Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.

572 Chapter
6
inset) was indexed employing
TREOR
and using 16 peaks below 20
=
40" in
the hexagonal crystal system with a
=
13.255,
c
=
5.265 8L,
V
=
801.1 8L3.
The
FN
figure of merit,
FI6
=
335(0.0021, 23), is extremely high. IT0
indexing produces the same result but in a C-centered orthorhombic lattice
with aortho
=
ahex, cortho
=
chex, and bortho
=
d3ahex. Unit cell refinement using
150 reflections observed below 20
=
130" results in highly accurate unit cell
parameters (see Table
6.43
on page 574) and a sample displacement of
-0.1 12(3) mm for a 250 mm goniometer radius.' The analysis of systematic
absences
-
points
-
to the following possible space groups P63/mmc, P63m~,
20 30 40
50
60 70
80
90 100 110 120 130
Bragg angle,
20
(deg.)
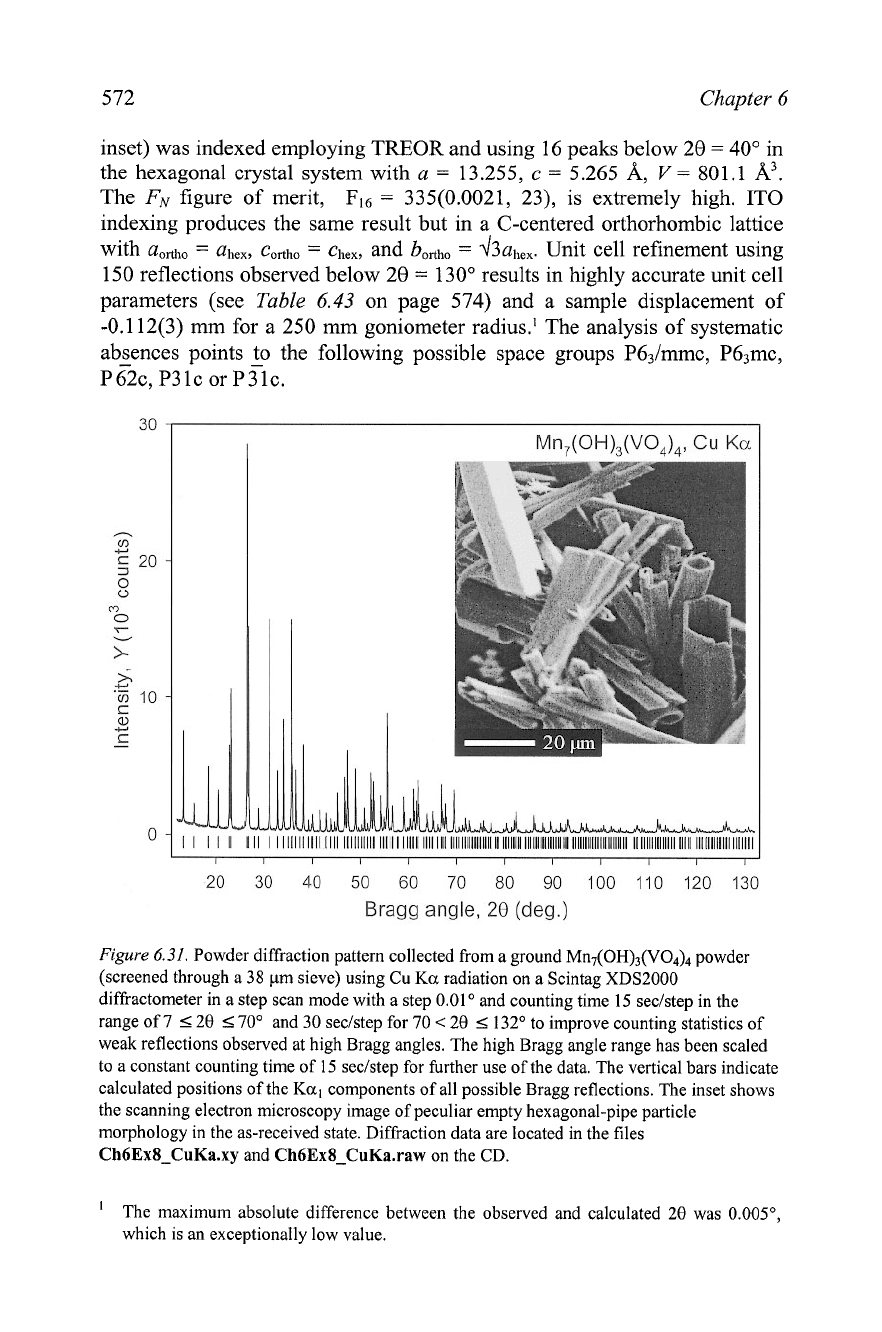
Figure
6.31.
Powder diMaction pattern collected from a ground MII~(OH)~(VO~)~ powder
(screened through a 38 pm sieve) using Cu
Ka
radiation on a Scintag XDS2000
diMactometer in a step scan mode with a step 0.01 and counting time
15
seclstep in the
range of 7 520
5
70•‹ and 30 seclstep for 70
<
20
5
132' to improve counting statistics of
weak reflections observed at high Bragg angles. The high Bragg angle range has been scaled
to a constant counting time of
15
seclstep for further use of the data. The vertical bars indicate
calculated positions of the
Kal
components of all possible Bragg reflections. The inset shows
the scanning electron microscopy image of peculiar empty hexagonal-pipe particle
morphology in the as-received state. Diffraction data are located
in
the
files
Ch6Ex8-CuKa.xy
and
Ch6Ex8-CuKa.raw
on the CD.
'
The maximum absolute difference between the observed and calculated 20 was 0.005',
which is an exceptionally low value.

Crystal structure solution
573
Thermogravimetric analysis resulted in complex traces in both the
oxygen and nitrogen atmospheres with gradual
-2
and -4
%
weight losses,
respectively. The powder diffraction pattern of the thermal decomposition
product can be identified as a mixture of Mn2V207 and Mn203. Available
data only allow a qualitative assumption about the absence of organic or
water molecules, simultaneously pointing to the presence of a small amount
of hydroxyl groups because of the continuous weight loss.'
