Qiu X.G. (Ed.) High Temperature Superconductors
Подождите немного. Документ загружается.

Electron-doped cuprates as high-temperature superconductors 211
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
6.2 Structure
6.2.1 214 cuprates: RE
2
CuO
4
The rare earth copper oxides of the general chemical formula RE
2
CuO
4
possess a
richness of structural and physical properties because of the wide range of the ion
size of RE
3+
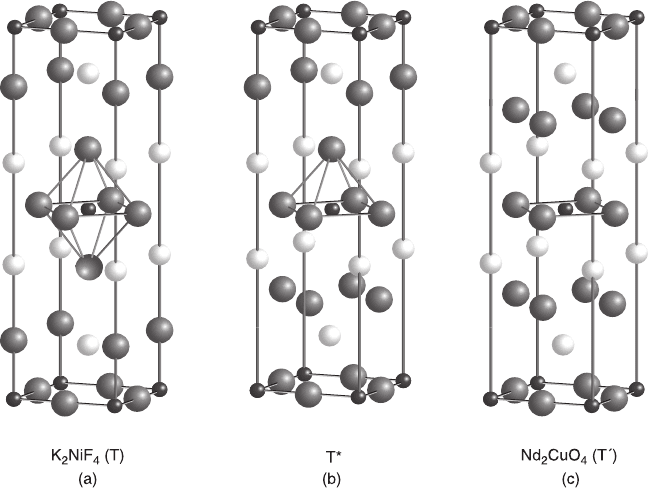
. There are two closely related structures as shown in Fig. 6.3(a) and
6.3(c): the K
2
NiF
4
(abbreviated as T) and Nd
2
CuO
4
(abbreviated as T
'
) structures.
The structural difference between T and T
'
can be viewed as the difference in the
RE-O arrangements: rock-salt-like versus fluorite-like; or alternatively as the
difference in the Cu-O coordination: octahedral CuO
6
versus square-planar CuO
4
.
The key parameter determining which of these structures is formed is the ionic
radius of the RE
3+
ion. The T structure is formed with large La
3+
whereas the T
'
structure is formed with smaller RE
3+
ions such as RE = Pr, Nd, Sm, Eu, and Gd.
By employing high-pressure synthesis, the T
'
structure is also formed with Dy to
Tm as well as Y (Okada et al., 1990; Bordet et al., 1992). The T–T
'
boundary lies
between La
3+
and Pr
3+
. In a mixed-lanthanide system (La,RE)
2
CuO
4
, a third
structure (denoted T*, Fig. 6.3(b)), which consists of alternating stacking of T- and
T
'
-slabs, is observed (Sawa et al., 1988). The stabilization of this structure requires
6.3 Three crystal structures of rare-earth copper oxides: (a) K
2
NiF
4
(abbreviated as T) structure, (b) T* structure, and (c) Nd
2
CuO
4
(abbreviated as T
'
) structure.

212 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
two RE ions with significantly different ionic sizes: one large La
3+
and the other
smaller RE
3+
. All of the three types of 214 cuprates show superconductivity with
T
c
of 30–40 K as shown in Table 6.1. It has been empirically claimed that T and T*
cuprates accept only hole doping whereas T
'
cuprates accept only electron doping.
6.2.2 Tolerance factor
The crystal chemistry, mentioned above, on the structure versus the ionic radius
in the rare earth copper oxides has been explained by Bringley et al. (1990) and
Manthiram et al. (1990) in terms of the crystallographic tolerance factor (t), which
is defined as
t = [r
i
IX
(RE
3+
) + r
i
VI
(O
2–
)]/[√2* [r
i
VI
(Cu
2+
) + r
i
VI
(O
2–
)], [6.1]
where r
i
IX
(RE
3+
), r
i
VI
(Cu
2+
), and r
i
VI
(O
2–
) are the empirical room-temperature
ionic radii by Shannon and Prewitt (1969) for RE
3+
, Cu
2+
, and O
2–
ions with the
coordination indicated by superscript Roman numerals. The tolerance factor (t)
was initially proposed to argue the stability of the perovskite structure (ABO
3
) by
Goldshimidt (1926). It represents the bond length matching between AO layers
and BO
2
layers. Ideal matching corresponds to t = 1, and the perovskite structure
is stable for ~ 0.8 < t < 1.0. This factor can also be used to argue the stability of
the T and T
'
structure. The calculated values for t are listed in Table 6.2. Figure
6.4(a) gives a schematic illustration for the bond length mismatch between Cu-O
and La-O in the case of T-La
2
CuO
4
. The cell size of the CuO
2
layer (4.26 Å) is
apparently larger than that of the LaO layer (3.70 Å).
