Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

ASPARTASES 565
Woods, S.A., Miles, J.S. and Guest, J.R. (1988). Sequence homologies between argininosuccinase,
aspartase, and fumarase: a family of structurally-related enzymes. FEMS Microbiol Lett 51, 181–186.
Wu, C.Y., Lee, H.J., Wu, S.H., Chen, S.T., Chiou, S.H. and Chang, G.G. (1998). Chemical mechanism
of the endogenous argininosuccinate lyase activity of duck lens delta2-crystallin. Biochem J 333(Pt 2),
327–334.
Yoon, M.Y., Thayer-Cook, K.A., Berdis, A.J., Karsten, W.E., Schnackerz, K.D. and Cook, P.F. (1995).
Acid/base chemical mechanism of aspartase from Hafnia alvei. Arch Biochem Biophys 320, 115–122.
Yumoto, N., Mizuta, K., Tokushige, M. and Hayashi, R. (1982). Studies on aspartase VIII. Protease-
mediated activation: comparative survey of protease specificity for activation and peptide cleavage.
Physiol Chem Phys 14, 391–397.
Yumoto, N., Okada, M. and Tokushige, M. (1982). Biospecific inactivation of aspartase by L-aspartic-
beta-semialdehyde. Biochem Biophys Res Commun 104, 859–866.
Yumoto, N., Tokushige, M. and Hayashi, R. (1980). Studies on aspartase. VI. Trypsin-mediated activation
releasing carboxy-terminal peptides. Biochim Biophys Acta 616, 319–328.
Zhang, H.Y., Zhang, J., Lin, L., Du, W.Y. and Lu, J. (1993). Enhancement of the stability and activity
of aspartase by random and site-directed mutagenesis. Biochem Biophys Res Commun 192, 15–21.
CHAPTER 32
TRANSGLUTAMINASES
MARÍA JESÚS ARRIZUBIETA
∗
Departamento de Ciencias del Medio Natural, Universidad Pública de Navarra, Pamplona, Spain
∗
mjesus.arrizubieta@unavarra.es
1. INTRODUCTION
Transglutaminases (TG: EC 2.3.2.13, protein glutamine:amine -glutamyl-
transferase) are a group of thiol enzymes that catalyse the post-translational modifi-
cation of proteins mainly by protein to protein cross-linking, but also through
the covalent conjugation of polyamines, lipid esterification, or the deamidation of
glutamine residues (Folk and Chung, 1973; Lorand and Conrad, 1984; Aeschlimann
and Paulsson, 1994; Nemes et al., 1999). Transglutaminases are widely distributed
among bacteria, plants and animals. Mammalian transglutaminases comprise a
group of structurally and phylogenetically related multidomain enzymes strictly
dependent on calcium for their activity (Grenard et al., 2001). These enzymes are
related to different physiological processes and diseases and consequently have
raised medical and pharmacological interests in their potential as therapeutic targets
or diagnostic aids (Griffin et al., 2002). In contrast to mammalian transglutami-
nases, the microbial transglutaminase from Streptomyces is a monomeric enzyme
that does not require calcium for activity. Phylogenetically this enzyme is not
related to mammalian transglutaminases and presents a novel catalytic mechanism
(Kashiwagi et al., 2002). Its discovery has enabled a diversity of industrial appli-
cations in the food and textile industries. Transglutaminase activity has also been
found in higher and lower plants (Del Duca and Serafini-Fracassini, 2005), and
the available data indicate that plant transglutaminases are similar in overall
structure and catalytic mechanism to those of mammals (Villalobos et al., 2004).
2. ENZYME ACTIVITY AND MECHANISM OF CATALYSIS
TGs catalyse the acyl-transfer reaction between the -carboxyamide group of a
peptide-bound glutamine residue and a primary amine. In this reaction the glutamine
side chain serves as the acyl donor, whereas the primary amine functions as acceptor.
567
J. Polaina and A.P. MacCabe (eds.), Industrial Enzymes, 567–581.
© 2007 Springer.