In
general it may
be assumed that this compound contains Mn cations, OH- groups and
individual or shared comer [vo413- tetrahedra, as in V2O7, V4012 or (VO3)n.
The latter conclusion is based on the color, since all other oxidation states or
coordinations of V would result in black, dark green or dark blue crystals.
This reasoning is provided here to show how various chemical and physical
information may be used when considering composition, predicting,
proposing, or solving the structure.
An identification attempt using the Powder Diffraction File failed as no
acceptable matches were found. Undoubtedly, such high quality of the
powder diffraction data should be sufficient to solve the structure from first
principles using either Patterson or direct methods. Yet, a structure solution
is not fully automated and therefore, the ICSD database was searched in the
following order:
-
All compounds containing oxygen and one or both of the metals, Mn and
V,
resulted in 3413 entries.
All hexagonal and primitive trigonal systems were considered, thus
reducing the number of entries to 204.
Search for the unit cell volume in the range between 700 and 900 A3, i.e.
within -100
A3
of the title compound, shortened the list to 16
compounds.
12 of them belong to a different diffraction class and two have different
cla
ratios, where
c
is much greater than
a.
Two remaining entries belong to the P63mc space group symmetry and
have similar unit cell dimensions as shown in
Table
6.43.
Note that Mn6-x(OH)3(HP03)4 has a unit cell volume and dimensions
close to those of the title material.
In
fact, much closer than the Zn-
containing compound, and it is also present in the PDF file. Theoretically, it
may have been found by a powder pattern search-match. The search,
conducted among all inorganic compounds with a narrow (0.04') window
and 5 matching reflections, failed in this example because of the relatively
large discrepancies in the unit cell dimensions and, therefore, peak positions.
The Zn-containing structure may be easily modified to represent the
crystal structure of Mn-OH-V04 (the composition derived above) by
'
Compounds containing organic molecules or water of crystallization usually demonstrate
rapid weight loss, while hydroxyl groups are lost slowly over a broad temperature range.
5
74
Chapter
6
substituting Zn with Mn and
S
with
V.
Therefore, it was chosen as the initial
model in the Rietveld refinement (see
Figure
6.32
and
Table
6.44).
The
second structure does not
look
promising, because it consists of Mn cations,
hydroxyl and
HP03
groups; the latter have a different geometry. It was not
tested at all because the first model results in a successful solution as will be
discussed in the next chapter, in section 7.10.
Table
6.43.
Unit cell dimensions of potentially closely related compounds identified as a
result of searching the ICSD database.
Compound
a,
'4
c,
A
V,
A'
ICSD PDF Ref.
Zn7(OH)3(V04)3S04 12.8 l3O(6) 5.1425(2) 73 1.15 402-888
I
Mn6.,(OH)3(HP03)4
13.1957(6) 5.1 VO(3) 780.68 75-269 47-868
Title compound
13.2294(1)
5.25529(7)
796.54 Refined from profile fitting
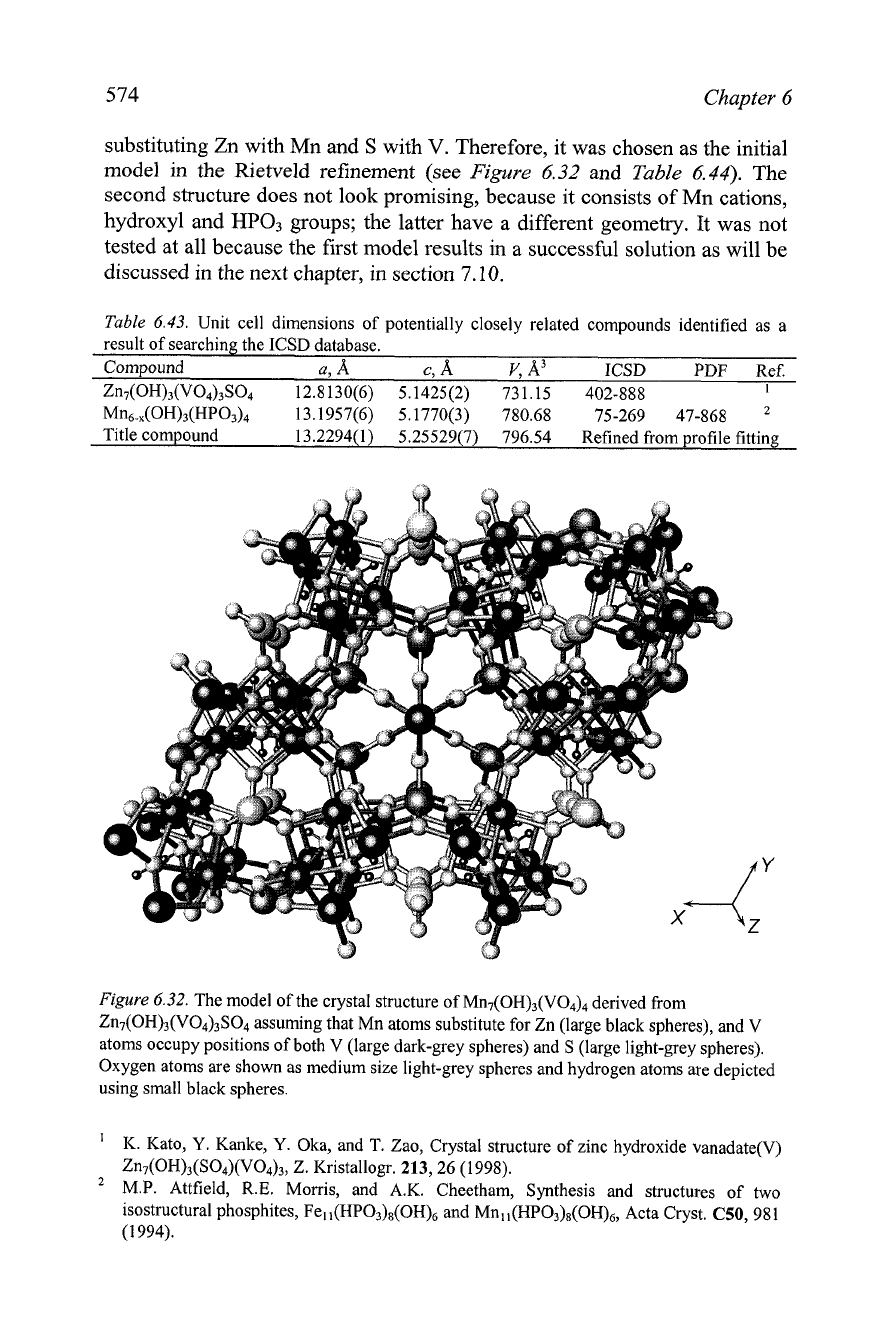
Figure
6.32.