Bringley et al. (1990) obtained the following empirical trend on the basis of the
systematic investigation on the mixed lanthanide systems La
2–x
RE
x
CuO
4
, in
which ‘average’ t can be varied continuously,
• For 0.87 ≤ t ≤ 0.99, the T structure is stable.
• For 0.83 ≤ t ≤ 0.86, the T
'
structure is stable. The critical value for the T to T
'
transition is t
c
R
= 0.865, where the superscript, R, denotes the room-temperature
value. See below for the temperature effect.
Table 6.1 Superconducting transition temperatures (T
c
) of rare earth copper
oxides (RE
2
CuO
4
)
Material T
c
(K) Ref.
T La
1.85
Ba
0.15
CuO
4
30 Bednorz and Müller, 1986
La
1.85
Sr
0.15
CuO
4
37 Kishio et al., 1987
T* Nd
1.32
Sr
0.41
Ce
0.27
CuO
4
28 Sawa et al., 1989
La
0.82
SmSr
0.18
CuO
4
37 Fisk et al., 1989
T
'
Nd
1.85
Ce
0.15
CuO
4
24 Tokura et al., 1989
La
1.9
Ce
0.1
CuO
4
30 Naito and Hepp, 2000
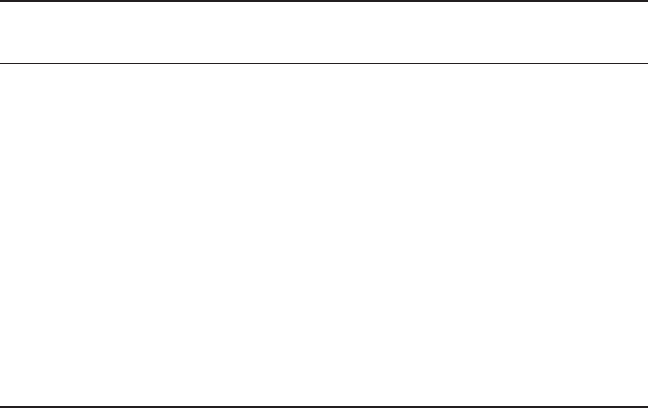
Electron-doped cuprates as high-temperature superconductors 213
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
• For t < 0.83, neither of the T nor T
'
forms. Instead the complicated, so-called,
‘Ho
2
Cu
2
O
5
’ structure forms (Freund and Müller-Buschbaum, 1977).
Similar geometrical considerations can be made for the T
'
stability. In this case,
the cell size of the CuO
2
layer is given by 2√2*r
i
VI
(O
2–
) = 3.96 Å. This is because
Cu
2+
for the square-planar 4-fold Cu-O coordination has r
i
= 0.57 Å, which is
substantially smaller than 0.73 Å for the 6-fold coordination, and fits in the space
surrounded by four oxygen ions, tangential to one another (see Fig. 6.4 (right)).
