Qiu X.G. (Ed.) High Temperature Superconductors
Подождите немного. Документ загружается.

Electron-doped cuprates as high-temperature superconductors 221
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
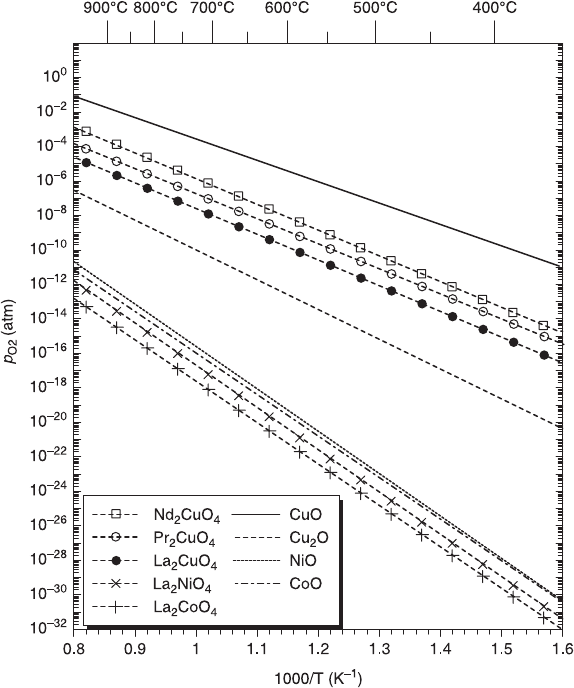
6.8 Comparison of the decomposition lines for cuprates with those
for nickelates and cobaltates. The decomposition lines for both simple
oxides and 214 oxides are included. The decomposition lines of 214
cuprates are located at much higher p
O2
than those of La
2
NiO
4
and
La
2
CoO
4
.
that the decomposition in 214 transition-metal (M) oxides is triggered by the
break of the M-O bond with much stronger La-O or RE-O bond intact.
Next we take a look at the RE dependence of the decomposition lines. T cuprates
decompose by the following reaction,
4RE
2
CuO
4
→ 4RE
2
O
3
+ 2Cu
2
O + O
2
[6.3]
or
4RE
2
CuO
4
→ 4RECuO
2
+ 2RE
2
O
3
+ O
2
. [6.4]
222 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
In either case, the Cu valence changes from +2 to +1 whereas the RE valence is
unchanged. The thermodynamic data (standard Gibbs energy change, standard
enthalpy change, standard entropy change) in decomposition [6.3] or [6.4] were
obtained by electrochemical measurements using galvanic cells by Tretyakov
et al. (1976), Petrov et al. (1989a, 1989b), and Idemoto et al. (1992). Table 6.5
summarizes the standard Gibbs energy change, standard enthalpy change, and
standard entropy change in decomposition [6.3] for T
'
-RE
2
CuO
4
with different
RE. The data for CuO and T-La
2
CuO
4
are also included in the table for a
comparison. All the data are normalized so as to desorb 1 mol of O
2
gas by
decomposition. The thermodynamic data in Table 6.5 can be converted to the
decomposition lines by the following equation (van’t Hoff equation),
[6.5]
which are also included in Table 6.5 and illustrated in Fig. 6.9(a). As seen in
Table 6.5, the standard enthalpy change in decomposition of T
'
-RE
2
CuO
4
is close
to the value (292 kJ/mol) for CuO. Furthermore ∆H° does not change by a subtle
difference in structure between the T and T
'
structure. This universal behavior
reconfirms that the decomposition in 214 cuprates is governed by the strength of
the Cu-O bond.
Taking a closer look at Table 6.5 and Fig. 6.9(a), one can notice the following trend
for the phase stability of T
'
-RE
2
CuO
4
, ‘the larger RE
3+
, the more stable’. This trend
Table 6.5 Thermodynamic data for decomposition reaction [6.3] in T
'
-RE
2
CuO
4
with
different RE
Material ∆H° ∆S° ∆G° log p
O2
Ref.