The model of the crystal structure of M~I~(OH)~(VO~)~ derived from
Zn7(OH)3(V04)3S04 assuming that Mn atoms substitute for Zn (large black spheres), and V
atoms occupy positions of both V (large dark-grey spheres) and
S
(large light-grey spheres).
Oxygen atoms are shown as medium size light-grey spheres and hydrogen atoms are depicted
using small black spheres.
K. Kato,
Y.
Kanke,
Y.
Oka, and
T.
Zao, Crystal structure of zinc hydroxide vanadate(V)
Zn7(OH)3(S04)(V04)3, Z. Kristallogr.
213,
26 (1998).
2
M.P. Attfield, R.E. Morris, and A.K. Cheetham, Synthesis and structures of two
isostructural phosphites, Fell(HP03)8(OH)6 and Mnll(HP03)8(OH)6, Acta Cryst.
C50,
981
(1 994).
Crystal structure solution
575
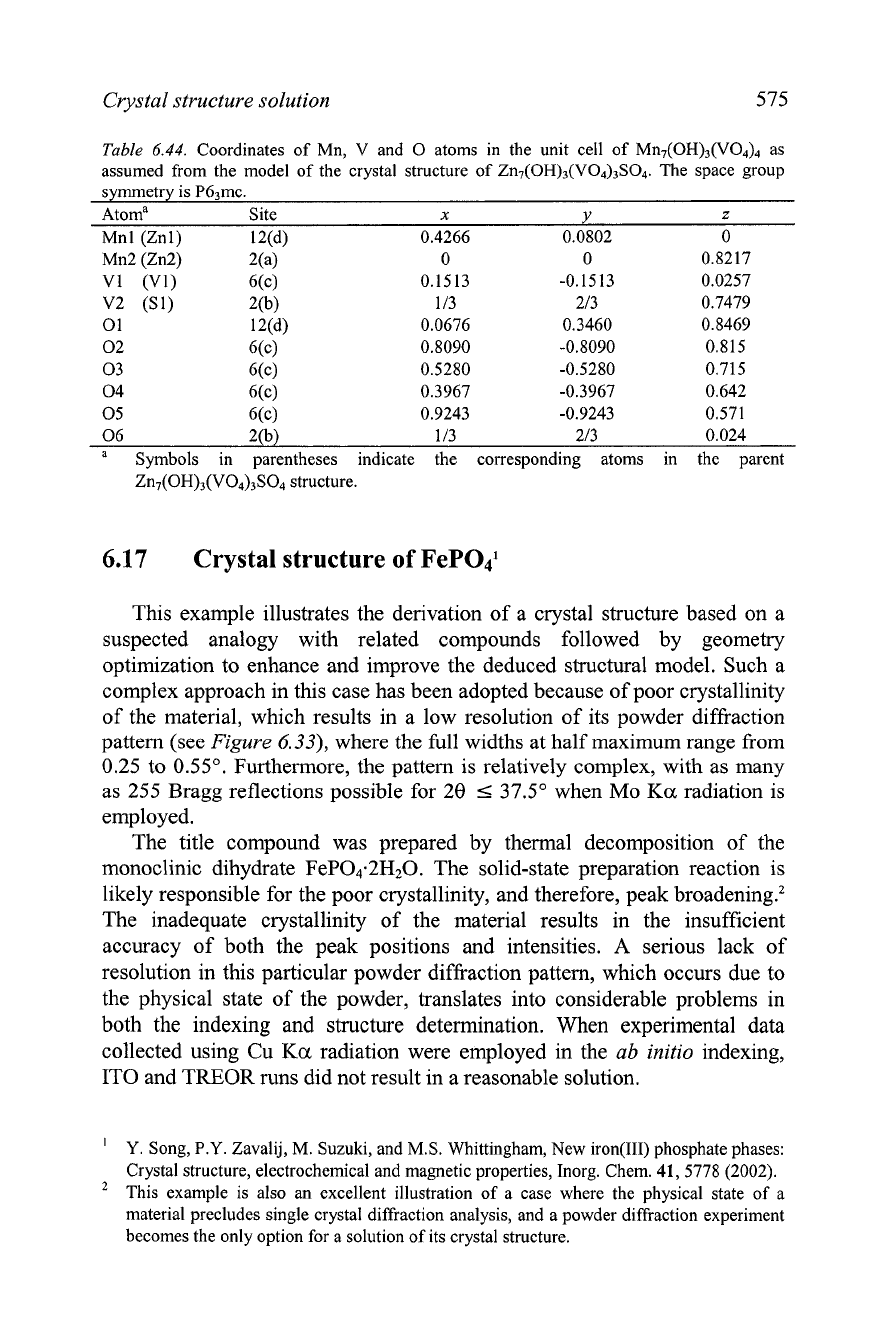
Table
6.44.
Coordinates of Mn, V and
0
atoms in the unit cell of MII,(OH)~(VO~)~ as
assumed from the model of the crystal structure of Zn7(OH),(VO,),SO4. The space group
symmetry is PG3mc.
Atoma Site
x
Y
z
Mnl (Znl) 12(d) 0.4266 0.0802 0
Mn2 (Zn2) 2(a) 0 0 0.8217
Vl (Vl) 6(c) 0.1513 -0.1513 0.0257
V2
(Sl) 2(b) 113 213 0.7479
0
1 12(d) 0.0676 0.3460 0.8469
02 G(c) 0.8090 -0.8090 0.815
03 6(c) 0.5280 -0.5280 0.715
04 6(c) 0.3967 -0.3967 0.642
05 6(c) 0.9243 -0.9243 0.571
06 2(b) 113 213 0.024
a
Symbols in parentheses indicate the corresponding atoms in the parent
Zn7(OH)3(V04)3S04 structure.