The evaluation of the cell size of the RE
2
O
2
layer is made by assuming the ideal
fluorite structure, namely oxygen ion is surrounded by four RE ions with regular
tetrahedron (the bond angle of RE-O-RE is arccos(–1/3) = 109.5°). Then the cell
size of the RE
2
O
2
layer is given by 2√6*[r
i
VIII
(RE
3+
) + r
i
IV
(O
2–
)]/3, and varies
from 4.15 Å (La) to 3.88 Å (Tm), which should be compared with the cell size of
the CuO
2
layer of 3.96 Å. It should be noted that O
2–
in the RE
2
O
2
layer is
surrounded only by four RE
3+
whereas O
2–
in the CuO
2
layer is surrounded
by four RE
3+
and two Cu
2+
, namely the coordination number is different for two
O
2–
. The resultant tolerance factor for the T
'
stability is given by
t
'
= [r
i
VIII
(RE
3+
) + r
i
IV
(O
2–
)]/[√3* r
i
VI
(O
2–
)], [6.2]
and is also listed in Table 6.2.
Table 6.2 Room-temperature ionic radius of RE
3+
for 9- and 8-fold coordinations and
corresponding tolerance factors for T and T
'
stability calculated from eq. [6.1] and [6.2]
RE
3+
r
i
IX
(RE
3+
) (Å) r
i
VIII
(RE
3+
) (Å) Tolerance factor Tolerance factor for
for T stability t T
'
stability t
'
La
3+
1.216 1.160 0.8685 1.0392
Ce
3+
1.196 1.143 0.8618 1.0322
Pr
3+
1.179 1.126 0.8562 1.0252
Nd
3+
1.163 1.109 0.8509 1.0182
Pm
3+
1.144 1.093 0.8445 1.0116
Sm
3+
1.132 1.079 0.8406 1.0058
Eu
3+
1.120 1.066 0.8366 1.0005
Gd
3+
1.107 1.053 0.8323 0.9951
Tb
3+
1.095 1.040 0.8283 0.9897
Dy
3+
1.083 1.027 0.8243 0.9844
Ho
3+
1.072 1.015 0.8206 0.9794
Er
3+
1.062 1.004 0.8173 0.9749
Tm
3+
1.052 0.9940 0.8140 0.9708
Yb
3+
1.042 0.9850 0.8107 0.9671
Lu
3+
1.032 0.9770 0.8074 0.9638
Y
3+
1.075 1.019 0.8216 0.9811
In calculating the tolerance factor, one should use r
i
IX
(RE
3+
) corresponding to the T
structure for eq. [6.1] and r
i
VIII
(RE
3+
) corresponding to the T
'
structure for eq. [6.2].
For Cu
2+
and O
2–
, the following ionic radii are used: r
i
IV
(Cu
2+
) = 0.57 Å, r
i
V
(Cu
2+
) =
0.65 Å, r
i
VI
(Cu
2+
) = 0.73 Å and r
i
IV
(O
2–
) = 1.36 Å, r
i
VI
(O
2–
) = 1.40 Å.
214 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
6.4 Sliced view of CuO
2
and La
2
O
2
layers in T-La
2
CuO
4
(left) and
T
'
-La
2
CuO
4
(right).
6.2.3 Thermal expansion of bond length
The above discussion neglects the temperature effect on the bond length, as the
bond length expands with temperature. The room-temperature ionic radii are used
above in calculation of the tolerance factor although the values at synthesis
Electron-doped cuprates as high-temperature superconductors 215
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
temperatures (~1000 °C) should be used in discussion of the phase stability.
As pointed out by Manthiram and Goodenough (1991), the ‘ionic’ RE-O bond
has a larger thermal expansion coefficient than the ‘covalent’ Cu-O bond. The
different thermal expansion coefficients (‘thermal-expansion mismatch’) of
the RE-O and Cu-O bond length leads to the increase of t with temperature
(t = t
0
+
α
T,
α
~ 2–3 × 10
–5
by rough estimate), where t
0
is the tolerance factor
at 0 °C (≈ room temperature) and T is measured in °C, and thereby plays
an important role in the T versus T
'
stability. Then the actual threshold for
the T to T
'
transition will be t
c
= 0.875–0.880 instead of the above room-
temperature value, t
c
R
= 0.865. Therefore, if one could synthesize La
2
CuO
4
at
room temperatures, one would get T
'
-La
2
CuO
4
since t = 0.8685 of La
2
CuO
4
is
smaller than t
c
= 0.875–0.880.