(kJ/mol) (kJ/mol/K) (kJ/mol) (atm)
CuO 292.0 0.2050 292–0.205T 10.637–15153/T
T-La
2
CuO
4
303.0 0.1548 303–0.155T 8.086–15828/T Kanai et al.,
1997
T
'
-Pr
2
CuO
4
329.0 0.1828 329–0.183T 9.549–17186/T Petrov et al.,
1988b
T
'
-Nd
2
CuO
4
298.3 0.1806 298–0.181T 9.434–15582/T Tretyakov
et al., 1976
T
'
-Sm
2
CuO
4
300.1 0.1961 300–0.196T 10.243–15676/T Tretyakov
et al., 1976
T
'
-Eu
2
CuO
4
240.6 0.1613 241–0.161T 8.426–12568/T Tretyakov
et al., 1976
T
'
-Gd
2
CuO
4
234.4 0.1613 234–0.161T 8.426–12244/T Tretyakov
et al., 1976
The data for the decomposition of CuO and T-La
2
CuO
4
are also included for
comparison. All the values are normalized so as to desorb 1 mol of O
2
gas by
decomposition.
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
223
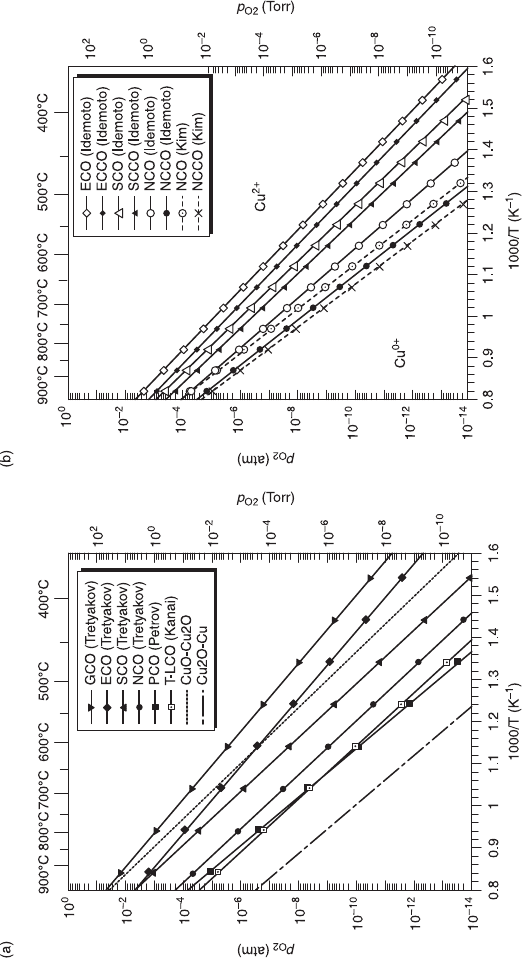
6.9 (a) RE dependence of the decomposition lines for T
'
-RE
2
CuO
4
. The trend ‘the larger RE
3+
, the more stable’ can be noticed.
(b) Comparison of the decomposition lines between T
'
-RE
2
CuO
4
and T
'
-RE
1.85
Ce
0.15
CuO
4
with different RE. In any RE, the
phase stability field of T
'
cuprates is enlarged by Ce substitution of x = 0.15. Both of the figures are constructed based on
the results of the electrochemical measurements by Tretyakov et al. (1976), Petrov et al. (1988a, 1988b), and Idemoto et al.
(1992). The results of thermogravimetric measurements on T-La
2
CuO
4
by Kanai et al. (1997), and on T’-Nd
2
CuO
4
and
T’-Nd
1.85
Ce
0.15
CuO
4
by Kim and Gaskell (1993) are also included.
224 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
may be explained partly from the stronger oxygen affinity of larger RE
3+
. RE
3+
is
coordinated (surrounded) by eight oxygen atoms, four of which are in the CuO
2
layer. Hence larger RE
3+
takes a firmer grip on weakly bound oxygen atoms in the
CuO
2
layer.