568 ARRIZUBIETA
The outcome is the generation of a covalent bond between the two substrates and the
release of ammonia. In most cases described, the primary amine is the -amino group
of a lysine residue, and the reaction results in the formation of -(-glutamyl)lysine
linkages. Depending on the nature of the substrates (proteins or peptides), the reaction
may result in protein cross-linking or peptide conjugation to proteins. When primary
amines are not available, water can function as the acyl group acceptor, with the
consequent deamidation of the glutamine residue (Folk and Chung, 1973; Lorand and
Conrad,1984).Inaddition, thehydroxylgroupsof hydroxyceramidescanalsofunction
as acyl acceptor substrates resulting in ester bond formation between the glutamine
side chains of proteins and hydroxylipids (Nemes et al., 1999).
The transglutaminase reaction is reversible and proceeds via a modified double-
displacement mechanism in which an acyl-enzyme intermediate is formed between
the acyl portion of the glutamine substrate and the sulfhydryl group of the catalyti-
cally active cysteine residue on the enzyme. An acyl acceptor, like water or a primary
amine,reactswiththisintermediateresultingindeamidationorcross-linkingreactions,
respectively(Folk,1969;FolkandChung,1973).ThefirstcrystalstructureofaTG,that
of blood coagulation factor XIII (FXIII), revealed striking similarities to cysteine-
proteases at the active site, leading to the proposal of a similar catalytic mechanism
for the TG-catalysed cross-linking reaction (Pedersen et al., 1994). According to this
mechanism, Cys-314 acts as the nucleophile and His-373 as the acid/base catalyst.
Asp-396 is proposed to play a secondary role, stabilizing the protonated form of His-
373 and providing a favourable orientation of this residue. Based on the structural
and mechanistic similarities between FXIII-like TGs and cysteine-proteases, it has
been proposed that these two groups of enzymes, catalysing almost opposite reactions,
evolved from a common ancestor (Pedersen et al., 1994).
TGs are synthesized as inactive zymogens that require activation before exhibiting
their transamidating activity (Lorand and Conrad, 1984). The mechanisms for
controlling the transamidating activity varies from enzyme to enzyme and involves
activation by proteolytic cleavage, calcium binding, GTP binding, substrate seques-
tration, and often combinations of two or more of these mechanisms (Lorand and
Conrad, 1984; Griffin et al., 2002).
Different enzymes display specificity differences towards substrate proteins. This
constitutes an important aspect of their biological function and it is also relevant to
their biotechnological applications. In general, there is more stringent specificity for
the glutamine substrate whereas the specificity for the primary amine substrate is
broader. The nature of the amino acids surrounding the sensitive glutamine residues
and the degree of the latters’ exposure to solvent appear to be the main determinants
of substrate specificity (Coussons et al., 1992).
3. ENZYME TYPES
3.1. Mammalian Transglutaminases
Nine different transglutaminase encoding genes have been characterized in
mammals (Ichinose et al., 1990; Aeschlimann and Paulsson, 1994; Aeschlimann
TRANSGLUTAMINASES 569
et al., 1998). The products of eight of them are active enzymes whereas the
remaining one is a non-catalytic protein, the erythrocyte protein band 4.2. These
genes present a high degree of sequence similarity and share the same genetic
organization.
All characterized mammalian TGs bind calcium and function as metal-
enzyme complexes in different biological processes (Aeschlimann and Paulsson,
1994). Blood coagulation factor XIII catalyses covalent cross-linking between
fibrin molecules during blood clot formation (Pisano et al., 1968; Ichinose
et al., 1990). There are six different TGs expressed during epidermal differ-
entiation. These enzymes cross-link the structural proteins that constitute the
protective cornified cell envelop of differentiating keratinocytes (Nemes and
Steinert, 1999; Candi et al., 2005). Tissue transglutaminase, or TG2, besides
being a cross-linking enzyme functions also as a GTPase, and participates in
processes as diverse as apoptosis, development, intracellular signalling, cell-
matrix interactions and cell migration (Akimov et al., 2000; Piacentini et al.,
2000; Mangala et al., 2005; Sarang et al., 2005). Prostate transglutaminase,
or TG4, is an extracellular TG secreted by the prostate gland into the
seminal fluid and is responsible for the production of the rodent vaginal plug
(Aeschlimann and Paulsson, 1994).