Manthiram and Goodenough (1991) succeeded in the selective stabilization of
T versus T
'
in the La
2 – y
Nd
y
CuO
4
system by changing the synthesis temperature
(T
s
). La
1.5
Nd
0.5
CuO
4
(t = 0.8641 at room temperature) is stabilized as single-
phased T
'
below T
s
= 625 °C or single-phased T above T
s
= 775°. A two-phase
mixture of T and T
'
is obtained in between 625 °C and 775 °C. In their experiments,
coprecipitation powders were employed to promote chemical reaction at firing
temperatures as low as 500 °C. At T
s
= 500 °C, even La
2
CuO
4
becomes not single-
phased T but a two-phase mixture of T and T
'
. By extrapolation of the
T/T
'
phase boundary in the La
2 – y
Nd
y
CuO
4
system to y = 0, it is predicted that
La
2
CuO
4
can be stabilized as the T
'
structure below T
s
= 425 °C, which is too low
for bulk synthesis. By means of thin-film synthesis, however, the reaction
temperature can be lowered significantly, since reactants are much smaller in size
and also more reactive than in bulk synthesis. Success in synthesizing the T
'
phase
of pure La
2
CuO
4
by reactive coevaporation technique was achieved by Tsukada
et al. (2002).
6.2.4 Structural parameters and interstitial oxygen
As mentioned above, T-La
2
CuO
4
is located at the borderline of the K
2
NiF
4
stability, and hence it distorts to the orthorhombic structure (LTO: low-temperature
orthorhombic phase) at temperatures below 550 K so as to accommodate the
large bond length mismatch by tilting of CuO
6
octahedra. On the other hand,
T
'
-RE
2
CuO
4
shows no distortion and keeps the original tetragonal (I4/mmm)
structure except for T
'
-Gd
2
CuO
4
, which is located at the borderline of the
Nd
2
CuO
4
stability. Table 6.3 shows the structural parameters obtained from
the powder neutron diffraction experiments on oxygenated and reduced
Nd
1.9
Ce
0.1
CuO
4
by Petrov et al. (1999). There are two regular oxygen sites in the
T
'
structure: the planar site (O1) and the out-of-plane site (O2). In addition, T
'
cuprates have a strong tendency to have excess oxygen atoms at the interstitial
apical site (O
ap
). As mentioned in section 6.1, ‘reduction’ is required to achieve
superconductivity in T
'
cuprates. The reduction is not intended for further electron

216 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
doping by oxygen deficiencies, but for removal of impurity oxygen atoms at O
ap
,
which is very harmful to high-T
c
superconductivity. As seen from Table 6.3, in
the oxygenated sample (annealed in air at 950 °C, and then quenched to
room temperature), a significant amount of oxygen (~0.08 atoms/formula unit)
is present at O
ap
. On the other hand, in the reduced sample (annealed in
p
O2
= 4.57 × 10
–2
atm at 1200 °C, and then quenched to room temperature), the
occupancy of O
ap
is reduced to ~0.04 atoms/formula unit. With regard to the
regular oxygen sites, the occupancy of O2 is essentially full (2.00) and unchanged
by reduction whereas the occupancy of O1 is slightly less than 2.00 even in the
oxygenated sample. In the experimental results by Petrov et al. (Table 6.3), there
appears to be essentially no change by reduction in the O1 occupancy, but
many results indicate that oxygen vacancies at O1 increase by reduction,
especially in samples with no or low Ce doping (Radaelli et al., 1994). The results
can be summarized as follows: oxygen vacancies at O1 and interstitial oxygen
atoms at O
ap
coexist with their occupancies in thermal equilibrium. This
makes quite complex the oxygen chemistry of T
'
cuprates, resulting in the defect
structure dependent on the temperature, oxygen partial pressure, and even cerium
content.
