Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Applications of Fluorinated Amino
Acids and Peptides to Chemical
Biology and Pharmacology
Fluorine in Medicinal Chemistry and Chemical Biology Edited by Iwao Ojima
© 2009 Blackwell Publishing, Ltd. ISBN: 978-1-405-16720-8

15
Application of Artifi cial Model
Systems to Study the Interactions
of Fluorinated Amino Acids within
the Native Environment of
Coiled Coil Proteins
Mario Salwiczek , Toni Vagt , and Beate Koksch
15.1 Introduction
Because of the unique physicochemical properties of carbon - bound fl uorine and due to
the fact that it does not appear within the pool of ribosomally encoded amino acids, fl uo-
rinated analogues can be used as powerful analytical labels to investigate protein structure
and protein – ligand interactions [1] . Furthermore, fl uorine ’ s impact on the structure, bio-
logical activity, and stability of polypeptides makes it an interesting substituent for protein
modifi cation [2 – 5] as well as for the de novo design of artifi cial proteins [6] . In this context,
fl uorinated analogues of hydrophobic amino acids show great promise as modulators for
the stability and self - organization of protein folding motifs whose interactions are largely
based on complementary hydrophobic side - chain packing. Global substitution of apolar
residues by fl uorinated analogues within the hydrophobic core of α - helical coiled coils
usually results in a strong thermodynamic stabilization and the specifi c formation of fl uo-
rous interfaces that strongly direct the self - sorting of these peptides [7] . In addition to such
“ tefl on ” proteins [8] per se, an interesting task is also to investigate how fl uorinated amino
Fluorine in Medicinal Chemistry and Chemical Biology Edited by Iwao Ojima
© 2009 Blackwell Publishing, Ltd. ISBN: 978-1-405-16720-8
392 Fluorine in Medicinal Chemistry and Chemical Biology
acids interact with their naturally occurring counterparts. However systematic approaches
toward this goal have been published only very recently [9] . Numerous attempts at inves-
tigating the interactions of comparably small nonpeptidic organic molecules with enzymes
indicate that, in addition to the hydrophobicity of fl uoroalkyl groups, their polarity also
plays an important role [10 – 14] . Polar interactions of carbon bound fl uorine were shown
to induce a favorable binding of a potential inhibitor to the thrombin active site [13] .
Moreover, fl uorine - induced polarity may result in disadvantageous, inverse fl uorous effects
such as a decrease in lipophilicity [10, 15] . Regarding polar interactions of fl uorine, it is
also important to note that fl uorine scientists have not yet fully agreed on whether carbon -
bound fl uorine may accept hydrogen bonds, especially from the functional groups of pro-
teins [16] . Accordingly, to this day, there is no consistent opinion on fl uorine ’ s behavior
as a nonnative “ functional group ” in amino acid side - chains. Such a specifi cation, however,
would be the most important precondition for enabling a directed application of fl uorine ’ s
unique properties in the engineering of peptides and proteins and their interactions with
one another.
Scientifi c approaches that attempt to rationalize fl uorine ’ s effects on the interaction
of polypeptides with native proteins usually rely on model systems with a precisely defi ned
interaction pattern that mimics a native environment. With the objective of unraveling the
effects of even single fl uorine substitutions, the structural homogeneity and stability of
such models are indispensable prerequisites. It is also very important that the chosen model
system and its analogues are easy to synthesize. Although successful attempts at incorpo-
rating fl uorinated amino acids by diverse protein expression methods have been reported