Next we see how the phase stability changes with Ce doping. Figure 6.9(b)
compares the decomposition lines of T
'
-RE
2
CuO
4
and T
'
-RE
1.85
Ce
0.15
CuO
4
for
RE = Nd, Sm, and Eu. In any RE, the phase stability field of T
'
cuprates is enlarged
by Ce substitution of x = 0.15. The enhanced phase stability by Ce substitution
can be explained similarly to the above RE dependence of the phase stability. Ce
is nearly tetravalent in T
'
cuprates, and takes a firmer grip on surrounding O
2–
ions
by stronger electrostatic force than trivalent RE.
6.3.2 Oxygen nonstoichiometry
Oxygen nonstoichiometry is a phenomenon common to cuprates, which is due to
the weak nature of a Cu-O bond and to the multivalent character of Cu (Cu
0
, Cu
1+
,
Cu
2+
, Cu
3+
, etc.). Oxygen loss in the CuO
2
layer starts to occur in low p
O2
prior to
decomposition, and this loss, in general, degrades and eventually kills high-T
c
superconductivity. In high p
O2
, excess oxygen atoms occupy the interstitial site in
the RE
2
O
2
layer in 214 cuprates: the tetrahedral site in T (the position close to O2
in T
'
) and the apical site (O
ap
) in T
'
. It is well known that interstitial excess oxygen
atoms in T-La
2
CuO
4
provide the CuO
2
layer with holes, achieving high-T
c
superconductivity without Sr substitution. In contrast, interstitial oxygen atoms in
T
'
cuprates are strong pair breakers, and harmful to high-T
c
superconductivity.
Therefore accurate data on oxygen nonstoichiometry in T
'
cuprates are required,
but not available. Although there are dozens of articles, the results do not concur
(Takayam-Muromachi et al., 1989; Kawashima et al., 1994; Moran et al., 1989;
Wang et al., 1990; Idemoto et al., 1990, 1991; Suzuki et al., 1990; Yamaguchi
et al., 1991; Kim and Gaskell, 1993; Klamut, 1993; Zhu et al., 1994a, 1994b,
1995; Radaelli et al., 1994; Schultz et al., 1996; Prado et al., 1995, 1999; Petrov
et al., 1999; Kang et al., 2007; Tanaka et al., 2008).
Even the presence of excess oxygen atoms is not supported by some articles.
Early iodometry titration experiments, employed for evaluation of the absolute
value (y) of oxygen in T
'
-(RE,Ce)
2
CuOy, often concluded y < 4.0. The behavior of
conductivity of T
'
cuprates, increasing monotonically with lowering p
O2
, is also
typical of n-type oxides with oxygen deficiencies. However, the presence of excess
oxygen atoms at the apical site (O
ap
) is in no doubt, as systematic photoemission
spectroscopy from Yamamoto et al. (1997) shows. The contradiction between
the conclusions by this study and others can be traced to the unique feature
of interstitial oxygen in T
'
cuprates. In fact, there is no space sufficient for a
large free O
2–
to reside at O
ap
, which may indicate the formation of a peroxide ion
(O
2
2–
) with neighboring regular oxygen atoms. In this case, interstitial excess
oxygen atoms will neither provide holes to the CuO
2
layer nor change the
Electron-doped cuprates as high-temperature superconductors 225
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
Cu valence. Since iodometry titration is, in principle, not to determine the oxygen
content but to determine the Cu valence, it will give a misleading conclusion on the
oxygen content in the case that a peroxide O
2
2–
ion exists. There has been no
experiment performed to take account of such a complicated situation. Hence,
based on several well researched articles, the author gives his understanding for the
oxygen nonstoichiometry in T
'
cuprates, specifically T
'
-Nd
2 – x
Ce
x
CuO
y
, as follows.
• Oxygen deficiency and excess oxygen coexist in T
'
cuprates. Even with
y = 4.00, the oxygen sublattice is not perfect. It is very difficult (almost
impossible) to remove O
ap
impurities without introducing oxygen deficiencies
in the CuO
2
layer.
