Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

ASPARTASES 555
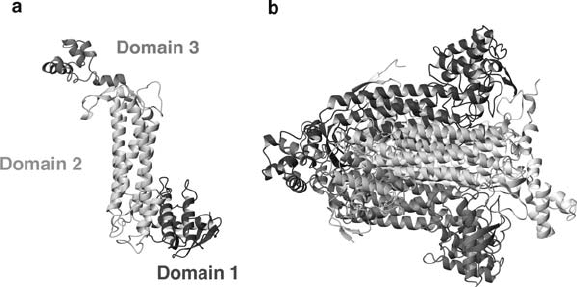
Figure 3. X-ray crystal structure of Escherichia coli aspartase (Shi et al., 1997). (a) Domain composition
of the E. coli aspartase subunit. (b) Structure of the aspartase tetramer
another, Ser 143, has been implicated to act as a general acid catalyst that mediates
the protonation of the departing ammonium group. Still missing from this molecular
view of the aspartase active site is the residue that acts as a general base to remove
a proton from C3 of L-aspartate to form the carbanion intermediate. Although a
number of candidates have been probed to date, notably His 26 and Asp 10, neither
of these residues satisfied the criteria of a general base catalyst.
3. BACILLUS sp. YM55-1 ASPARTASE
Relatively recently a new member of the aspartase family was isolated from
an aerobic, moderately thermophilic bacterium, Bacillus sp. YM55-1 (Kawata
et al., 1999). This aspartase displayed a number of characteristics that were
different from those of the mesophilic aspartases. Firstly, the specific activity of
Bacillus sp. YM55-1 aspartase was significantly (∼5 fold) higher than that of
the other mesophilic aspartases under identical conditions (assayed at 30
C). This
enhancement was much more pronounced when the enzyme was assayed at its
optimum temperature of 55
C, yielding a specific activity of 2200 units/mg protein,
which is among the highest specific activities known for an aspartase. The stability
of this aspartase against thermal and chemical denaturation was also significantly
better than that of E. coli aspartase. This has been attributed to increased numbers
of intersubunit hydrogen bonds and ion pairs in the Bacillus sp. YM55-1 enzyme
(Fujii et al., 2003). Another interesting difference between the Bacillus sp. YM55-
1 aspartase and other mesophilic aspartases is that the former does not display
enhanced activity at alkaline pH in the presence of divalent cations, nor does it
undergo substrate activation by L-aspartate. The enzyme kinetics of Bacillus sp.
YM55-1 aspartase are hyperbolic at all pH values tested, and cooperativity is notably
lacking.
Cloning and overproduction of Bacillus sp. YM55-1 aspartase in E. coli (Kawata
et al., 2000) and crystallization/determination of its structure (Fujii et al., 2003)
556 MIZOBATA AND KAWATA
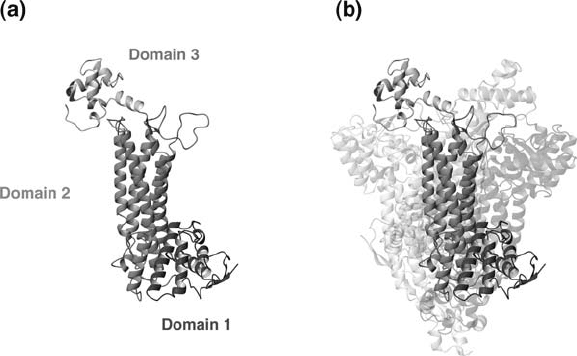
followed soon after (Fig. 4). The general structure of Bacillus sp. TM55-1 aspartase
is essentially identical to that of E. coli aspartase and E. coli fumarase: a central
subunit interface consisting mainly of -helices, and active sites flanking this
central structure (compare Figs. 3 and 4). Upon closer examination a number of
additional similarities, and some notable differences, are apparent between these
three molecular structures.