[17, 18] , most synthetic strategies for peptides bearing nonnatural substitutions rely on
solid - phase peptide synthesis (SPPS). While linear SPPS is restricted by the achievability
of long sequences [19] , convergent synthetic routes applying various peptide ligation
methods [20] as well as expressed protein ligation [21] pave the way to large modifi ed
proteins. Nevertheless, fast synthetic approaches are desirable for the synthesis of a broad
variety of different modifi ed peptides. In addition, comparably small model systems allow
for a more comprehensive interpretation of experimental data. Consequently, model
systems are often signifi cantly smaller than natural proteins and, thus, have to be very
carefully designed to effi ciently mimic a natural protein environment. In this respect, α -
helical coiled coil peptides have greatly gained in importance in recent years [7] . Coiled
coils are ubiquitous small proteins that show broad biological activities [22] . As the struc-
tural components of many DNA - binding proteins, they play an important role in gene
transcription, cell growth, and proliferation. Larger coiled coil assemblies provide molecu-
lar scaffolds and networks for the cytoskeleton as well as important structural components
of so - called “ motor proteins ” [23] . Such naturally occurring coiled coils are usually com-
posed of two to fi ve monomeric α - helices whose primary structure is characterized by a
repetitive alignment of seven amino acids ( abcdefg )
n
called a “ heptad repeat ” . Positions
a and d are mostly hydrophobic and harbor leucine, valine, and in some cases isoleucine
and methionine. In the folded state, these positions point to one side of the helix, whereas
the predominantly hydrophilic positions b , c , and f point to the other side. This spatial
separation of hydrophobic and hydrophilic residues imparts signifi cant amphiphilicity to
the molecule. Due to segregation of hydrophobic surface area from the aqueous solvent,
the helices usually fold into left - handed superhelical oligomers that bury the hydrophobic
residues within the so - called “ hydrophobic core ” (Figure 15.1 ). In dimeric coiled coils,
Application of Artifi cial Model Systems 393
positions e and g are usually occupied by charged residues such as glutamic acid, lysine,
or arginine. These residues additionally stabilize the folded structure by attractive interheli-
cal coulomb interactions. Besides its stabilizing role, this charged interaction domain is
an important determinant of folding specifi city [24] . This detailed knowledge about their
structure enables the de novo design of coiled coils that predictably fold into a specifi c
oligomeric structure that is best suited for a certain investigation. In the subsequent
sections, we will summarize how the coiled coil can be used to study fl uorine as a
side - chain substituent in different native - like polypeptide environments. The incorporation
of fl uorinated amino acids at different sites within the heptad repeat allows one to probe
the impact of fl uorination in both hydrophobic and polar environments. Furthermore,
the position of fl uorinated residues as well as the folding specifi city of the coiled coil
determines the orientation of the side - chains. Accordingly, the interactions of fl uorinated
amino acids depend not only on the nature of the environment but also on the way they
“ look ” at it.
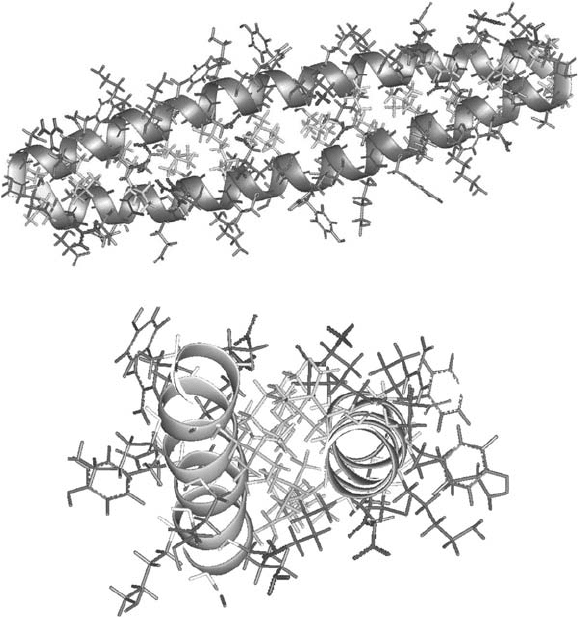
Figure 15.1 Schematic representation of a modelled antiparallel coiled coil homodimer in
both a side view (top) and a view along the superhelical axis (bottom). Hydrophobic side -
chains (Leu) are represented in yellow, complementary salt bridges in red (Glu), and blue
(Lys or Arg). See color plate 15.1.