• The maximum occupancy at O
ap
is ~0.10 at x = 0.00 and decreases to ~0.05 at
x = 0.15. Impurity oxygen atoms of 0.02–0.04 at O
ap
remain, even after
reduction.
• The maximum amount of oxygen deficiency in the CuO
2
layer is ~0.05 at
x = 0.00 decreasing to ~0.02–0.03 at x = 0.15. Oxygen deficiencies may not
be filled up completely even after annealing in 1 atm of oxygen.
Oxygen loss in the CuO
2
layer is a precursor of the decomposition. This is
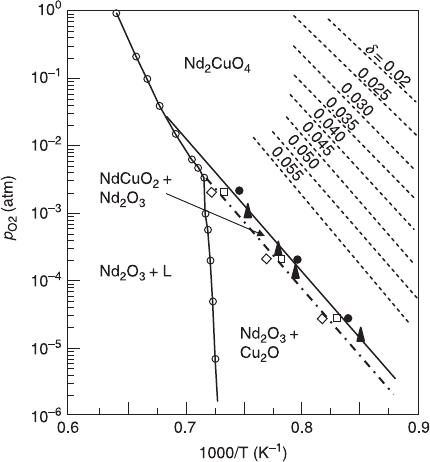
illustrated in Fig. 6.10, which plots the iso-composition lines (a line connecting
6.10 Phase diagram for T
'
-Nd
2
CuO
4 –
δ
. The iso-composition lines
connecting the same oxygen loss (
δ
) are also plotted, and they are
essentially parallel to the decomposition line. (Adapted from Kim and
Gaskel, 1993.)
226 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
the same oxygen loss,
δ
) in the p
O2
-vs-1/T plane. These lines were obtained by the
thermogravimetric analysis for T
'
-Nd
2
CuO
4
by Kim and Gaskel (1993) (excess
oxygen is not taken into account in their study, therefore the absolute value of the
oxygen deficiency may not be reliable). All the iso-composition lines with
different values of
δ
are essentially parallel to the decomposition line. The slope
of the iso-composition lines gives the standard enthalpy change, ∆H°, to desorb
1 mol of O
2
gas by the following process,
2RE
2
CuO
4
–>2 RE
2
CuO
4 –
δ
+
δ
O
2.
[6.6]
∆H° is 300–400 kJ/mole regardless of
δ
, which is essentially the same as ∆H° for
the decomposition, indicating that the oxygen loss and the decomposition are
governed by the same mechanism, namely the break of the Cu-O bond.
Finally, I point out two empirical trends about the RE dependence of the oxygen
chemistry:
1 The binding energy of O1 in the CuO
2
layer is larger for larger RE
3+
, which is
explained in section 6.3.1.
2 The binding energy of O
ap
in the RE
2
O
2
layer is smaller for larger RE
3+
(Zhu
and Manthiram, 1994b, 1995).
Both of the trends suggest that more perfect oxygen sublattice can be obtained
with larger RE
3+
such as Pr
3+
, Nd
3+
, which agrees with the trend to have better
superconductivity in T
'
cuprates. Ce substitution also affects the oxygen chemistry
and increases the binding energy of O1, thereby allowing stronger reduction than
for pristine compounds. Oxygen diffusion is also an important factor in low-
temperature reduction. The reduction time required for homogeneous oxygen is a
function of the sample size/thickness, grain size, etc. Thin films with small grain
size may be best to achieve homogeneous oxygen distribution in a short time.
6.4 Sample preparation
6.4.1 Bulk single crystal versus epitaxial thin films
Early sintered pellets of T
'
cuprates showed high resistivity (10–100 mΩcm),
semiconducting behavior, and a broad superconducting transition in most cases.