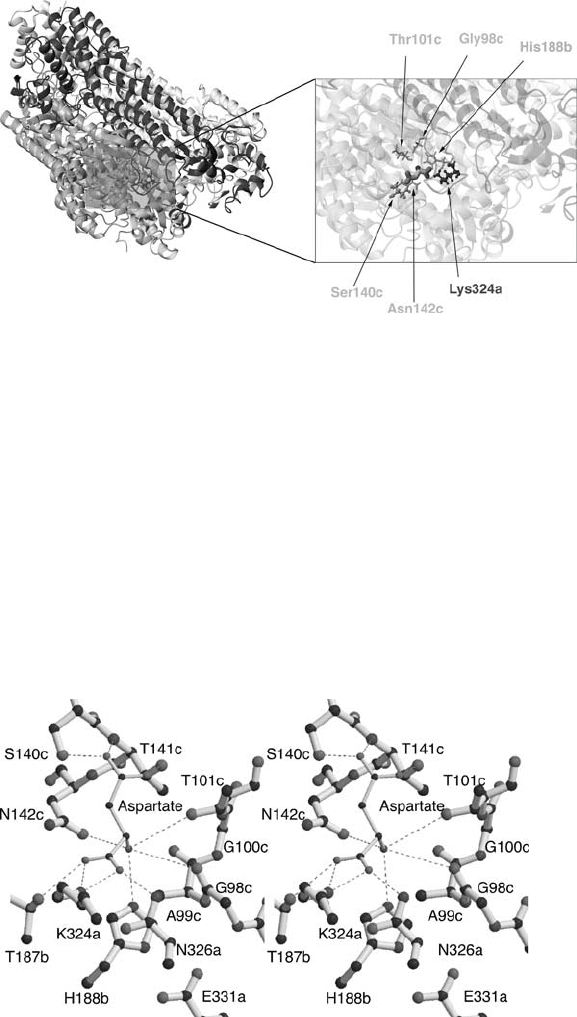
A docking simulation of aspartate bound to Bacillus sp. YM55-1 aspartase was
performed in order to ascertain the orientation and possible active site residues in this
enzyme (Figs.5 and 6; Fujii etal., 2003). Lys 327, theresidue that binds the -carboxyl
group in E. coli aspartase, corresponds to Lys 324 in the Bacillus sp. structure, and this
residue was postulated to act in a similar role in this thermostable enzyme. The amino
group was arranged to be bound through three separate residues (Fig. 6): Asn142 (E.
coli Asn 145), His 188 (E. coli Gln 191) and either Gly 98 (E. coli Gly 100) or Thr 101
(E. coli Thr 104). Docking was based on the finding that residues homologous to these
threeinE. coli fumarase (Ser98, Thr 100, Asn141 and His 188)were found complexed
with a water molecule. This water molecule is regarded as being very important in the
catalytic mechanism of fumarase, and an analogy may be derived that implicates these
residues in aspartase catalysis as well. The -carboxyl group of docked L-aspartate
was found to interact with Ser 140 (E. coli Ser 143) in the completed docking model
(Fig. 6). This residue is located at the N-terminal end of a helix dipole (helix 6 in the
subunit structure) and the positive charge provided by this dipole is thought to assist
in binding.
Figure 4. X-ray crystal structure of Bacillus sp. YM55-1 aspartase (Fujii et al., 2003). (a) The domain
composition of the aspartase subunit, oriented in a similar manner to that of the E. coli aspartase subunit
shown in Fig. 3a. (b) Structure of the Bacillus sp. YM55-1 aspartase tetramer. In this figure the tetramer
was arranged in a manner such that the relative positions of the domains in a given subunit is apparent.