394 Fluorine in Medicinal Chemistry and Chemical Biology
15.2 Hydrophobicity, Spatial Demand and Polarity – Fluorine in a
Hydrophobic and a Polar Polypeptide Environment
A rationally designed antiparallel, homodimeric α - helical coiled coil peptide served as a
model system for the fi rst systematic approach to investigating the interaction character-
istics of fl uorinated amino acids with native residues (Figure 15.2 ). The hydrophobic core
of the model is exclusively composed of leucine, whereas complementary coulomb inter-
actions between positions e and e ′ as well as g and g ′ control the antiparallel alignment
of the helices within the dimer [25] . In both strands, position a9 in the hydrophobic core
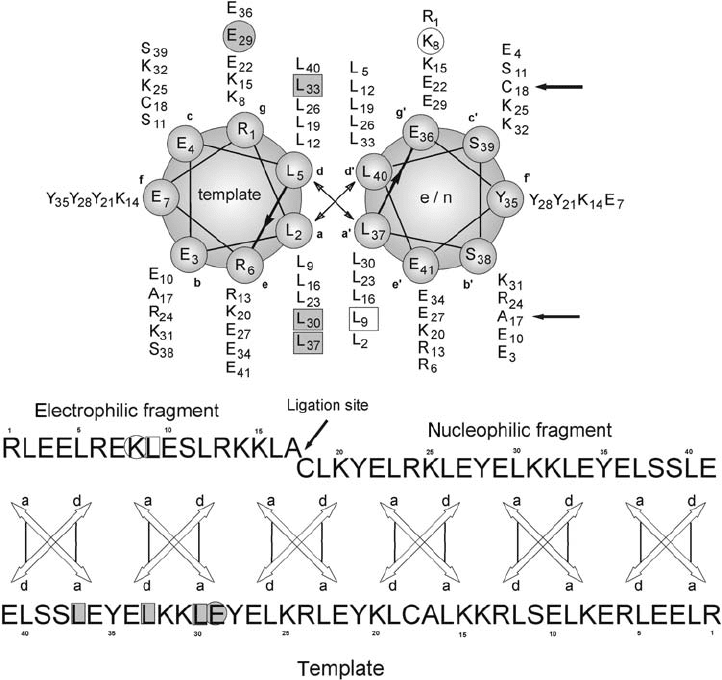
Figure 15.2 Helical wheel and sequence representation of the antiparallel, dimeric model
system. The substitution positions are highlighted in one strand with an open square for the
hydrophobic core and an open circle for the charged domain. Their direct interaction partners
in the opposite strand are shaded in gray squares or circles, respectively. The ligation site is
marked with an arrow.
Application of Artifi cial Model Systems 395
and position g8 in the charged interface served as substitution positions for fl uorinated
amino acids. This substitution pattern enabled an investigation of the effects of fl uorination
in both a hydrophobic and a hydrophilic coiled coil environment. The antiparallel orienta-
tion of the two 41 - amino - acid peptide chains ensures that fl uorinated residues interact
exclusively with native residues in the opposite strand. As the peptides were to be used
in an assay of the self - replication rate of these peptides (see next section), a convergent
synthesis strategy based on native chemical ligation [26] was applied. Three f - positions
contain tyrosine as an analytical label.
The strength of hydrophobic interactions largely correlates with the hydrophobic
surface area but also depends on packing effects of the interacting residues. Regarding the
hydrophobic core of the coiled coil model, the fi rst important step was to choose the amino
acids that would be appropriate for a systematic study of the impact of fl uorination. Single
fl uorine substitutions for hydrogen often behave bio - isosterically to hydrogen [27] , that
is, they alter neither the conformation nor the activity of the molecule. Nevertheless, there
is no linear correlation between the degree of fl uorination and the spatial demand of fl uo-
rinated alkyl groups. A comparison of the van der Waals volumes shows a trifl uoromethyl
group (42.8 Å
3
) to be approximately twice as large as a methyl group (24.5 Å
3
). These
volumes are calculated on a per - molecule basis according to published procedures [28] .
Furthermore, the steric effects of a trifl uoromethyl group, which are determined by both
spatial demand and conformational fl exibility, show similarities to an isopropyl group [29]
rather than to a methyl group.
Accordingly, the investigations were based on ( S ) - aminobutyric acid (Abu) deriva-
tives. Stepwise fl uorination of the Abu side - chain yields ( S ) - difl uoroethylglycine (DfeGly)
and ( S ) - trifl uoroethylglycine (TfeGly). The spatial demand of the side - chain can be further
increased by elongation by one methyl group, yielding ( S ) - difl uoropropylglycine (DfpGly),
which may be expected to have a spatial demand that is comparable to that of leucine.
Alanine, with the smallest side - chain in this series, served as a control substitution. Figure
15.3 summarizes the discussion of steric size/effects and the resulting substitution
pattern.
The analysis of temperature - induced unfolding monitored by circular dichroism (CD)
spectroscopy yields the midpoint of thermal denaturation. The melting point ( T
m
) is defi ned
as the temperature at which 50% of the oligomer is unfolded. Given that the coiled coil
maintains its folding specifi city when single substitutions are performed, this melting point
serves as a qualitative, yet not absolute, measure of stability. Small differences in T
m
do not
necessarily imply a difference in thermodynamic stability. Signifi cant changes, however,
may be interpreted in terms of stabilization or destabilization when comparing structurally
equivalent coiled coils since, in many cases, an increase in T
m
is associated with an increase
in thermodynamic stability [30] . Figure 15.4 shows the melting curves of all variants that
carry substitutes for leucine at position a9 within the hydrophobic core. The respective
melting points are given in Table 15.1 . With the exception of the DfpGly - variant, the
thermal stability in this series of dimers increases along with the increasing spatial demand
of the side - chain in position a9 in the order Ala > Abu > DfeGly > TfeGly > Leu.