3.2. Microbial Transglutaminases
The first characterized microbial transglutaminase (MTG) was that of the bacterium
Streptomyces mobaraensis (previously termed Streptoverticillium mobaraensis)
(Ando et al., 1989). This enzyme is secreted as a zymogen that is sequen-
tially processed by two endogenous enzymes to yield the mature form (Zotzel
et al., 2003). The mature enzyme is a monomeric protein 331 amino acids
long and contains a single cysteine (Cys-64) which is the catalytic residue
(Kanaji et al., 1993; Pasternack et al., 1998). The amino acid sequence of MTG
bears little significant similarity to the FXIII-like TGs or to any other known
sequence. MTG does not require calcium for activity, shows broad substrate
specificity and can be produced at relatively low cost. These properties are
advantageous for industrial applications. Currently the enzyme is produced by
fermentation using a Streptomyces strain; however, a lot of effort has been
directed towards achieving production of recombinant MTG in a heterologous host
(Yokoyama et al., 2004).
TG activity has also been found in Bacillus species (Kobayashi-K et al.,
1996). The enzyme purified from B. subtilis catalyses the cross-linking of the
spore coat protein GerQ thereby contributing to the physical and chemical resis-
tance of the bacterial spore (Ragkousi and Setlow, 2004). In addition, bacterial
virulence factors such as Escherichia coli cytotoxic necrotizing factor and Borde-
tella dermonecrotic toxin present transglutaminase activity. They exert their
cytotoxic effects by activating the small intracellular GTPases of the Rho family by
570 ARRIZUBIETA
deamidation of specific Gln residues or by cross-linking the GTPases with
polyamines (Horiguchi, 2001).
3.3. Plant Transglutaminases
Plant transglutaminases are present in various compartments such as chloro-
plasts, mitochondria, cell walls and cytoplasm. Their functions are related to plant
growth, cell division, differentiation, programmed cell death, fertilization and stress
(Serafini-Fracassini et al., 1995). So far only three TG have been characterized at
the molecular level, one in Arabidopsis (Della Mea et al., 2004) and two proteins
of 39 and 58 kDa in the chloroplast of Zea mays (Della Mea et al., 2004; Villalobos
et al., 2004). The expression and activity of the corn enzymes is light dependent
and it has been proposed that they might play a role in the light-harvesting process
in thylakoids (Della Mea et al., 2004).
4. ENZYME STRUCTURE
4.1. Factor XIII
The structure of the zymogen of the A subunit of factor XIII (FXIIIA) has been
published (Yee et al., 1994). The solved structures correspond to a homodimer. The
region corresponding to the mature enzyme is composed of four domains. Starting
from the N-terminus, there is a -sandwich domain (residues 43–184) followed by
a catalytic core (residues 185–515) and two -barrels - barrel 1 (residues 516–628)
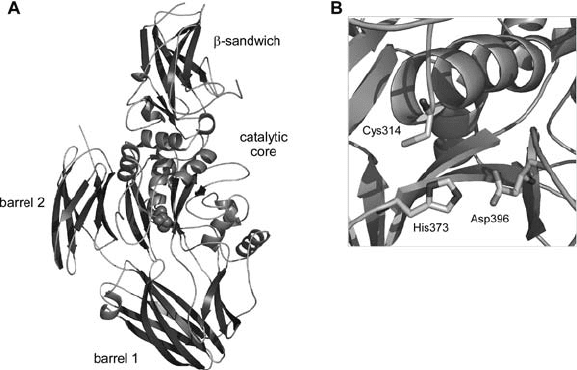
Figure 1. A) 3D structure of factor XIIIA. Only one subunit of the dimer is represented. The catalytic
cysteine is represented in space-filling mode. B) Detail of the catalytic triad
TRANSGLUTAMINASES 571
and barrel 2 (residues 629–727) - at the C-terminus (Fig. 1). The core domain
includes 10 segments of -helix arranged around a twisted -sheet of six antiparallel
strands. The two C-terminal barrels have a seven-stranded -barrel fold, with a
fibronectin-like structure. Residues Cys-314, His-373 and Asp-396 constitute the
catalytic triad of factor XIIIA and they are located at the base of a cavity bounded by
the core and barrel 1 domains. The catalytically active cysteine, Cys-314, sits at the
N-terminus of a long helix in the core domain and is hydrogen bonded to His-373,
which in turn forms a hydrogen bond with Asp-396. Both His-373 and Asp-396 sit
on two -strands of the same sheet. In addition, Cys-314 is also hydrogen bonded
to Tyr-560 located in one of the loops of the barrel 1 domain.