Images were created using a coordinate file of the Bacillus aspartase tetramer (provided generously by
Drs. T. Fujii and Y. Hata, Kyoto University)
ASPARTASES 557
Figure 5. Close-up view of the active site of Bacillus sp. YM55-1 aspartase with an L-aspartate molecule
in the postulated binding site. The left image shows a view of the aspartase tetramer with different
shading assigned to individual subunits. Following the naming convention of Fujii et al. (2003) individual
subunits are shaded dark to light in the following sequence: subunit a, subunit b, subunit c. Subunit d,
which does not participate in the formation of the active site, is the most lightly shaded. The right image
shows a zoomed view of the portion of the left panel. The relative orientation of the two images is
identical. The amino acids that are postulated to participate in the catalytic mechanism of aspartase are
drawn in the zoomed view in either stick or ball-and-stick form, and the relative shading of the amino
acid residues denotes the subunit from which each residue is derived. The docked L-aspartate residue is
shown in thick stick form
From numerous studies of members of the aspartase-fumarase superfamily it has
been suggested that residues at the position corresponding to His 188 in Bacillus
sp. YM55-1 aspartase – modeled to interact with the amino group of L-aspartate in
Figure 6. Stereo view of the active site of Bacillus sp. YM55-1 aspartase, reprinted from Fujii et al. (2003)
558 MIZOBATA AND KAWATA
Figs. 5 and 6 – not only participate in substrate binding but also actively participate
in the catalytic mechanism as a general acid catalyst (Blanchard and Cleland, 1980;
Weaver and Banaszak, 1996; Weaver et al., 1997; Wu et al., 1998; Lee et al.,
1999). His 188 is highly conserved among most of the members of the fumarase
superfamily, including the aspartase from Bacillus sp. YM55-1. However, that is
not the case in E. coli aspartase where a glutamine residue occurs at this position
(Gln 191 in E. coli). Although this difference in a crucial residue may indicate
that this position in the active site of the aspartase-fumarase superfamily is not an
essential functional position, the homology between Bacillus sp. YM55-1 aspartase
and the other members of the fumarase family may warrant a more detailed scrutiny
of this interesting residue.
Another residue that was proposed to be an active participant in the catalytic
mechanism of the E. coli aspartase was Ser 143 (Ser 140 in Bacillus sp. YM55-1
aspartase). Ser 143 was postulated to be the general acid residue that protonates the
ammonia leaving group (Jayasekera et al., 1997). The position of Ser 140 in Bacillus
sp. YM55-1 aspartase is, however, comparatively distant from the candidate active
site region and the role of general acid catalyst seemed difficult to achieve in this
enzyme (Fujii et al., 2003). Instead, Ser 140 is postulated to bind the -carboxyl
group of L-aspartate, thus filling an important role in substrate binding for this
residue (Fig. 6). An alternate residue that could assume the role of general acid
catalyst in place of Ser 140 was not readily apparent in the Bacillus sp. YM55-1
structure. The detailed understanding of the catalytic mechanism of Bacillus sp.
aspartase is therefore far from complete.
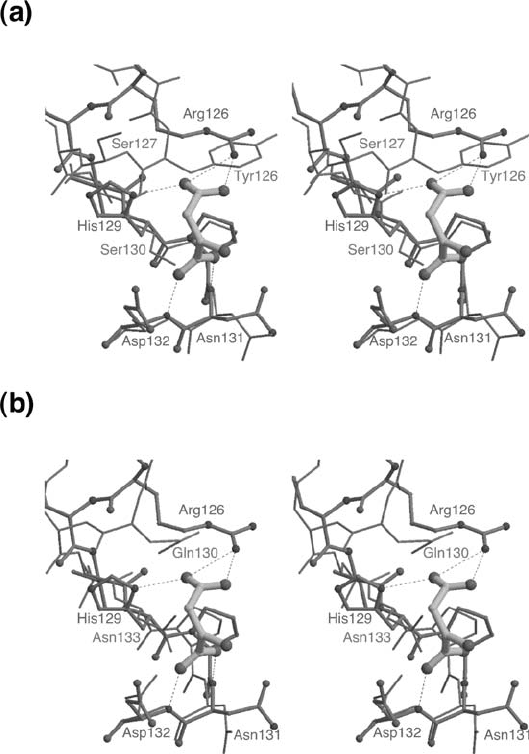
With regard to the substrate activation effect that is seen in both E. coli
aspartase and fumarase but not in Bacillus sp. YM55-1 aspartase, structural
comparison has revealed that a site corresponding closely to the B-site in
E. coli fumarase (where a L-malate molecule was found to be bound by crystal
structure analysis (Weaver and Banaszak, 1996)) also exists in the Bacillus sp.
