to trucks.
It can be afrustrating experiencetooperate the line efficiently at highspeed, and then stop and
wait for 20 to 30 min for acrane or forklift truck to load the next coil or to move the completed
product. For efficient operation, it is importanttoreducethe unproductive “down time”ofthe lines.
Therefore, it is advisable to makethe line as self-sufficient and independent from the plant material
handling equipment as possible. Entryend and in-line coil storage and handling devices, as well as exit
end product handling and in-line temporarystorage facilities, can keep the roll forming lines running
for hours.
8.2 Coil Handling and Storage
8.2.1 Receiving Coils
With the exception of afew sheet-fed lines, coils are the starting form for roll formed products. Most of
the time, coils are shipped to the manufacturing plants on open bed trucks. Closed trucks and railway
cars are seldom used for transportation.
Incoming material is sometimes unloaded from the transportvehicle outside the plant by crane or
forklift truck. However,itispreferable to movethe material into storage or to the rollforming line with
the same equipment in one handling.
Inside plant unloading is most common because it reduces coil handling time and minimizes the effect
of weather.Open bed trucks or railwaycars inside the plant can be unloaded with crane or with forklift
trucks. Closed vans and the very seldom used boxcars stationed at the dock level are unloaded with low
mast forklift trucks.
Coils should be visually inspected for damage, marked, entered into the inventory, and then moved
into the storage area. Checking weight, thickness, camber,waviness, surface appearance, and mechanical
properties may be apartofthe receiving procedure.
8.2.2 Coil Storage
The selection of coil storage method and the coil storage area layout has along lasting influence on
product cost. Incorrect storage method and layout can be expensive, either because of unnecessary
investment or the time wasted by searching for,moving,and accidentally damaging coils for manyyears
or decades.
Coils with either horizontal or vertical axes can be stored in storage racks or in multiple heights on the
floor.
8.2.3 Storage Racks
The primarysteel manufacturers and steel centers, frequently store coils in racks (see Figure 8.1). Racks
provide easy access to anycoil and minimize the damage to them. Coil loading and removal can be
automated, using stacker cranes. However,storage racks are expensiveand corridors between the racks
occupy considerable floor space. Usually,rollforming plants can neither afford nor fully utilize storage
racks.
8.2.4 Storing Coils with HorizontalAxis on the Floor
This is the most inexpensive and effective waytostorecoils. It is applicable to hot and coldrolled,
galvanized, or frequently to prepainted steel. Wide, 10-ton coils can be stacked4to 5highasshown in
Coil Processing, Material Handling, and Plant Layout 8 -3

Figure8.2 if the strength of the plant floor and the height of the crane hook permits it. Depending on soil
conditions, about 8to10-in. (200 to 250-mm) thick, reinforced concrete floor is usually sufficient for coil
storage.
To prevent the sideways rolling and collapse of the coil “pyramid,”sufficient supportshould be
provided to the twooutside (end) coils at each bottom row(see Figure 8.3).
Coil storage on floor is a“dense”arrangement, and storage areaiseffectively utilized. However,more
time is required to “search” for coilsin“mixed coil rows.”The chance of damage to the coils is also
higher and this method is not well suited to store of leftovercoils with only afew inches of wraps.
However,inmost places, this is still the most economic method of storing coils with horizontal axis.
FIGURE 8.1 Coil storage rack loaded with stacker crane. (Courtesy of Palmer Shile Company.)