The reasons for such poor properties are high-resistance impurity phases at grain
boundaries and residual O
ap
impurities. At present, bulk single crystals of T
'
cuprates
can be grown by the flux method (Hidaka and Suzuki, 1989) or the traveling-solvent
floating zone (TSFZ) method (Tanaka et al., 1991) and epitaxial thin films can be
prepared by various methods such as molecular beam epitaxy (MBE), pulsed laser
deposition (PLD), sputtering, metal organic decomposition (MOD), etc. However,
good superconducting properties are still difficult to obtain for T
'
cuprates. The
problem is the removal of O
ap
impurities without introducing oxygen deficiencies
into the CuO
2
layer. The amount of residual O
ap
impurities significantly affects the
Electron-doped cuprates as high-temperature superconductors 227
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
experimental results, even entirely changing the most fundamental properties such as
the electronic phase diagram, which will be discussed in the next section.
Bulk single crystals eliminate grain boundaries but make O
ap
removal more
difficult due to limited paths of oxygen diffusion. Better superconducting
properties can be obtained with epitaxial thin films. In general, epitaxial films of
cuprates are not single-crystalline in a rigorous sense since they consist of
submicron grains aligned in and out of plane. Such microstructure, however, is
favorable in obtaining good properties for T
'
cuprates since oxygen diffusion is
prompt and grain boundaries with a small misorientation angle do not generate
resistance. So far the best quality samples have been obtained in films grown by
MBE and MOD, which are briefly described below.
6.4.2 Molecular beam epitaxy growth of T
'
cuprates
Molecular beam epitaxy (MBE) is the primary tool used for the deposition of
III–V semiconductors, such as GaAs and InP, employed in a variety of optoelectronic
devices. The volatility of the group V elements (e.g., As and P) has been a key for
the stoichiometric growth of these compounds, which has resulted in retarding the
development of composition control techniques. Oxide MBE is a field emerging
after the discovery of high-temperature superconductors. The growth of multi-
component oxides by MBE poses many challenges, the primary one being the
precise composition control of the constituent elements in an oxidizing environment.
The demands of oxide MBE have led to the development of real-time in situ
composition control techniques such as atomic absorption spectroscopy (AAS),
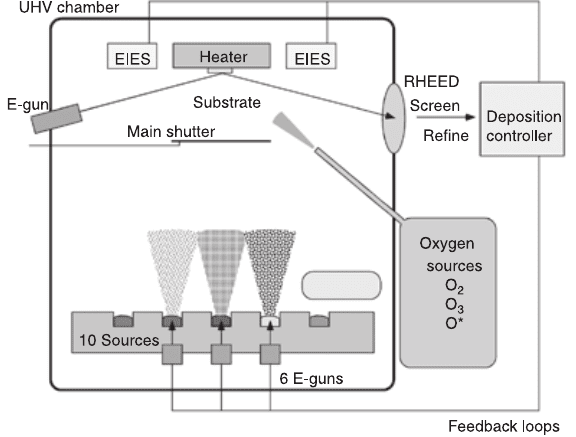
electron impact emission spectroscopy (EIES), etc.
MBE growth of RE
2 – x
Ce
x
CuO
4
films were performed by electron beam
coevaporation from metal sources in a customer designed UHV chamber as
shown in Fig. 6.11 (Naito et al., 1997). The main feature of this MBE system is
the precise control of evaporation flux based on EIES. The EIES sensors are
calibrated against the quartz crystal microbalance, which can be inserted at the
substrate holder position with the substrate holder swung up. The accuracy of
control is ~ 10
–3
Å/s for Cu and 10
–1
to 10
–2
Å/s for RE other than Ce. The flux of
Ce is controlled by a quartz crystal microbalance (not the same one for calibration,
but one located near the Ce source) since Ce has no sharp and strong atomic
emission line. The actual composition of films was confirmed by inductively
coupled plasma spectroscopy (ICP).