YM55-1 and E. coli aspartases (Fig. 7). A detailed comparison of this region
(corresponding to residues 123–128 in Bacillus sp. aspartase) showed that the
structure of Bacillus sp. YM55-1 aspartase more closely resembles the structure
of E. coli fumarase C (Fig. 7a) rather than the apo structure of E. coli aspartase
(Fig. 7b). It may be that the Bacillus sp. aspartase is locked in an active confor-
mation similar to that seen for ligand-bound E. coli fumarase. An additional
striking finding was that in Bacillus sp. YM55-1 aspartase, a tyrosine side chain
(Tyr 126) was found in a position which partially occluded this activation site
(Fig. 7a). This may be the reason why the enzyme does not display a prominent
substrate activation effect and is locked in a permanently active conformation.
Related to this finding, Fujii and co-workers showed that the structure of apo
E. coli aspartase differs markedly from the structures of Bacillus sp. YM55-1
aspartase and E. coli fumarase (liganded form) in the regions corresponding to
residues 40–45, 80–84, 228–241 and 265–272. A detailed examination of these
regions may yield additional clues about substrate-activated catalysis in E. coli
aspartase.
ASPARTASES 559
Figure 7. Superimposed comparisons of the active site and activator binding site regions of E. coli
aspartase, E. coli fumarase and Bacillus sp. YM55-1 aspartase reprinted from Fujii et al. (2003). In both
panels the docked L-aspartate molecule is shown by a thick ball-and-stick model. (a) Superposition of
Bacillus sp. YM55-1 aspartase (wire model) with E. coli fumarase (thin ball-and-stick model). Note the
extension of Tyr126 into the activation site region. (b) Superposition of E. coli aspartase (wire model)
with E. coli fumarase (thin ball-and-stick model)
4. MOLECULAR ENGINEERING OF ASPARTASE
AND INDUSTRIAL APPLICATIONS
In industry, aspartase is used mainly in the enzyme-mediated production of
L-aspartate, an important starting compound for generating food additives and
560 MIZOBATA AND KAWATA
artificial sweeteners. A survey of patents awarded for the application of aspartase
reveals a number of interesting methods that have been used to alter the activity of
the enzyme with a view toward its utilization in various industrial processes. In this
section, we summarize the numerous methods that have been used in the industrial
application of aspartase, and also provide some hints toward future efforts.
4.1. C-terminal Deletion of Amino Acid Residues
Early studies on aspartase discovered that the specific activity of this enzyme
could be enhanced by brief treatment with certain proteases such as trypsin or
subtilisin (Yumoto et al., 1980; Yumoto et al., 1982). Analysis showed that
C-terminal truncation was responsible for this enhancement of activity. Jayasekera
and co-workers (Jayasekera et al., 1997) undertook a detailed analysis of C-terminal
deletion mutants of E. coli aspartase and found that a mutant aspartase truncated
after Tyr 472 displayed a greater than two-fold enhancement of k
cat
and a minimal
increase in the K
M
, yielding an enzyme with enhanced activities. The results of this
study have been patented for use in industrial applications (US Patent 5,993,807).
A similar patent (US Patent 6,015,704) was later awarded to Tsai and co-workers
who combined the effects of C-terminal truncation with the substitution of selected
histidine residues by glutamine (His 25, 123, 421 or 463). These two patents open
the way toward the creation of novel aspartase proteins with improved enzymatic
activities suitable for industrial applications.
4.2. Random Mutagenesis and Directed Evolution
The difficulties in identifying the specific residues that are important for aspartase
function have led to the utilization of random mutagenesis as a viable method for
obtaining aspartases having enhanced activities. Zhang and co-workers success-
fully used this method to obtain a mutant E. coli aspartase whose specific activity
was increased 4-fold and which also showed slightly improved thermostability
(Zhang et al., 1993). The characteristics of these enzymes were compared to those
of another mutant aspartase produced by site-directed mutagenesis, Lys126Arg.