Roll Forming Handbook8 -4
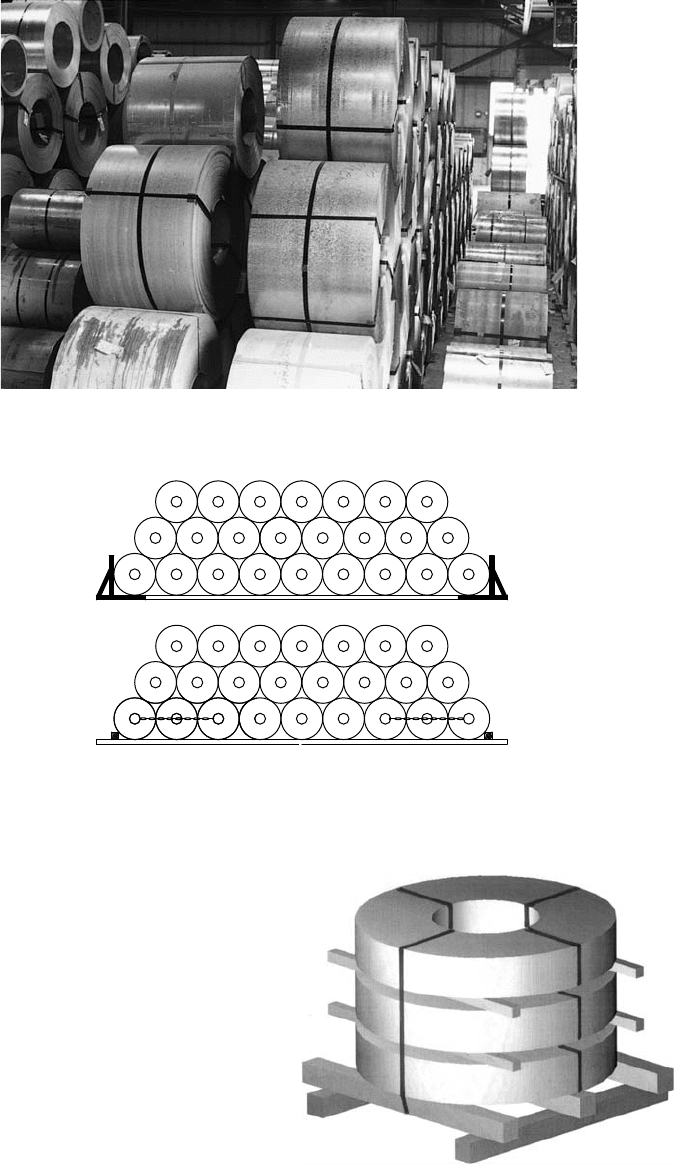
8.2.5 Storing Coils with Vertical
Axis on the Floor
Coils fastened to pallets, as shown in Figure8.4,
are frequently stored in multiple heights. Advan-
tagesand disadvantagesofthismethodare
similartothosepertainingtothe method
mentioned above, but storage spaceutilization
is less effective than storing coils with horizontal
axis.
Narrowcoils, strapped to pallets or skids, are
usually stored in this wayatmost roll forming
plants. Narrowcoils, stored with vertical axes
(“eye in the sky”) for safe handling,are “flipped”
over when they are placed on the uncoilers.
FIGURE 8.2 Storing coils on the floor.
(b)
(a)
FIGURE 8.3 Coil stops (a) or chains (b) prevent the “rolling-out”ofthe end coils.
FIGURE 8.4 Storing coils with “eye in the sky.”
Coil Processing, Material Handling, and Plant Layout 8 -5
Wide coils shipped with horizontal axes are stored and loaded onto the uncoilers in the same position.
Wide coils received with vertical axes (eye in the sky) can be stored in this position or with their axes
rotated 908 ,usually with an upender,tothe horizontal position beforeplacing on the line. Alternatively,
they can be “upended” beforeplacing in the storage area. Local conditions will determine the more
effectiveprocedure with the least handling time and travel distance.