Several factors have to be taken into account for a conclusive interpretation of these
results. Recent investigations have shown that some fl uorinated amino acids exhibit less
favorable helix - forming propensities [31] . Thus, structural perturbations of the helical
backbone may contribute to some extent to the general destabilization upon incorporation
396 Fluorine in Medicinal Chemistry and Chemical Biology
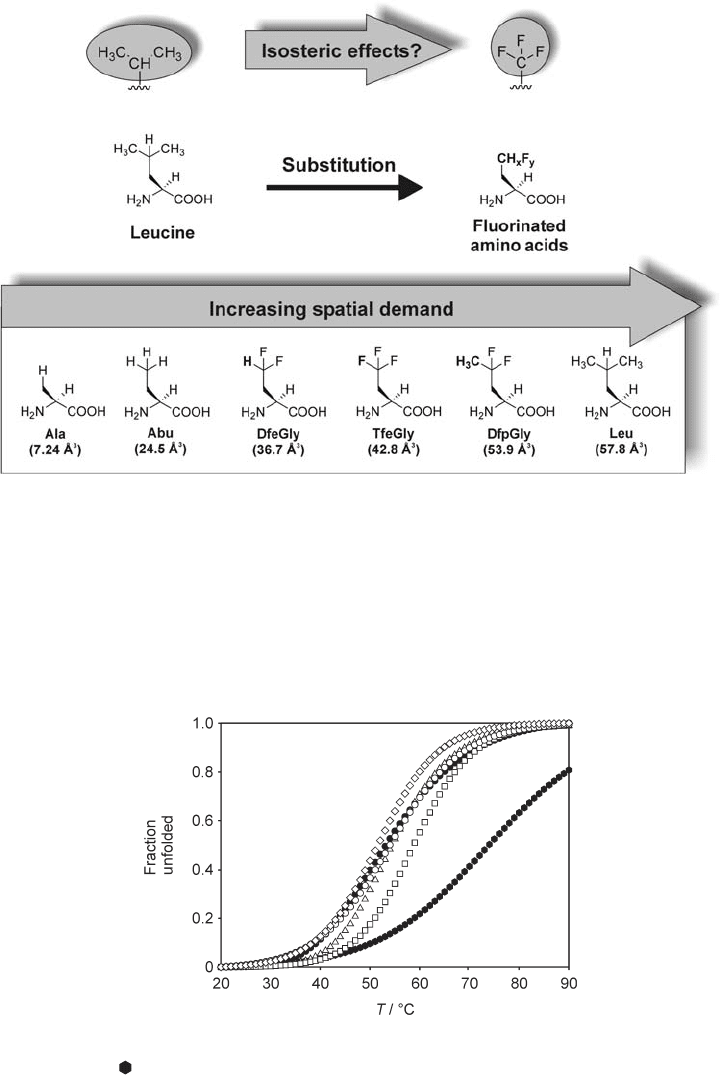
Figure 15.3 Based on the proposed comparability of isopropyl and trifl uoromethyl, one
native leucine residue within the hydrophobic core is substituted by amino acids bearing fl uo-
rinated ethyl side - chains. Starting from Abu the spatial demand was increased by stepwise
fl uorination and elongation of the side - chain from the left to the right. Alanine served as a
control substitution. The van der Vaals volumes in parentheses were calculated according to
reference [28] and refer to the group attached to the β - carbon of the side - chain.