Although both enzymes displayed similar specific activities, it is not known
whether the enzyme derived from random mutagenesis is similar or identical to
the Lys126Arg mutant. Another enhanced aspartase enzyme was obtained using
a directed evolution approach comprising random mutagenesis followed by gene
shuffling (Wang et al., 2000). The enzyme obtained had the following amino acid
substitutions; Asn217Lys, Thr233Arg and Val367Gly. Alteration of these three
amino acids resulted in an enzyme with a k
cat
/K
M
that was increased 28-fold
compared to wild type, a K
M
that was decreased 4.6 fold, and improved thermosta-
bility such that 61% of its initial activity was retained after a 30 min incubation
at 50
C. Under similar conditions the wild type enzyme only retained 17% of its
activity. This improved aspartase would be an ideal candidate for application in
industry.
ASPARTASES 561
4.3. Isolation of Thermostable Aspartases from Thermophilies
and Psychrophiles
As outlined above, the aspartase isolated from Bacillus sp. YM55-1 possesses many
characteristics that are desirable for industrial applications, including high specific
activity and thermostability. Other examples of thermostable aspartases have also
been reported. Kimura and co-workers have been awarded a patent (US Patent
4,391,910) outlining their discovery of thermophilic aspartases isolated from various
Bacillus species such as Bacillus aminogenes and Bacillus thermoaminophilus. The
enzymes described in the patent are characterized by very high specific activity,
much like that of the aspartase from Bacillus sp. YM55-1. However, unlike the latter,
the aspartases described in the patent are activated by the presence of divalent cations
such as magnesium ion. This interesting difference in enzymatic characteristics may
suggest that the finer characteristics of aspartases may differ considerably even
among members of the same genus.
Recently, the isolation of a novel thermostable aspartase from a marine
psychrophile, Cytophaga sp. KUC-1 (Kazuoka et al., 2003) has been reported
whose optimal temperature of growth is 15
C. This aspartase displays enzymatic
characteristics that are similar to those of the E. coli enzyme, such as activation by
Mg
2+
and L-aspartate. However, the thermostability of the Cytophaga enzyme is
considerably higher: about 80% of its activity is retained after a 60 min incubation at
50
C. Thus the enzyme from Cytophaga KUC-1 represents an interesting example
of an aspartase whose characteristics are a composite of those derived from aspar-
tases from mesophilic and thermophilic sources. A comparative study of these three
enzyme types should be very enlightening as regards the elucidation of the various
molecular mechanisms which underlie the functional aspects of aspartase.
4.4. Immobilization of Aspartase and Aspartase-producing Bacteria
within Solid Gel Matrices
Industrial production of L-aspartate using aspartase and aspartase-producing
organisms is an excellent example of the successful application of an enzymatic
activity in industrial processes. Since the early 1970’s various methods have been
developed to utilize the activity of aspartase to produce L-aspartate in bulk quantities
(Sato and Tosa, 1993). Early attempts involved the immobilization of purified
aspartase enzyme in polyacrylamide lattices (Tosa et al., 1973) and was followed by
immobilization of aspartase-producing bacteria themselves in gel matrices such as
polyurethane (Fusee et al., 1981) and polyacrylamide. The immobilization of living
cells in gel matrices provides certain advantages over immobilizing pure enzymes
such as enhanced stability of the enzymatic activity and the saving of labour
costs associated with enzyme purification. Future applications in this direction may
involve utilizing bacteria that produce enhanced forms of the aspartase, for example
E. coli cells expressing the aspartase from Bacillus sp. YM55-1.
562 MIZOBATA AND KAWATA
4.5. Other Studies
A number of interesting studies on aspartase provide hints toward future work on
applications for this enzyme. Early studies by Murase and co-workers have shown
that aspartase is partially active in a dimeric state induced by the addition of a
denaturant (Murase et al., 1993). In support of this finding, recent work by Kong
and co-workers succeeded in constructing a novel form of aspartase in which two
original subunits of E. coli aspartase were joined by a suitable peptide linker to
produce a dimer-mimicking, monomeric form of the enzyme that was active (Kong
et al., 2002). This monomeric form of aspartase displayed improved stability at
high temperatures compared to the wild type enzyme. Such approaches toward
stabilizing the structure and ultimately the activity of aspartase represent a novel
alternate method for improving the high efficiency of this enzyme.