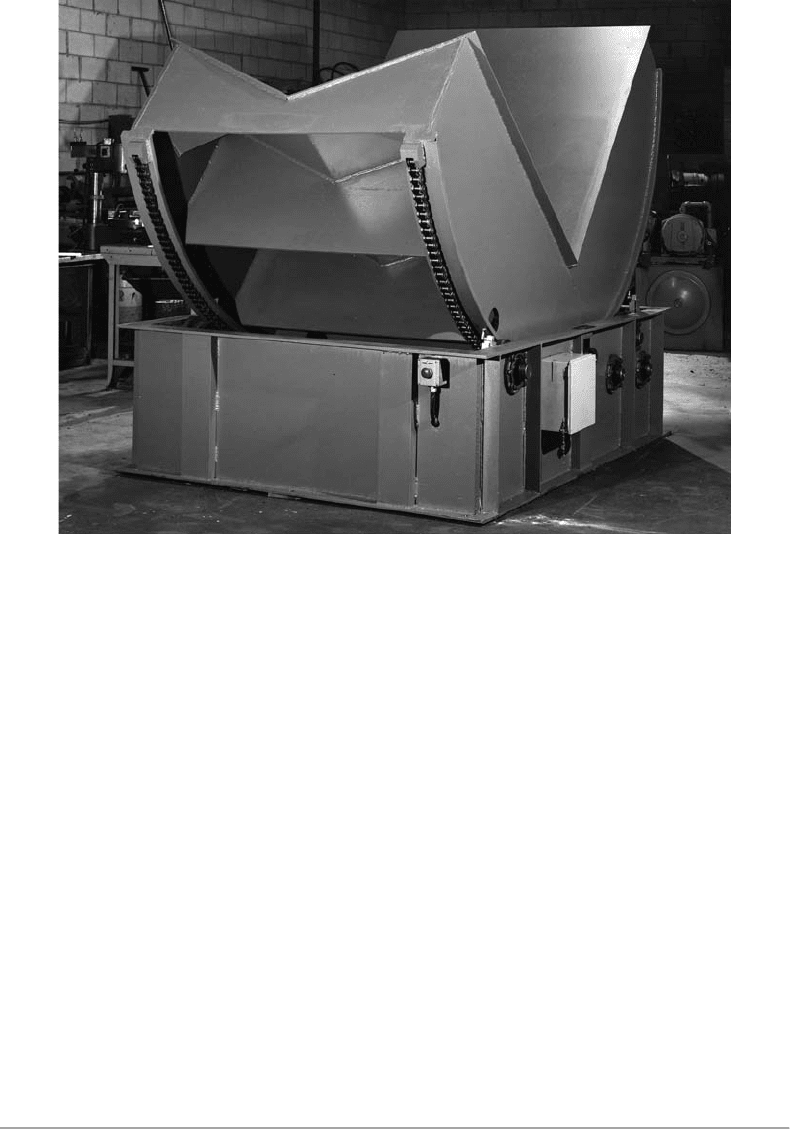
8.2.6 Coil Upender
All uncoilers and reels, except the pallet decoilersand the continuous coil stacks, are loaded with coils
having horizontal axis, while either all or frequently alarge portion of coils are shipped to the plants with
vertical axes. In most cases, the vertical axis is rotated to horizontal one with an upender (see Figure 8.5)
and the coils are loaded/unloaded by forklift trucks or cranes.
To install an upender for each line would be too expensive; therefore, most plants haveone upender
only.All incoming coils with vertical axes must be placed on the upender,either when received or when
moved to the roll forming equipment. The extra trip to the upender increases the traveling distanceof
each coil, but the handling time increases even more drastically.Palletized coils withvertical axes must
be unloaded with fork attachment while handling of coils with horizontal axes is preferred with “C”
hooks or coil grabs. To comply withthis requirement, the crane attachments are changed after each
upending.
8.3 Sheet Handling and Storage
Only asmall percentage of the material processed throughthe roll forming lines are in sheet form. Sheets
received on skids or in bundles are handled by trucks, sheet grabs or with another crane attachments
using forks. In most cases, sheets are stored on the floor,inmultiple bundle heights.
FIGURE 8.5 Coil upender.(Courtesy of BradburyCo. Inc.)
Roll Forming Handbook8 -6

8.3.1 Placing Bundles into Feeding Position
Bundles of sheets or prepierced, notched blanks are usually placed into the feeding position by material
handling equipment (crane, truck etc.). Production delaycaused by waiting for handling equipment can
be eliminated by loading several bundles side by side on aroller or chain conveyor.When the last sheet of
abundle is removed, the next bundle can be moved into the feeding position by achain conveyors or
pushers (Figure8.6).
Bundles are occasionally movedinto position by trolleys, or several bundles are placed on top of each
other on alifting table. The table can automatically lift the top sheet of the bundle into the right feeding
height.
8.3.2 Separating and Grabbing Sheets
Moving asheet usually causes fewer problems than separating it from the next sheet in the bundle. Trying
to grab asing le sheet on the top of abundle can be cumbersome for the operator wearing protective
gloves. Oily sheets may create avacuum effect, “sticking” the sheets together.Rust or greasecan make the
separation even moredifficult.
The simplest method of separating or “fanning” steel and certain stainless steel sheets is to use simple,
permanent magnets (Figure8.7). The edge of the separated top sheet can be easily grabbed and moved.
FIGURE 8.7 “Fanning” magnets separate top sheets of the bundle.
FIGURE 8.6 In-line bundle storage eliminates crane/forklift truck waiting time.
Coil Processing, Material Handling, and Plant Layout 8 -7