In the zymogen structure the active site of the molecule is inaccessible to solvent,
and thus to substrate, due to both intra- and inter-subunit interactions. Access to
the catalytic Cys-314 residue is blocked by Tyr-560, and movement of the -barrel
1 domain is required to allow the substrate to approach the nucleophilic Cys-314.
In addition, the structure of the dimer shows that the activation peptide of each
A-subunit crosses the dimer interface and partially occludes the opening of the
active site in the catalytic core of the other subunit. Only after removal of this
pro-peptide is the active site accessible to substrates. Crystallographic studies have
shown that neither calcium binding (Fox et al., 1999) nor pro-peptide cleavage
alone (Yee et al., 1995) causes the large conformational changes required to expose
the active site. Based on these results it has been postulated that binding of both
Ca
2+
and the acyl donor substrate is required to trigger the conformational change
necessary to expose the active site of the enzyme.
4.2. Other Animal Transglutaminases
Besides human FXIIIA, the X-ray structures of three other animal TGs have been
solved: human tissue transglutaminase, TG2 (Liu et al., 2002), human epithelial
transglutaminase, TG3 (Ahvazi et al., 2002) and the fish-derived TG, fTG (Noguchi
et al., 2001). All these TGs present the same overall four domain structure as
FXIIIA: -sandwich, / catalytic core, barrel 1 and barrel 2. The position of the
catalytic triad Cys-His-Asp is conserved in the three enzymes, and in all of them
the active site is shielded from contact with the solvent thus requiring major struc-
tural changes for enzyme activation. The conservation of these features indicates
a common mechanism of catalysis and a common phylogenetic origin for the
members of this family of enzymes. Nevertheless, the solved structures of these
additional TGs have revealed the structural basis for the biochemical peculiarities
of these enzymes, such as the GTP regulation of TG2 activity (Liu et al., 2002)
and the mechanism of calcium activation of TG3 (Ahvazi et al., 2002).
4.3. Streptomyces mobaraensis Transglutaminase
The crystal structure of the microbial transglutaminase from Streptomyces
mobaraensis (Kashiwagi et al., 2002) revealed a completely different structure
572 ARRIZUBIETA
from that of FXIIIA and related TGs. Furthermore, the structure of MTG seems to
present a novel fold since it does not resemble any structure published to date. The
configuration of the catalytic residues also suggests a distinct catalytic mechanism
that might account for the peculiar biochemical characteristics of this enzyme.
In contrast to the multidomain structure of the FXIII-like TGs, MTG presents a
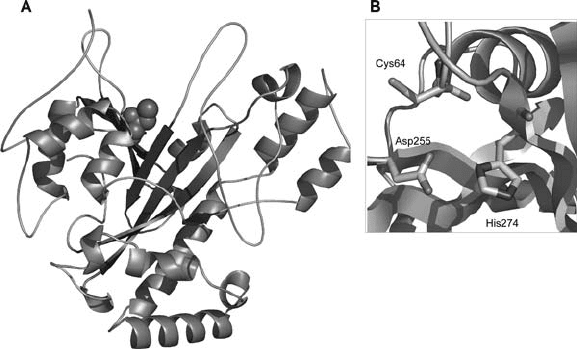
single domain having a disk-like shape (Fig. 2). The active site is located at the
bottom of a deep cleft at the edge of the disk. The structure of the MTG belongs to
the + folding class, containing 11 -helices and 8 -strands. These secondary
structure elements are arranged so that a central -sheet with seven antiparallel
strands is surrounded by the -helices.