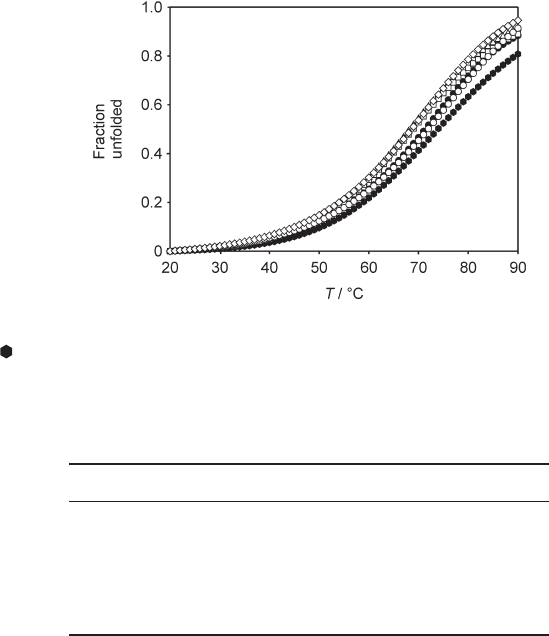
Figure 15.4 Melting curves of the coiled - coil dimers substituted in position a9 . Parent
peptide (Leu) (
), Ala ( 䊉 ), Abu ( 䊊 ), DfeGly ( 䉭 ),TfeGly ( ⵧ ), and DfpGly ( 䉫 ) .Recorded at
peptide concentration of 20 µ M at a pH of 7.4 (phosphate buffer containing 5 M GdnHCl).

Application of Artifi cial Model Systems 397
of fl uorinated residues. However, with the exception of TfeGly these effects have not been
studied for the amino acids used here. Moreover, helix - forming propensity is not the sole
determinant of the stability of the coiled coil folding motif whose structure formation is
based on intermolecular interactions. The thermodynamic driving force for the oligomer-
ization of coiled coils largely originates from hydrophobic interactions and side - chain
packing effects within the hydrophobic core. Due to such stabilizing intermolecular inter-
actions, the effects of helical propensity are often smaller within the interface of coiled
coils then they are in single helices [32] . In addition, many investigations have shown that
helix - forming propensity can only partly account for conformational preferences of amino
acids and that other interactions such as hydrophobic side - chain packing may also play a
key role in this regard [33] . For example: the helix propensity for Abu is 1.22 while the
value for TfeGly is only 0.05 [31] . Nevertheless, the thermal stability of the folding motif
presented here increases upon replacing Abu by TfeGly. The increase in surface area and
hydrophobicity upon stepwise fl uorination of the Abu side - chain has a favorable effect on
hydrophobic interactions and thus appears to stabilize the folding motif. The DfpGly
variant is excluded from this trend as, surprisingly, it represents the most destabilizing
substitution. Although DfpGly bears a side - chain closest to leucine regarding steric size,
its presence in the hydrophobic interior results in an even stronger destabilization than that
caused by alanine in the same position.
This effect can be ascribed to the highly polarized methyl group at the γ - carbon
(Figure 15.3 ). Even though the side - chain should be bulky enough to gain suffi cient
packing within the hydrophobic core, its polarity appears to prevent a favorable interaction
in this nonpolar environment. The interpretation of this result gains support from two other
fi ndings. The formal γ - difl uorination of the Abu side - chain, although it increases the spatial
demand, shows only a marginal effect on T
m
( ∆ T
m
= +0.4 K). One could argue that the
stabilizing effect of increased steric bulk is offset by the destabilization that is introduced
by a more polarized γ - hydrogen atom. Furthermore, the TfeGly substitution, although
expected to show steric effects comparable to leucine, results in a dimer that is roughly
15 K less stable than the parent peptide. As the strong inductive effect of fl uorine affects
the β - methylene groups, this fi nding can also be explained by the polarization of hydrogen
atoms within the side - chain.
Nevertheless, another important aspect regarding the stability of the folded state must
be taken into consideration. In comparison to leucine, TfeGly may exhibit a comparable
spatial demand but it lacks two carbon atoms within the side - chain. Therefore, the con-
formational fl exibility and, consequently, the entropy and stability of the folded dimer are
Table 15.1 Melting points of the Leu9 variants
Amino acid at position a9 T
m
( ° C)
Leu 73.9
Ala 53.2
Abu 54.0
DfeGly 54.4
TfeGly 59.9
DfpGly 51.6
398 Fluorine in Medicinal Chemistry and Chemical Biology
reduced. However, as the thermal stability of the model system decreases along with the
increasing number of polarized hydrogen atoms buried within the hydrophobic core, the
interpretation in terms of polarity is conclusive. The stability follows the order TfeGly > -
DfeGly > DfpGly. DfpGly bears the highest number of polarized hydrogen atoms and,
accordingly, represents the most destabilizing substitution.