Another interesting approach that has been tested involves the construction of
hybrid and/or chimeric proteins. By ingenious utilization of gene fusion and in vivo
selection for the desired activity Sheng et al. succeeded in constructing a 74-kDa
polypeptide hybrid enzyme combining the activities of -aspartyl dipeptidase (85%
of wild type) and aspartase (87% of wild type) into a single polypeptide (Sheng
et al., 2005). As an added bonus, this novel hybrid enzyme displayed improved
thermostabilities with regard to both activities. This astonishing example of protein
engineering is indicative of the potential that still remains to be tapped in aspartase.
We too have recently begun to study various chimeric constructs of aspartase genes
with a view to generating novel aspartases having improved thermostabilities and
enzymic activities.
5. CONCLUDING REMARKS
The accumulation of knowledge on the structure and function of aspartase provides
us with a detailed view of the mechanisms that underlie the unique functional
aspects of this intriguing enzyme. Nevertheless, experimentation on aspartase never
fails to produce exciting new developments that promise to lead to previously
unsuspected applications and novel concepts. Exploration of the functional and
structural aspects of aspartase is work in progress, and future studies will further
deepen our understanding of this enzyme.
REFERENCES
Acuna, G., Ebeling, S. and Hennecke, H. (1991). Cloning, sequencing, and mutational analysis of the
Bradyrhizobium japonicum fumC-like gene: evidence for the existence of two different fumarases. J
Gen Microbiol 137, 991–1000.
Beeckmans, S. and Van Driessche, E. (1998). Pig heart fumarase contains two distinct substrate binding
sites differing in affinity. J Biol Chem 273, 31661–31669.
Blanchard, J.S. and Cleland, W.W. (1980). Use of isotope effects to deduce the chemical mechanism of
fumarase. Biochemistry 19, 4506–4513.
ASPARTASES 563
Burland, V., Plunkett, G., 3rd, Sofia, H.J., Daniels, D.L. and Blattner, F.R. (1995). Analysis of the
Escherichia coli genome VI: DNA sequence of the region from 92.8 through 100 minutes. Nucleic
Acids Res 23, 2105–2119.
Chen, H.H., Chen, J.T. and Tsai, H. (1996). Site-directed mutagenesis of cysteinyl residues in aspartase
of Escherichia coli. Ann N Y Acad Sci 799, 70–73.
Cook, R.P. and Woolf, B. (1928). The deamination and synthesis of L-aspartic acid in the presence of
bacteria. Biochem J 22, 474–481.
Ellfolk, N. (1953a). Studies on aspartase. I. Quantitative separation of aspartase from bacterial cells, and
its partial purification. Acta Chem Scand 7, 824–830.
Ellfolk, N. (1953b). Studies on aspartase. II. On the chemical nature of aspartase. Acta Chem Scand 7,
1155–1163.
Ellfolk, N. (1954). Studies on aspartase. III. On the specificity of aspartase. Acta Chem Scand 8,
151–156.
Falzone, C.J., Karsten, W.E., Conley, J.D. and Viola, R.E. (1988). L-aspartase from Escherichia coli:
substrate specificity and role of divalent metal ions. Biochemistry 27, 9089–9093.
Fujii, T., Sakai, H., Kawata, Y. and Hata, Y. (2003). Crystal structure of thermostable aspartase from
Bacillus sp. YM55-1: structure-based exploration of functional sites in the aspartase family. J Mol
Biol 328, 635–654.
Fusee, M.C., Swann, W.E. and Calton, G.J. (1981). Immobilization of Escherichia coli Cells Containing
Aspartase Activity with Polyurethane and Its Application for l-Aspartic Acid Production. Appl Environ
Microbiol 42, 672–676.
Guest, J.R., Roberts, R.E. and Wilde, R.J. (1984). Cloning of the aspartase gene (aspA) of Escherichia
coli. J Gen Microbiol 130, 1271–1278.