6.17
Crystal structure
of
FePO4I
This example illustrates the derivation of a crystal structure based on a
suspected analogy with related compounds followed by geometry
optimization to enhance and improve the deduced structural model. Such a
complex approach in this case has been adopted because of poor crystallinity
of the material, which results in a low resolution of its powder diffraction
pattern (see
Figure
6.33), where the full widths at half maximum range from
0.25 to
0.55O.
Furthermore, the pattern is relatively complex, with as many
as
255
Bragg reflections possible for 28
5
37.5" when Mo
Ka
radiation is
employed.
The title compound was prepared by thermal decomposition of the
monoclinic dihydrate FeP04.2H20. The solid-state preparation reaction is
likely responsible for the poor crystallinity, and therefore, peak broadening2
The inadequate crystallinity of the material results in the insufficient
accuracy of both the peak positions and intensities. A serious lack of
resolution in this particular powder diffraction pattern, which occurs due to
the physical state of the powder, translates into considerable problems in
both the indexing and structure determination. When experimental data
collected using Cu
Ka
radiation were employed in the
ab initio
indexing,
IT0 and TREOR runs did not result in a reasonable solution.
'
Y.
Song,
P.Y.
Zavalij, M. Suzuki, and MS. Whittingham, New iron(II1) phosphate phases:
Crystal structure, electrochemical and magnetic properties, Inorg. Chem.
41,
5778 (2002).
This example is also an excellent illustration of a case where the physical state of a
material precludes single crystal diffraction analysis, and a powder diffraction experiment
becomes the only option for a solution of its crystal structure.
576
Chapter
6
FePO,:
Cu Ka and
Mo
Ka
Bragg angle,
28
(deg.)
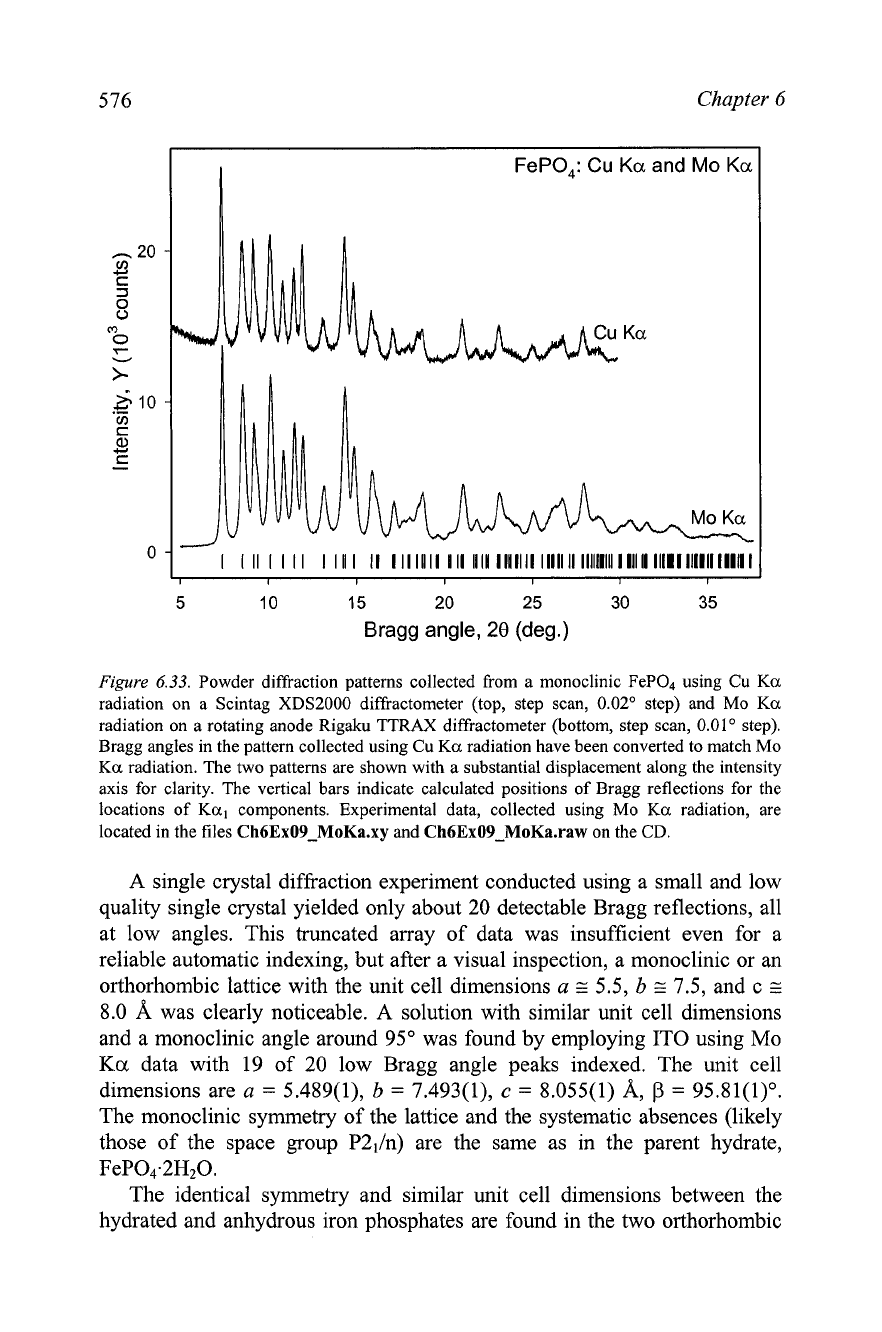
Figure
6.33.
Powder diffraction patterns collected from a monoclinic FeP04 using Cu
Ka
radiation on a Scintag XDS2000 diffractometer (top, step scan, 0.02" step) and Mo
Ka
radiation on a rotating anode Rigaku TTRAX diffractometer (bottom, step scan, 0.01' step).