6.2.5 Lattice parameters
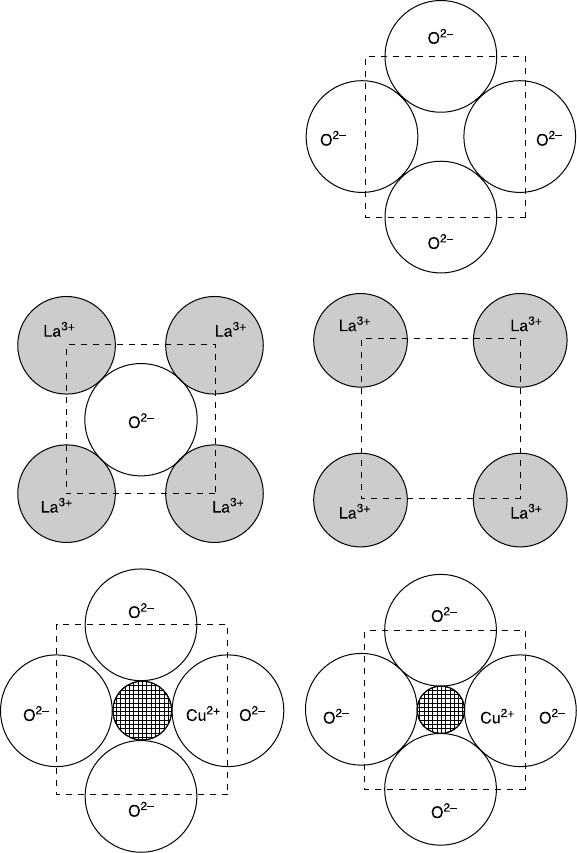
First, we take a look at the RE dependence of the lattice parameters of the
parent compounds, T
'
-RE
2
CuO
4
. Since the ionic radius of RE
3+
changes from
1.160 Å (La) to 0.977 Å (Lu), the lattice parameters of T
'
-RE
2
CuO
4
change
accordingly. One can investigate a systematic variation in both physical and
chemical properties with the lattice parameters, which is one benefit to the
research of T
'
cuprates. Figure 6.5 and Table 6.4 summarize the RE dependence
of the a-axis and c-axis lattice parameters (a
0
and c
0
). The a
0
changes by ~5 %
Table 6.3 Structural parameters (atomic position (z) and occupancy (n))
determined by the powder neutron diffraction on oxygenated and reduced
Nd
1.9
Ce
0.1
CuO
4 +
δ
Atom z/n Oxygenated (
δ
= 0.03) Reduced (
δ
= 0.00)
Nd/Ce z 0.3519 0.3520
n 2 2
Cu n 1 1
O1 n 1.95 1.96
O2 n 2.00 2.00
O
ap
z 0.1904 0.1905
n 0.08 0.04
The atomic positions are: Nd 4e[0,0,z]; Cu 2a[0,0,0]; O1 4c[1/2,0,0]; O2
4d[0/1/2,1/4]; O
ap
4e[0,0,z]. After Petrov et al. (1999).
Electron-doped cuprates as high-temperature superconductors 217
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
6.5 RE dependence of the c-axis (a) and a-axis (b) lattice parameters of
T
'
-RE
2
CuO
4
. The data are collected from Okada et al. (1990) (+), Bordet et
al. (1992) (
), Uzumaki et al. (1992) (♦), and Chou et al. (1990) (×).
from 4.025 Å (La) to 3.830 Å (Tm) and the c
0
changes by ~8 % from 12.55 Å
(La) to 11.58 Å (Tm). Both of the a
0
and c
0
do not show a linear dependence
on the ionic radius, but show a weaker dependence from Sm to Tm. This
behavior may be due to interstitial O
ap
impurities, which are contained more in
heavier RE
3+
.
Next, we take a look at the Ce doping dependence of the lattice constants.
In general, Ce can be trivalent as well as tetravalent. Which valence Ce takes
depends on the environment around Ce ions in lattice and also on the synthesis
condition. The early chemical analysis (iodometry titration) by Idemoto et al.