The size of MTG (331 amino acids) is similar to that of the core domain of FXIIIA
(330 amino acids). Although they both belong to the + folding class, their
overall folding patterns are considerably different. Nevertheless, the arrangements
of the secondary structure elements around their active sites are very similar. Both
active site cysteines occur near the N-termini of -helices, and in each TG this
-helix is flanked by a four-stranded -sheet that contains the other two residues
of the catalytic triad.
Unlike the catalytically active cysteine groups of animal TGs, the sulfhydryl
group of Cys-64 is partially exposed to the solvent and can readily react with
substrates, which may explain the higher reaction rate of MTG. Besides Cys-64,
residues Asp-255 and His-274 constitute the catalytic triad. The most striking
difference between MTG and the FXIII-like TGs is that the positions occupied
by Asp-255 and His-274 in MTG superimpose well on the positions of His-373
and Asp-396 in FXIIIA, respectively. In other words, in the MTG molecule the
relative positions of the catalytically important His and Asp residues are reversed
Figure 2. A) 3D structure of the microbial TG from Streptomyces mobaraensis. The catalytic cysteine
is represented in space-filling mode. B) Detail of the catalytic triad
TRANSGLUTAMINASES 573
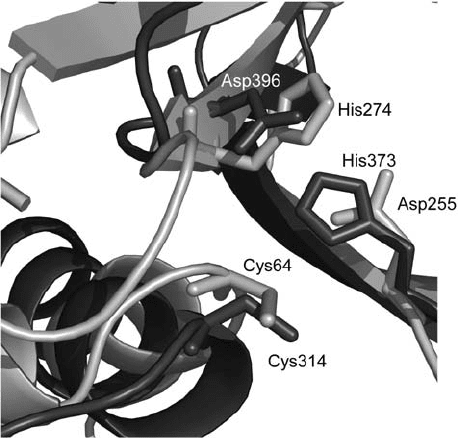
Figure 3. Comparison of the active centres of factor XIIIA (dark grey) and MTG (light grey). Organi-
zation of secondary structure elements and catalytic residues at the active site
relative to the Cys residue (Fig. 3). These results have been taken to indicate that in
the catalytic mechanism of MTG, Asp-255, and not His-274, acts as the acid/base
catalyst. It has been suggested that the negatively charged state of Asp-255 during
the reaction might be responsible for the weaker deamidation activity of MTG
compared to the FXIII-like TGs since this would favour positively charged groups
acting as acyl acceptors instead of neutral species such as water.
The similarities between the active site structures and the differences in the
overall structures between MTG and the FXIII-like TGs suggest that the relationship
between these enzymes is a special case of convergent molecular evolution.
5. INDUSTRIAL APPLICATIONS OF TRANSGLUTAMINASES
TG-mediated cross-linking of proteins has dramatic effects on their physical and
chemical properties. This has triggered the use of these biocatalysts in a wide range
of industrial sectors, from cosmetics to the food industry. In addition, and arising
from the involvement of this group of enzymes in many physiological and patho-
logical processes, they have found important applications in the pharmaceutical
industry. Their biotechnological potential is best reflected in the rapidly growing
number of patent applications regarding this group of enzymes (Griffin et al., 2002).
The first assays to test possible industrial uses of TG were carried out to modify
food proteins using mammalian enzymes (Matheis and Whitaker, 1987). Despite
the interest in these enzymes for modulating food rheological properties, limited
574 ARRIZUBIETA
supply hindered their commercial utilization. The situation changed in 1989 with the
purification of the microbial TG from Streptomyces mobaraensis (Ando et al., 1989)
which could be produced relatively cheaply by fermentation methods. Subsequently,
the application of TG in industry, and especially in the food industry, began to
increase at a steady rate. It is remarkable that sixteen years later, the microbial
transglutaminase obtained from Streptomyces is still the only commercial source of
MTG. The use of microbial transglutaminase in food processing has been covered
in a series of reviews (Nielsen, 1995; Motoki and Seguro, 1998; Kuraishi et al.,
2001; Yokoyama et al., 2004).
5.1. Meat and Fish Products
Muscle proteins, especially myosin, are polymerised by TG (Huang et al., 1992) and
the resulting cross-links strengthen the protein network in muscle-derived products.