Bragg angles in the pattern collected using Cu
Ka
radiation have been converted to match Mo
Ka
radiation. The two patterns are shown with a substantial displacement along the intensity
axis for clarity. The vertical bars indicate calculated positions of Bragg reflections for the
locations of
Ka,
components. Experimental data, collected using Mo
Ka
radiation, are
located in the files
Ch6Ex09-MoKa.xy
and
Ch6Ex09-MoKa.raw
on the
CD.
A single crystal diffraction experiment conducted using a small and low
quality single crystal yielded only about 20 detectable Bragg reflections, all
at low angles. This truncated array of data was insufficient even for a
reliable automatic indexing, but after a visual inspection, a monoclinic or an
orthorhombic lattice with the unit cell dimensions
a
G
5.5,
b
=
7.5, and c
z
8.0
ki
was clearly noticeable. A solution with similar unit cell dimensions
and
a
monoclinic angle around 95" was found by employing IT0 using Mo
Ka
data with 19 of 20 low Bragg angle peaks indexed. The unit cell
dimensions are
a
=
5.489(1),
b
=
7.493(1),
c
=
8.055(1)
A,
P
=
95.81(1)".
The monoclinic symmetry of the lattice and the systematic absences (likely
those of the space group P2Jn) are the same as in the parent hydrate,
FeP04.2H20.
The identical symmetry and similar unit cell dimensions between the
hydrated and anhydrous iron phosphates are found in the two orthorhombic
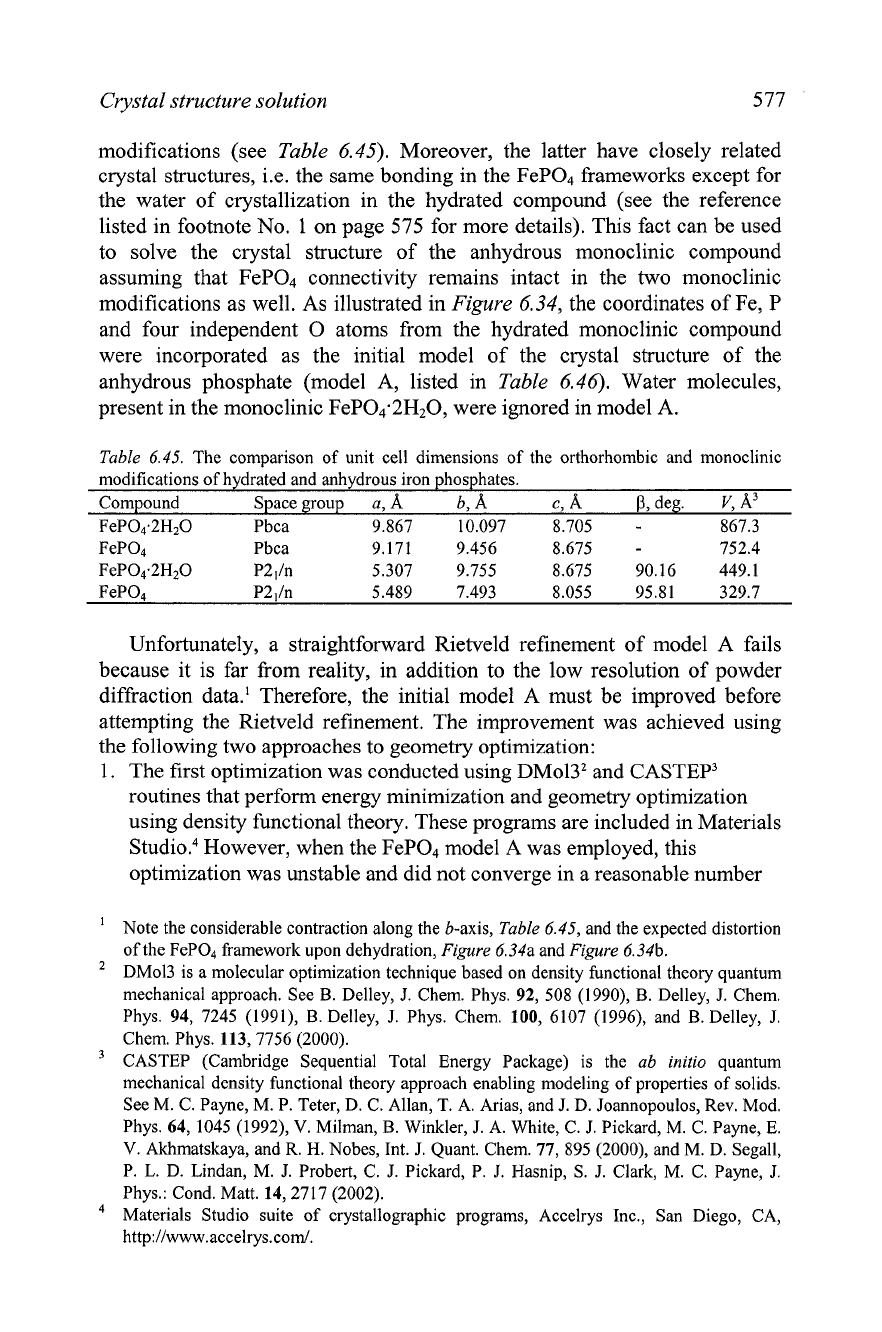
Crystal structure solution
5
77
modifications (see
Table
6.45). Moreover, the latter have closely related
crystal structures, i.e. the same bonding in the FeP04 frameworks except for
the water of crystallization in the hydrated compound (see the reference
listed in footnote No.
1
on page
575
for more details). This fact can be used
to solve the crystal structure of the anhydrous monoclinic compound
assuming that FeP04 connectivity remains intact in the two monoclinic
modifications as well. As illustrated in
Figure
6.34, the coordinates of Fe, P
and four independent
0
atoms from the hydrated monoclinic compound
were incorporated as the initial model of the crystal structure of the
anhydrous phosphate (model A, listed in
Table
6.46). Water molecules,
present in the monoclinic FePO4.2H2O, were ignored in model A.
Table 6.45. The comparison of unit cell dimensions of the orthorhombic and monoclinic
modifications of hydrated and anhydrous iron phosphates.
Compound Space group a,
A
b,
A
c,
A
8,deg.