The next important step was to investigate how the same amino acids would behave
as substitutes for lysine in position g8 of the charged domain. This substitution replaces
the salt - bridge to glutamic acid in the opposite helix by introducing fl uorinated amino
acids as noncharged interaction partners. As shown in Figure 15.5 , the resulting impact
on the thermal stability of the dimer is much lower than that observed for the hydrophobic
core (and see Table 15.2 ).
The decrease in melting temperature compared to the Lys - variant ranges from 2 K
for Abu to roughly 6 K for DfeGly. There is no clear trend pointing to a correlation between
solvent - exposed hydrophobic surface area and thermal stability. However, there are very
interesting effects arising from fl uorination of the Abu side - chain. Fluorine substitutions
in position g8 generally result in a somewhat stronger destabilization than the incorpora-
tion of Ala and Abu. As shown for substitutions within the hydrophobic core, fl uorinated
Figure 15.5 Melting curves of the coiled coil dimers substituted in position g8 . Parent peptide
(Lys) (
), Ala ( 䊉 ), Abu ( 䊊 ), DfeGly ( 䉭 ),TfeGly ( ⵧ ), and DfpGly ( 䉫 ) . Recorded at peptide
concentrations of 20 µ M at pH 7.4 (phosphate buffer in 5 M GdnHCl).
Table 15.2 Melting points of the Lys8 variants
Amino acid at position g8 T
m
( ° C)
Lys 73.9
Ala 71.3
Abu 71.9
DfeGly 68.3
TfeGly 68.9
DfpGly 68.6
Application of Artifi cial Model Systems 399
alkyl groups exhibit the unique property of being polar and hydrophobic. This “ polar
hydrophobicity ” [34] of fl uorinated compounds is one reason for the preferential formation
of fl uorous phases. Following this argument, the fl uorine - substituted peptides may unfold
more readily than their nonfl uorinated analogues to enable fl uorous interactions between
the unfolded peptide chains. This assumption gains support from investigations on the
self - replication properties of the model system [9] presented in the next section.
15.3 Do Fluorine – Fluorine Interactions Interfere with Coiled Coil
Association and Dissociation?
The analysis of temperature - induced unfolding as described in the preceding section
explains how single substitutions of proteinogenic residues by fl uorinated amino acids
affect the dimer ’ s overall stability. The study of the impact of fl uorine substitutions on the
replicase activity of coiled coils provides some valuable additional insight. Primary struc-
tures based on the heptad repeat motif exhibit the ability to promote the condensation of
two monomeric fragments whose amino acid sequence follows a complementary heptad
repeat. Thus, depending on the primary structure of the fragments, coiled coil peptides
can act as either ligases or replicases [35] . The investigations presented in this section are
based on the replicase activity, which explains the convergent route for the synthesis of
the model peptide (see Section 15.2 ).
For the establishment of such a replicase cycle, two peptide fragments are required,
one of which carries a cysteine at its N - terminus (nucleophilic fragment). The electrophilic
fragment usually carries a C - terminal benzyl thioester [36] . A noncatalyzed peptide bond
formation between the nucleophilic and the electrophilic fragment that yields a full - length
monomer initiates the process of self - replication. The fi rst step in this reaction is a thioes-
ter - exchange reaction between the C - terminal benzylthioester moiety of the electrophilic
fragment and the N - terminal cysteine of the nucleophilic fragment followed by an irrevers-
ible S → N acyl migration to yield a native peptide bond (native chemical ligation) [26] .
Based on the complementary coiled coil interactions, the monomer catalyzes the fi rst cata-
lytic cycle, acting as a template for the annealing of the nucleophilic and the electrophilic
fragments. The specifi c association of the fragments with the template brings their reactive
functional groups into close proximity and thereby promotes the fragment condensation
as described above. The cycle is fi nalized by the dissociation of the oligomer, releasing a
newly formed template for further reaction cycles. For the investigation of replicase activ-
ity, the proteinogenic residues in both substitution positions ( g8 and a9 ) of the model
peptide were replaced by three different amino acids that bear a side - chain of identical
length and vary only in fl uorine content (Abu, DfeGly, and TfeGly). Figure 15.6 shows
the turnover for peptides that contain these residues within the hydrophobic as well as
within the charged interaction domain.
The substitution of Abu for leucine in the hydrophobic core position a9 shows that
a signifi cant decrease in spatial demand and hydrophobicity that thermally destabilizes the
folding motif also reduces the rate of product formation. The γ - di - and trifl uorination
of Abu further decelerates rather than accelerates the reaction. This trend correlates
with the number of fl uorine atoms within the side - chain. Interestingly, the same kind of