The oxidation during growth is performed by either O
3
gas (nondistilled, ~ 10%
concentration) from a commercial ozone generator or O* generated from a
commercial RF-activated source (HD25, Oxford Applied Research) operated at
250–300 W. In either case, the gas flow is typically 1–2 c.c./min, and the resultant
chamber pressure during growth is, at highest, 1–2 × 10
–5
Torr, which enables
stable rate control. The growth temperature is 650 °C to 750 °C, growth rate is
228 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
~1.5 Å/s, and the total thickness is typically 1000 Å. The substrates used are
SrTiO
3
(001) in most cases and REScO
3
(110) in some cases (REScO
3
has the
GdFeO
3
structure, distorted perovskite structure, and the (110) face of the GdFeO
3
structure is equivalent to the (001) face of the perovskite structure). Real-time in
situ RHEED observations show streaky diffraction patterns, which are evidence
of the growth of smooth epitaxial films.
The reduction of O
ap
impurities is a crucial step to optimizing the
superconducting properties of films. After growth, by cutting oxygen, the films
were held in vacuum (p
O2
~ 1 × 10
–8
Torr) for 10 min at the optimum reduction
temperature (T
r
) of 600 °C to 650 °C. The optimization for T
r
is performed using
RHEED observations. The films were slowly heated in vacuum with observation
of the film surface by RHEED. The appearance of diffraction spots due to
impurity phases shows the decomposition. The optimum T
r
is about 20 to 30 °C
lower than the decomposition temperature.
6.4.3 Metal organic decomposition growth of T
'
cuprates
Metal organic decomposition (MOD) is a rather inexpensive and easy-to-
implement thin film process, which is in contrast to MBE. The MOD process to
synthesize T
'
cuprate films is summarized in Table 6.6 (Matsumoto et al., 2008).
6.11 Schematic picture of the oxide MBE system (Naito et al., 1997).
The main feature is the precise control of evaporation flux based on
electron impact emission spectrometry (EIES) sensors, which are
located very close to the substrate holder.
Electron-doped cuprates as high-temperature superconductors 229
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
Table 6.6 (Matsumoto et al., 2008) MOD process to synthesize T
'
cuprate
films
MO RE and Cu naphthenates
Substrates DyScO
3
(110) for RE = Pr, Nd
SrTiO
3
(001) for RE = Nd, Sm, Eu, Gd
Calcination 400°C/30 min/in air
Firing 850–900°C/60 min/in N
2
/O
2
mixture (p
O2
= 10
–4
~ 10
–2
atm)
Reduction 400–450°C/10 min/in vacuum
The stoichiometric mixture of naphthenate solutions was spin-coated on substrates.
The coated films were first calcined at 400 °C in air to obtain precursors, then fired
at 850–900 °C in a tubular furnace under a mixture of O
2
and N
2
, controlling the
oxygen partial pressure (p
O2
) from 4 × 10
–5
atm to 10
–2
atm. Finally the films
were ‘reduced’ in vacuum (< 10
–4
Torr ≈ 10
–7
atm) at various temperatures for O
ap
removal. As compared with the MBE growth conditions, the synthesis temperature
is higher, and the reduction temperature is lower. A comparison of the morphologies
of MBE and MOD films observed by AFM indicated that the MBE films consist
of 2D grains with the size of 2000–3000 Å whereas the MOD films consist of 3D
grains with the size of less than 1000 Å. The smaller grain size in MOD films
facilitates O
ap
removal at lower temperatures than in MBE films.
6.5 Electronic phase diagram
6.5.1 Early results
The first article reporting the discovery of ‘electron-doped’ superconductors
(Takagi et al., 1989) demonstrated the following results on T
c
as a function of Ce
doping (x) in Nd
2 – x
Ce
x
CuO
4
. Superconductivity of T
c
> 20 K suddenly appears at
x = 0.14, and the highest T
c
is achieved at x = 0.15. Further Ce doping lowers T
c
until superconductivity disappears at x = 0.18. The superconducting window in
Nd
2 – x
Ce
x
CuO
4
was quite narrow. It was revealed by later µSR experiments (Luke
et al., 1990) that the antiferromagnetic order develops in x < 0.14, which competes
with the superconducting order. This first report employed polycrystalline
specimens annealed in an Ar + O
2
mixture of p
O2
~ 10
–4
atm at 950–1000 °C for
10 hours, and then quenched to room temperature (it was reported that additional
low-temperature annealing in p
O2
~ 10
–4
– 1 atm at 550 °C improves the width of
a superconducting transition). The reduction field (950–1000 °C in p
O2
~ 10
–4
atm) is located almost on the phase stability line.