218 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
(1990) gave the Ce valence of +3.84 in T
'-
Nd
1.85
Ce
0.15
CuO
4
, which is close to +4.
The ionic radius of tetravalent Ce, r
i
VIII
(Ce
4+
), is 0.97 Å, which is smaller than
r
i
VIII
(RE
3+
) of trivalent RE ions. Therefore the lattice constants of T
'
cuprates
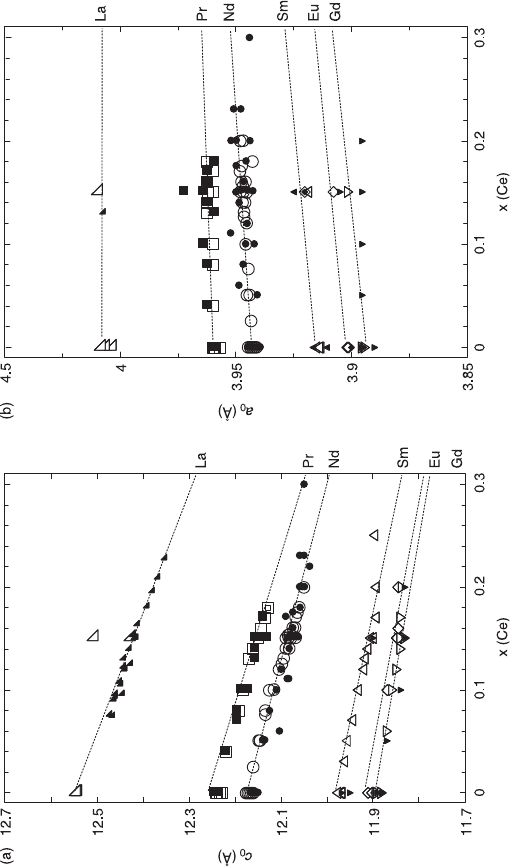
should decrease with substitution of Ce for RE. Figure 6.6 summarizes the a
0
and
c
0
for T
'
-RE
2 – x
Ce
x
CuO
4
for different RE as a function of the Ce content, x. The
filled symbols are the data for reduced samples, and the empty symbols for
oxygenated samples. They are essentially the same within the experimental
accuracy. The c
0
decreases with x in accordance with the above expectation
whereas the a
0
increase slightly with x, which is opposite to the expectation. The
increase of a
0
with electron doping is due to the stretching of the Cu-O bond by
filling electrons into the antibonding (
σ
*) orbitals, which makes the Cu-O covalent
bond less stable.
In Fig. 6.6, both a
0
and c
0
change linearly with x in low Ce doping, but they
show no change for x above a certain value, which is the solubility limit (x
c
) of Ce
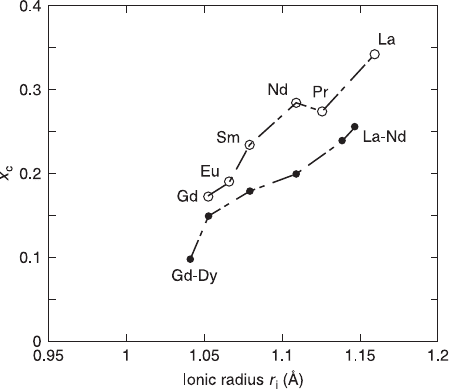
(Vegard’s law). Figure 6.7 summarizes the RE dependence of x
c
in bulk synthesis
and molecular beam epitaxy (MBE) growth. With any RE, the x
c
in MBE growth
is larger than x
c
in bulk synthesis. As shown in Fig. 6.7, the x
c
increases with the
ionic radius of RE
3+
, and is ~ 0.3 for La and ~ 0.1 for Gd. This RE dependence of
x
c
can be understood from the geometrical considerations as mentioned in section
6.2.2. As shown in Table 6.2, the tolerance factor (t
'
) for the T
'
structure is less
than 1.00 for small RE
3+
, namely the cell size of the fluorite RE
2
O
2
layer is smaller
than that of the CuO
2
layer. The Ce substitution decreases the average ionic radius
of RE
3+
2 – x
Ce
4+
x
and at the same time stretches the Cu-O bond, which reduces t
'
to a further smaller value and makes the T
'
phase less stable.