Ida, N. and Tokushige, M. (1984). Chemical modification of essential histidine residues in aspartase
with diethylpyrocarbonate. J Biochem (Tokyo) 96, 1315–1321.
Ida, N. and Tokushige, M. (1985). Assignment of catalytically essential cysteine residues in aspartase
by selective chemical modification with N-(7-dimethylamino-4-methylcoumarynyl) maleimide.
J Biochem (Tokyo) 98, 793–797.
Ida, N. and Tokushige, M. (1985). L-Aspartate-induced activation of aspartase. J Biochem (Tokyo) 98,
35–39.
Jayasekera, M.M., Saribas, A.S. and Viola, R.E. (1997). Enhancement of catalytic activity by gene
truncation: activation of L-aspartase from Escherichia coli. Biochem Biophys Res Commun 238,
411–414.
Jayasekera, M.M., Shi, W., Farber, G.K. and Viola, R.E. (1997). Evaluation of functionally important
amino acids in L-aspartate ammonia-lyase from Escherichia coli. Biochemistry 36, 9145–9150.
Jayasekera, M.M. and Viola, R.E. (1999). Recovery of catalytic activity from an inactive aggregated
mutant of L-aspartase. Biochem Biophys Res Commun 264, 596–600.
Karsten, W.E., Gates, R.B. and Viola, R.E. (1986). Kinetic studies of L-aspartase from Escherichia coli:
substrate activation. Biochemistry 25, 1299–1303.
Karsten, W.E., Hunsley, J.R. and Viola, R.E. (1985). Purification of aspartase and aspartokinase-
homoserine dehydrogenase I from Escherichia coli by dye-ligand chromatography. Anal Biochem
147, 336–341.
Karsten, W.E. and Viola, R.E. (1991). Kinetic studies of L-aspartase from Escherichia coli: pH-dependent
activity changes. Arch Biochem Biophys 287, 60–67.
Katoh, K., Kuma, K., Toh, H. and Miyata, T. (2005). MAFFT version 5: improvement in accuracy of
multiple sequence alignment. Nucleic Acids Res
33, 511–518.
Kawata, Y., Tamura, K., Kawamura, M., Ikei, K., Mizobata, T., Nagai, J., Fujita, M., Yano, S.,
Tokushige, M. and Yumoto, N. (2000). Cloning and over-expression of thermostable Bacillus sp.
YM55-1 aspartase and site-directed mutagenesis for probing a catalytic residue. Eur J Biochem 267,
1847–1857.
Kawata, Y., Tamura, K., Yano, S., Mizobata, T., Nagai, J., Esaki, N., Soda, K., Tokushige, M. and
Yumoto, N. (1999). Purification and characterization of thermostable aspartase from Bacillus sp.
YM55-1. Arch Biochem Biophys 366, 40–46.
564 MIZOBATA AND KAWATA
Kazuoka, T., Masuda, Y., Oikawa, T. and Soda, K. (2003). Thermostable aspartase from a marine
psychrophile, Cytophaga sp. KUC-1: molecular characterization and primary structure. J Biochem
(Tokyo) 133, 51–58.
Kong, X., Li, Z., Gou, X., Zhu, S., Zhang, H., Wang, X. and Zhang, J. (2002). A monomeric L-aspartase
obtained by in vitro selection. J Biol Chem 277, 24289–24293.
Lee, T.T., Worby, C., Bao, Z.Q., Dixon, J.E. and Colman, R.F. (1999). His68 and His141 are critical
contributors to the intersubunit catalytic site of adenylosuccinate lyase of Bacillus subtilis. Biochem-
istry 38, 22–32.
Mizuta, K. and Tokushige, M. (1975). Studies on aspartase. II. Role of sulfhydryl groups in aspartase
from Escherichia coli. Biochim Biophys Acta 403, 221–231.
Murase, S., Kawata, Y. and Yumoto, N. (1993). Identification of an active dimeric form of aspartase as
a denaturation intermediate. J Biochem (Tokyo) 114, 393–397.