V,A3
FeP04.2H20 Pbca 9.867 10.097 8.705
-
867.3
FeP04 Pbca 9.171 9.456
8.675
-
752.4
FeP04,2H20
P2Jn
5.307 9.755 8.675 90.16 449.1
FeP04
P2Jn 5.489
7.493 8.055 95.81 329.7
Unfortunately, a straightforward Rietveld refinement of model A fails
because it is far from reality, in addition to the low resolution of powder
diffraction data.' Therefore, the initial model A must be improved before
attempting the Rietveld refinement. The improvement was achieved using
the following two approaches to geometry optimization:
1.
The first optimization was conducted using DMo13' and
CASTEP3
routines that perform energy minimization-and geometry optimization
using density functional theory. These programs are included in Materials
St~dio.~ However, when the FeP04 model A was employed, this
optimization was unstable and did not converge in a reasonable number
Note the considerable contraction along the b-axis, Table 6.45, and the expected distortion
of the FeP04 framework upon dehydration, Figure 6.34a and Figure 6.34b.
DMol3 is a molecular optimization technique based on density functional theory quantum
mechanical approach. See
B.
Delley, J. Chem. Phys. 92, 508 (1990),
B.
Delley, J. Chem.
Phys. 94, 7245 (1991),
B.
Delley, J. Phys. Chem. 100, 6107 (1996), and
B.
Delley, J.
Chem. Phys. 113,7756 (2000).
CASTEP (Cambridge Sequential Total Energy Package) is the ab initio quantum
mechanical density functional theory approach enabling modeling of properties of solids.
See M. C. Payne, M. P. Teter, D. C. Allan,
T.
A. Arias, and J. D. Joannopoulos, Rev. Mod.
Phys. 64, 1045 (1992),
V.
Milman,
B.
Winkler, J. A. White, C.
J.
Pickard, M. C. Payne, E.
V.
Akhmatskaya, and
R.
H. Nobes, Int.
J.
Quant. Chem.
77,
895 (2000), and M. D. Segall,
P. L.
D.
Lindan,
M.
J.
Probert, C.
J.
Pickard, P. J. Hasnip, S. J. Clark, M. C. Payne, J.
Phys.: Cond. Matt. 14,2717 (2002).
Materials Studio suite of crystallographic programs, Accelrys Inc., San
Diego, CA,
http://www.accelrys.com/.
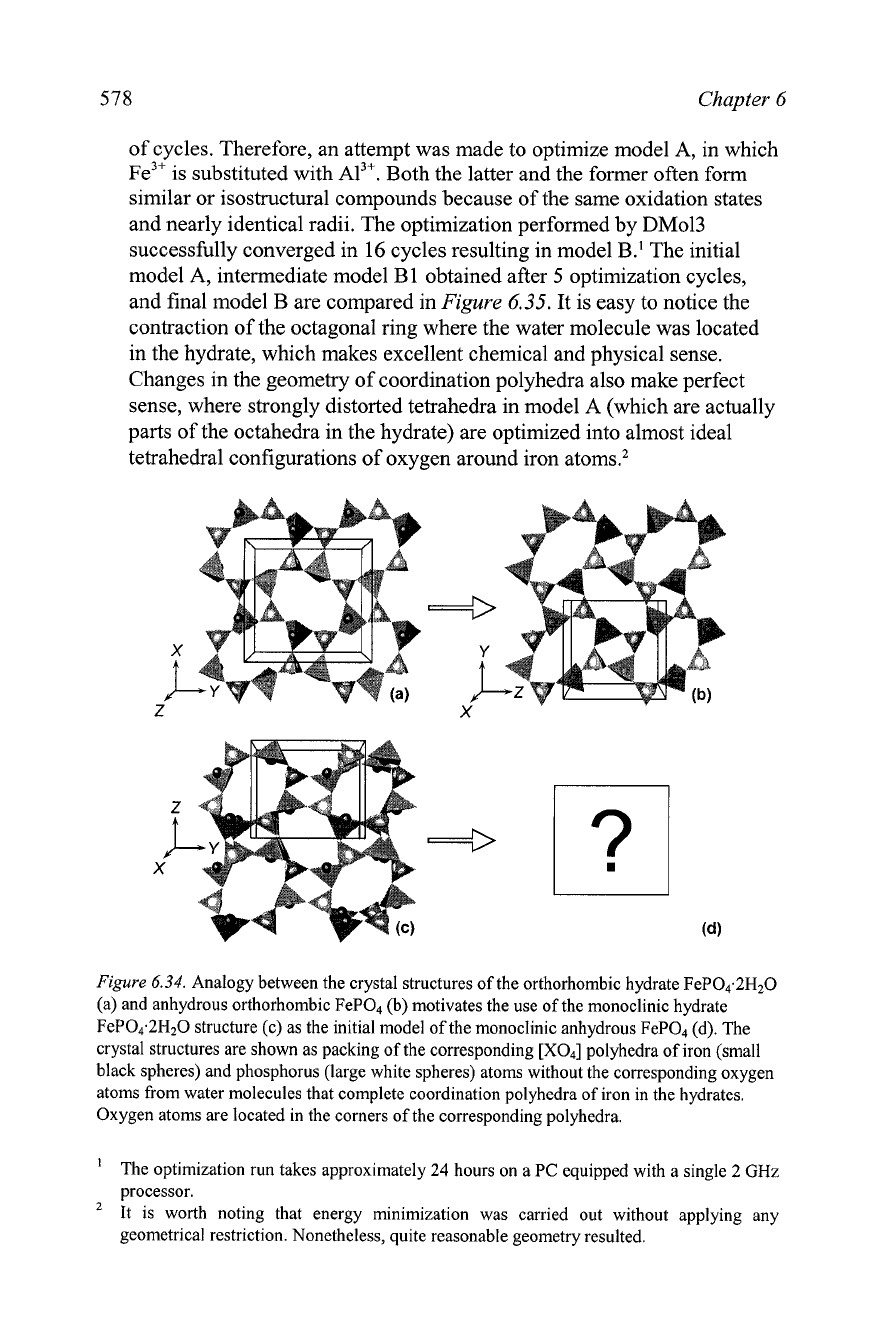
578
Chapter
6
of cycles. Therefore, an attempt was made to optimize model A, in which
~e~' is substituted with ~1~'. Both the latter and the former often form
similar or isostructural compounds because of the same oxidation states
and nearly identical radii. The optimization performed by
DM013
successfully converged in
16
cycles resulting in model B.' The initial
model A, intermediate model B
1
obtained after
5
optimization cycles,
and final model
B
are compared in
Figure
6.35.