Table 6.4 Lattice parameters (a
0
and c
0
) of T
'
-RE
2
CuO
4
with different RE
RE a
0
(Å) c
0
(Å)
La 4.005 12.550
Pr 3.958 12.288
Nd 3.943 12.163
Sm 3.905 11.929
Eu 3.894 11.882
Gd 3.888 11.859
Tb 3.880 11.815
Dy 3.869 11.771
Ho 3.861 11.721
Er 3.840 11.637
Tm 3.830 11.578
Y 3.861 11.721
The values for La are taken from Chou et al. (1990) whereas those for other
RE are from Bordet et al. (1992).
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
219
6.6 Ce doping dependence of the c
0
(a) and a
0
(b) lattice parameters of T
'
-RE
2 – x
Ce
x
CuO
4
with different RE. The data are
collected from:
La: Takayama-Muromachi et al. (1990), Chou et al. (1990), Yamada et al. (1994), Naito and Hepp (2001);
Pr: Markert et al. (1990), Matsuda et al. (1991), Uzumaki et al. (1992), Zhou et al. (1993), Kawashima et al. (1994);
Nd: Takagi et al. (1989), Huang et al. (1989a, 1989b), Hidaka and Suzuki (1989), Markert et al. (1990), Uzumaki et al. (1992),
Zhou et al. (1993), Kawashima et al. (1994);
Sm: Markert et al. (1990), Uzumaki et al. (1992), Zhou et al. (1993), Ishii et al. (1993), Kawashima et al. (1994);
Eu: Markert et al. (1990), Uzumaki et al. (1992), Ishii et al. (1993), Kawashima et al. (1994);
Gd: Markert et al. (1990), Uzumaki et al. (1992), Zhou et al. (1993), Ishii et al. (1993), Kawashima et al. (1994).
220 High-temperature superconductors
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
5
6
7
8
9
40
1
2
43X
© Woodhead Publishing Limited, 2011
6.3 Solid-state chemistry
6.3.1 Phase stability
Copper is an element with weak chemical bonding (affinity) to oxygen. Hence
copper oxides inevitably decompose at high temperature or in low oxygen partial
pressure. Roughly speaking, the decomposition line (phase stability limit) of all
high-T
c
cuprates is close to the decomposition line of the divalent simple oxide,
CuO, since Cu is essentially divalent in high-T
c
cuprates. Figure 6.8 shows a
closer comparison between the decomposition lines of 214 and simple copper
oxides (CuO and Cu
2
O). It also includes a similar comparison for Ni and Co
oxides. The decomposition lines of T-La
2
CuO
4
, T
'
-Pr
2
CuO
4
, and T
'
-Nd
2
CuO
4
are
all located in between those of CuO and Cu
2
O. In fact, the decomposition lines
of 214 cuprates are located substantially below that of CuO. One can imagine
the reason as follows. The oxygen desorption of the CuO
2
layer triggering
the decomposition in 214 cuprates is partially blocked by the RE
2
O
2
layers
sandwiching above and below. The decomposition lines of La
2
NiO
4
and La
2
CoO
4
are located at much lower p
O2
than those of 214 cuprates, but they almost coincide
with the decomposition lines of the simple oxides, NiO and CoO. The large
difference of the decomposition line between cuprates and Ni, Co oxides reflects
much stronger oxygen affinity of Ni or Co than of Cu. These observations indicate
6.7 Solubility limit (x
c
) of Ce in T
'
-RE
2 – x
Ce
x
CuO
4
with different RE. The
filled circles are from bulk synthesis (Zhu and Manthiram, 1994a) and
the open circles from our MBE growth. In both cases, the x
c
increases
with r
i
VIII
(RE
3+
).