Nuiry, II, Hermes, J.D., Weiss, P.M., Chen, C.Y. and Cook, P.F. (1984). Kinetic mechanism and location
of rate-determining steps for aspartase from Hafnia alvei. Biochemistry 23, 5168–5175.
Porter, D.J. and Bright, H.J. (1980). 3-Carbanionic substrate analogues bind very tightly to fumarase
and aspartase. J Biol Chem 255, 4772–4780.
Quastel, J.H. and Woolf, B. (1926). The equilibrium between L-aspartic acid, fumaric acid and ammonia
in the presence of resting bacteria. Biochem J 20, 545–555.
Rose, I.A. and Weaver, T.M. (2004). The role of the allosteric B site in the fumarase reaction. Proc Natl
Acad SciUSA101, 3393–3397.
Rudolph, F.B. and Fromm, H.J. (1971). The purification and properties of aspartase from Escherichia
coli. Arch Biochem Biophys 147, 92–98.
Saribas, A.S., Schindler, J.F. and Viola, R.E. (1994). Mutagenic investigation of conserved functional
amino acids in Escherichia coli L-aspartase. J Biol Chem 269, 6313–6319.
Sato, T. and Tosa, T. (1993). Production of L-aspartic acid. Bioprocess Technol 16, 15–24.
Schindler, J.F. and Viola, R.E. (1994). Mechanism-based inactivation of L-aspartase from Escherichia
coli. Biochemistry 33, 9365–9370.
Sheng, Y., Li, S., Gou, X., Kong, X., Wang, X., Sun, Y. and Zhang, J. (2005). The hybrid enzymes
from alpha-aspartyl dipeptidase and L-aspartase. Biochem Biophys Res Commun 331, 107–112.
Shi, W., Dunbar, J., Jayasekera, M.M., Viola, R.E. and Farber, G.K. (1997). The structure of L-aspartate
ammonia-lyase from Escherichia coli. Biochemistry 36, 9136–9144.
Sun, D.X. and Setlow, P. (1991). Cloning, nucleotide sequence, and expression of the Bacillus subtilis
ans operon, which codes for L-asparaginase and L-aspartase. J Bacteriol 173, 3831–3845.
Takagi, J.S., Ida, N., Tokushige, M., Sakamoto, H. and Shimura, Y. (1985). Cloning and nucleotide
sequence of the aspartase gene of Escherichia coli W. Nucleic Acids Res 13, 2063–2074.
Takagi, J.S., Tokushige, M. and Shimura, Y. (1986). Cloning and nucleotide sequence of the aspartase
gene of Pseudomonas fluorescens. J Biochem (Tokyo) 100, 697–705.
Tosa, T., Sato, T., Mori, T., Matuo, Y. and Chibata, I. (1973). Continuous production of L-aspartic acid
by immobilized aspartase. Biotechnol Bioeng 15, 69–84.
Viola, R.E. (2000). L-aspartase: new tricks from an old enzyme. Adv Enzymol Relat Areas Mol Biol
74, 295–341.
Virtanen, A.I. and Tarnanen, J. (1932). Die Enzymatische Spaltung und Synthese der Asparaginsaure.
Biochem Z 250
, 193–211.
Wang, L.J., Kong, X.D., Zhang, H.Y., Wang, X.P. and Zhang, J. (2000). Enhancement of the activity
of L-aspartase from Escherichia coli W by directed evolution. Biochem Biophys Res Commun 276,
346–349.
Weaver, T. and Banaszak, L. (1996). Crystallographic studies of the catalytic and a second site in
fumarase C from Escherichia coli. Biochemistry 35, 13955–13965.
Weaver, T., Lees, M. and Banaszak, L. (1997). Mutations of fumarase that distinguish between the
active site and a nearby dicarboxylic acid binding site. Protein Sci 6, 834–842.
Weaver, T.M., Levitt, D.G., Donnelly, M.I., Stevens, P.P. and Banaszak, L.J. (1995). The multisubunit
active site of fumarase C from Escherichia coli. Nat Struct Biol 2, 654–662.