The addition of the TG during the setting process of surimi results in a gel with
greater breaking stress and improved elasticity (Sakamoto et al., 1995), both of
which are principle determinants of the value of the product. Fish species with
high proteolytic activity were traditionally not capable of being used for surimi
production. In these under utilized fish species the addition of TG has been reported
to improve the gel characteristics of fish paste (Haejung et al., 1996) thereby
allowing their utilization in surimi production.
In the case of sausages, hams and other meat products the effect of TG addition
is improved texture with higher breaking strength or firmness, and increased defor-
mation and elasticity of the gel. This leads to an increase in manufacturers’ profits
due to reduced product loss during processing and slicing. On the other hand, the
use of TG results in the production of reduced salt/phosphate meat products with
improved water-holding capacity and texture, which constitutes a valuable health
benefit for the consumer. The covalent nature of the isopeptide bonds created by TG
determines that the changes in structure are resistant to both heating at high temper-
atures and freezing. Thus, manufacturing of canned or frozen products benefits
from the utilization of this enzyme. One of the most successful applications of
TG in the meat industry is probably the elaboration of restructured meat products.
Although other methods had been previously described and used for this purpose,
the combination of TG and caseinate has emerged as a very convenient and practical
method to obtain restructured meat products of high quality (Motoki and Seguro,
1998; Kuraishi et al., 2001).
5.2. Dairy Industry
Of the proteins present in milk, caseins, which have an open conformation, react
readily with TG, while globular whey proteins react only under conditions that
favour unfolding (Matsumura et al., 1996; Lorenzen et al., 1998). In yoghurt
manufacturing, TG treatment of milk results in an increase in firmness and viscosity
and reduced syneresis. In cheese manufacturing, TG treatment of milk results in an
TRANSGLUTAMINASES 575
increase in curd yield, a less dry texture and reduced whey separation. Processed
cheese products treated with the enzyme present higher heat stability, maintaining
viscosity when melting. In ice-cream manufacturing, TG application allows the
production of low calorie, sugar-free, ice-cream which is softer, smoother and easier
to scoop (Motoki and Seguro, 1998; Kuraishi et al., 2001). TG also has potential
commercial applications as a food-grade additive capable of improving the heat
stability of milk (Osullivan et al., 2002), thus conferring resistance to coagulation
at sterilization temperatures or gelation during storage. Enzymatic cross-linking has
also potential applications in controlling the stability of milk protein-containing
emulsions and foams (Dickinson and Yamamoto, 1996).
5.3. Bakery and Noodles
Gluten, the protein of wheat flour, can be cross-linked by TG and the action of the
enzyme reinforces the protein network structure changing the viscoelastic properties
of the dough (Larre et al., 2000). Consequently, pasta manufactured from TG-treated
dough has a higher breaking strength, or firmness. In addition, treated pasta releases
fewer solids into the boiling water, retaining its firmness and elasticity for a longer
period after cooking (Kuraishi et al., 2001). TG has also found use in the baking
industry as an improver of dough stability and loaf volume (Gerrard et al., 1998),
as well as an improver of the lift of puff pastry and the volume of yeasted croissants
(Gerrard et al., 2000). These effects are retained even after freezing offering a
potential solution to the problem of dough deterioration during frozen storage. In
addition, deep-fried dough products such as doughnuts absorb 25% less oil when the
dough has been treated with transglutaminase, with the concomitant health benefit
for the consumer (Kuraishi et al., 2001).
5.4. Soy Derivatives
TG cross-links soy globulins resulting in modification of the gelation and textural
characteristics of soybean products. In the case of tofu, a soybean curd product
obtained by coagulation of soybean proteins, TG treatment increases its water-
holding properties and results in tofu with a smoother and firmer texture (Nonaka
et al., 1994). In addition, treatment with the enzyme allows for better control of
the coagulation reaction and a reduction in weight loss during retort cooking. Soy
proteins are added to many processed foods such as sausages, ham and surimi to
improve their textural and nutritional characteristics. Thus, TG offers the potential
to modulate textural properties of those foods to which soy proteins are added
(Motoki and Seguro, 1998).
5.5. Bioavailability of Cross-linked Proteins
The wide application of MTG in food production has raised concerns about
the potential effects of the cross-linked proteins on consumer’s health and the