It is easy to notice the
contraction of the octagonal ring where the water molecule was located
in the hydrate, which makes excellent chemical and physical sense.
Changes in the geometry of coordination polyhedra also make perfect
sense, where strongly distorted tetrahedra in model A (which are actually
parts of the octahedra in the hydrate) are optimized into almost ideal
tetrahedral configurations of oxygen around iron
atoms.2
Figure
6.34.
Analogy between the crystal structures of the orthorhombic hydrate FeP04.2H20
(a) and anhydrous orthorhombic FeP04 (b) motivates the use of the monoclinic hydrate
FeP04.2H20 structure (c) as the initial model of the monoclinic anhydrous FeP04 (d). The
crystal structures are shown as packing of the corresponding
[X04] polyhedra of iron (small
black spheres) and phosphorus (large white spheres) atoms without the corresponding oxygen
atoms from water molecules that complete coordination polyhedra of iron in the hydrates.
Oxygen atoms are located in the corners of the corresponding polyhedra.
The optimization run takes approximately 24 hours on a PC equipped with a single
2
GHz
processor.
It is worth noting that energy minimization was carried out without applying any
geometrical restriction. Nonetheless, quite reasonable geometry resulted.
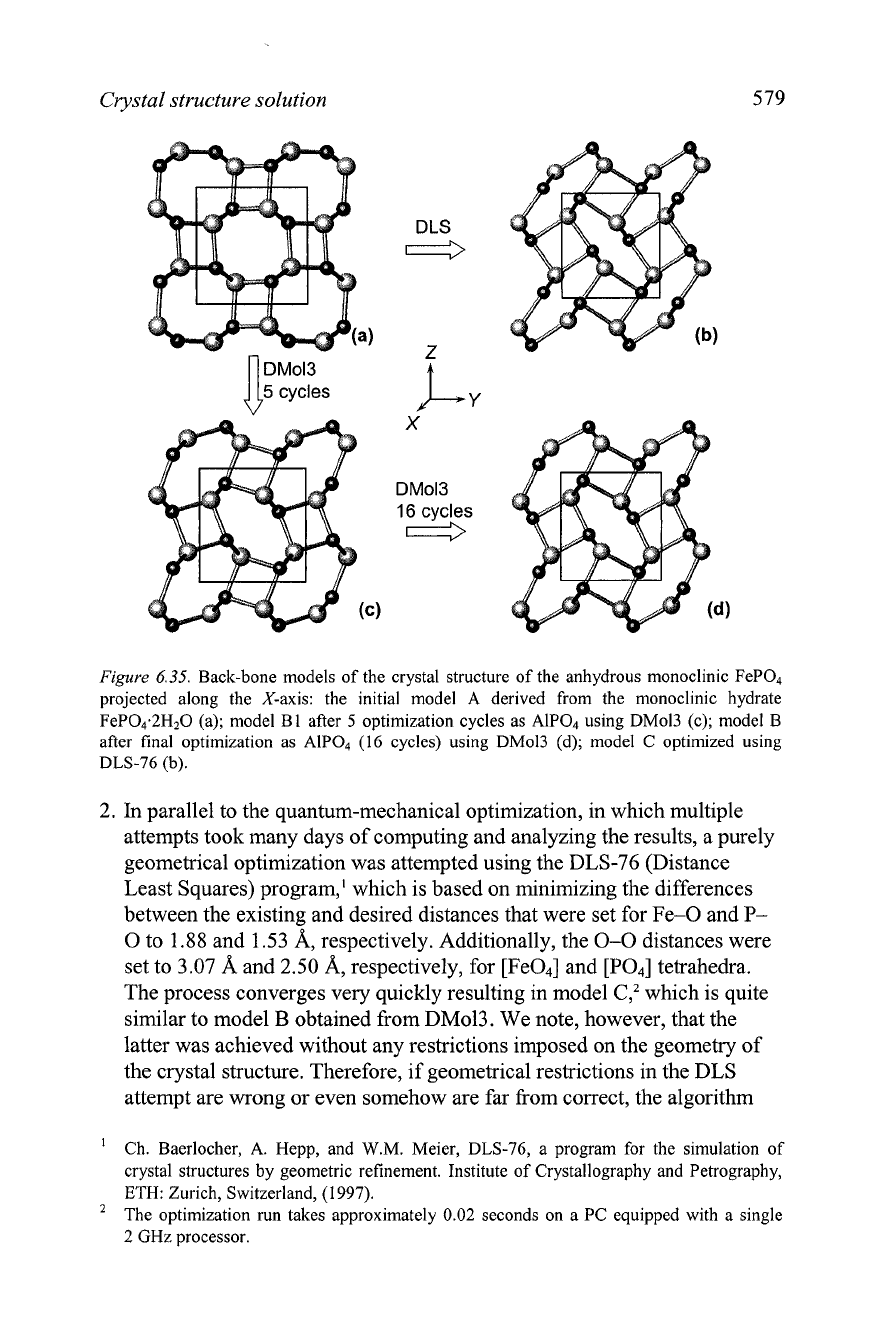
Crystal structure solution
DMol3
5
cycles
DLS
-
L
X
DMol3
16
cycles
e
Figure
6.35.
Back-bone models of the crystal structure of the anhydrous monoclinic FeP04
projected along the X-axis: the initial model A derived from the monoclinic hydrate
FeP04.2H20 (a); model B1 after
5
optimization cycles as AlP04 using DMol3 (c); model B
after final optimization as
AIP04
(16 cycles) using DM013 (d); model
C
optimized using
DLS-76
(b).
2.
In
parallel to the quantum-mechanical optimization, in which multiple
attempts took many days of computing and analyzing the results, a purely
geometrical optimization was attempted using the DLS-76 (Distance
Least Squares) program,' which is based on minimizing the differences
between the existing and desired distances that were set for Fe-0 and
P-
O to
1.88
and 1.53 A, respectively. Additionally, the
0-0
distances were
set to 3.07
A
and 2.50 A, respectively, for [Fe04] and [PO4] tetrahedra.