Specimens used in most of the early experiments were similar polycrystalline
pellets with a small superconducting volume fraction (~10%), a broad
superconducting transition, and high resistivity. Subsequent developments in
single-crystal growth techniques enabled production of single-crystalline
230 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
specimens with a full superconducting volume fraction and metallic low resistivity
(0.5 – 1 mΩcm at room temperature). However, the initial T
c
-vs-x as described
above has been believed to be ‘typical’ of T
'
cuprates over 20 years. The
superconducting window (x = 0.14 to 0.18) of T
'
-Nd
2 – x
Ce
x
CuO
4
is much
narrower than 0.05 < x < 0.30 of the hole-doped analog, T-La
2 – x
Sr
x
CuO
4
, but
electron-hole symmetry appears to hold roughly in that the T
c
-vs-x shows a dome
shape with the optimum doping of x = 0.15. Based on this ‘apparent’ electron-
hole symmetry, the currently accepted ‘doped Mott insulator’ scenario for high-T
c
superconductors has been developed.
6.5.2 Electronic phase diagram modified by more complete
removal of O
ap
impurities
As mentioned in section 6.4.1, O
ap
impurities entirely change the most fundamental
properties such as the electronic phase diagram. There have been some efforts
performed toward more complete removal of O
ap
impurities, which changed the
electronic phase diagram dramatically. In general, it takes longer to remove O
ap
impurities from single crystals due to limited paths of oxygen diffusion, than it
does for polycrystalline specimens. Raising the reduction temperature to shorten
the reduction time often results in partial decomposition at the surface of single
crystals but with a fair amount of O
ap
impurities remaining inside. For the purpose
of more homogeneous reduction, Brinkmann et al. (1995) proposed the following
reduction. They sandwiched Pr
2 – x
Ce
x
CuO
4
single crystals with polycrystalline
pellets of the same composition, and reduced them together in a mixture of Ar + O
2
at much higher temperatures (up to 1080 °C) for a much longer time (3 days) than
in the standard reduction. The result is shown in Fig. 6.12, which compares the
T
c
-vs-x by the improved (solid line) and standard (broken line) reduction. With the
improved reduction, the superconducting window expands down to x = 0.04 and
there is a clear tendency of an increasing T
c
with decreasing x.
Brinkmann et al. (1996) also pointed out the following correlation between
T
c
and residual resistivity (
ρ
R
) ‘the larger
ρ
R
, the lower T
c
’, which is demonstrated
in Fig. 6.13. Empirically, specimens containing a larger amount of residual O
ap
impurities show a more prominent upturn in low-temperature resistivity. Hence the
above correlation expresses that O
ap
impurities are a strong scatterer to increase
ρ
R
as well as a strong pair-breaker to lower T
c
. It appears that O
ap
impurities in T
'
cuprates behave like magnetic impurities in conventional superconductors.
Another way for more complete removal of O
ap
impurities is to employ thin films
as oxygen diffusion is prompt in thin films due to a large surface-to-volume ratio
and small grain size. Figure 6.14 summarizes the T
c
-vs-x curves for MBE-grown
RE
2 – x
Ce
x
CuO
4
thin films with different RE (Krockenberger et al., 2008). Table 6.7
is a summary of the optimum doping level (x
opt
) and highest T
c
(T
c
max
) for each RE.
T
'
-La
2 – x
Ce
x
CuO
4
shows superconductivity in 0.04 < x < 0.22 with x
opt
= 0.08 and
T
c
max
= 31.0 K (Naito and Hepp, 2000b, 2001). In general, the superconducting