The process converges very quickly resulting in model
C,'
which is quite
similar to model
B
obtained from DMol3. We note, however, that the
latter was achieved without any restrictions imposed on the geometry of
the crystal structure. Therefore, if geometrical restrictions in the DLS
attempt are wrong or even somehow are far from correct, the algorithm
Ch. Baerlocher, A. Hepp, and
W.M.
Meier, DLS-76, a program for the simulation of
crystal structures by geometric refinement. Institute of Crystallography and Petrography,
ETH:
Zurich, Switzerland, (1 997).
The optimization run takes approximately
0.02
seconds on a PC equipped with a single
2
GHz processor.
5
80
Chapter
6
may not (and highly likely will not) converge to a reasonable model. The
final model
C
is compared with the initial model
A
and models
B
1
and
B
from the DM013 optimization in
Figure
6.35.
This model was not tested
further because model
B
was successful.
Model
B,
obtained as a result of DM013 optimization, is shown in
Table
6.47
and it will be used as the initial approximation in Rietveld refinement
discussed in the next chapter, section 7.1 1.
Table
6.46.
Coordinates of Fe, P and
0
atoms in the unit cell of FePO,, model
A,
as assumed
from the model of the crystal structure of the monoclinic FeP04.2H20.
Atom Site
x
Y
z
Fe 4(e) 0.0914 0.6739 0.6916
P 4(e) -0.0870 0.3509 0.6839
0
1 4(e) -0.1 164 0.5068 0.6700
02 4(e) 0.1659 0.3221 0.7638
03
4(e) -0.0944 0.2814 0.5268
04
4(e) -0.3002 0.2937 0.783 1
Table
6.47.
Coordinates of Fe, P and
0
atoms in the unit cell of the anhydrous FePO,, model
B,
obtained from DMol3 ~ptimization.~
Atom Site
x
v
z
04 4iej 0.3450 0.3548 0.3720
a
In
order to directly compare models A and
B,
the atomic coordinates of the former should
be transformed as:
xtA
=
xA
+
0.5,
yIA
=
y,,
zIA
=
zA
-
0.5 because atomic coordinates
undergo several transformations during the optimization process.
6.18
Empirical methods of solving crystal structures
In
addition to reciprocal and direct space techniques considered in the
previous sections, a large variety of approaches may be employed to create a
model of the crystal structure in direct space. One of these, i.e. the
geometrical method, has been implicitly employed in section 6.9, where the
location of a single La atom in the unit cell was established from a simple
analysis of the unit cell dimensions and from the availability of low
multiplicity sites in the space group symmetry P61mmm. Here we consider
a
more complex example, i.e. the solution of several crystal structures
occurring in the series of Gd5(Si,Gel-,)4 alloys.' These examples illustrate
the power of the powder diffraction method in detecting subtle details of the
'
V.K. Pecharsky and K.A. Gschneidner, Jr., Phase relationships and crystallography in the
pseudobinary system Gd5Si4-Gd5Ge4, J. Alloys Comp. 260,98 (1997).

Crystal structure solution
581
atomic distribution in the unit cell in addition to highlighting how structural
information is an enabling step in establishing critical structure
-
properties
corre1ations.l It is worth noting that nearly always, empirical techniques
require extensive literature searches to find out as much as possible about the
crystal structures of closely related materials.'
According to Smith, Tharp and
John~on,~ both the silicide and germanide
of Gd at
5:4
stoichiometries belong to the same type of crystal structure; the
distributions of atoms in their unit cells are essentially identical to the
orthorhombic Sm5Ge4-type str~cture.~ Furthermore, as reported by
Holtzberg, Gambino and
McGuire,' extended solid solutions based on both
binary compounds exist in the GdS(SixGel.,)4 system in addition to the
formation of an intermediate phase with an unknown crystal structure near
the Gd5Si2Ge2 stoichiometry. The coordinates of atoms in the unit cell of
SmSGe4 are listed in
Table
6.48.
Powder diffraction patterns collected from three different samples, which
belong to three different phase regions in the Gd5(SixGel-J4 system are
shown in
Figure
6.36.
Both the similarities and differences are noteworthy:
the patterns have distinct clusters of Bragg peaks in the regions -10
<
28
<
-18"
and -21
<
29
<
-26", however, a conspicuous variation in peak
intensities from one pattern to another is also observed.
This simple visual analysis of powder diffraction patterns is usually a
good indicator that there are detectable changes in the atomic structures of
the materials in question but the overall structural motif remains closely
related. Based on this conclusion and assuming that at least one of the
materials belongs to the
SmSGe4 type, it should be possible to establish
details of atomic distributions in these three lattices, provided the quality of
diffraction data is ~ufficient.~
V.K. Pecharsky and K.A. Gschneidner, Jr., Gd5(SixGel.x)4: An extremum material, Adv.
Mater.
13,
683 (2001).
Examples considered in this section are also similar to the case of hydrated and anhydrous
FeP04 discussed in the previous section. The major difference is in the better crystallinity
of the Gd5(SixGel.,), materials and in the resulting higher quality of powder diffraction
data, which facilitate a straightforward Rietveld refinement of individual atomic and
profile parameters without the need for a preliminary quantum mechanical
and/or
geometrical optimizations.
G.S. Smith, A.G. Tharp, and
Q.
Johnson, Rare earth
-
germanium and -silicon compounds
at 5:4 and
5:3
compositions, Acta Cryst. 22, 940 (1967).
G.S. Smith,
Q.
Johnson, and A.G. Tharp, Crystal structure of Sm5Ge4, Acta Cryst. 22, 269
(1967).
F. Holtzberg, R.J. Gambino, and T.R. McGuire, New ferromagnetic
5:4
compounds in the
rare earth silicon and germanium systems, J. Phys. Chem. Solids 28,2283 (1967).
Holtzberg
et
al.,
J. Phys. Chem. Solids 28, 2283 (1967), also noted the differences in the
powder diffraction patterns of Gd5Ge4, Gd5Si2Ge2, and Gd5Si4. However, considering the
