Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Fluorine-18 Radiopharmaceuticals 369
instability under usual conditions of nucleophilic or electrophilic
18
F - fl uorination, or insol-
ubility in solvent systems typically used), or if the needed synthetic precursor for direct
substitution proves too hard to prepare. Examples of small aromatic molecules used
as prosthetic groups include 4 - [
18
F]fl uorophenacyl bromide [17] , N - succimidyl -
4 - [
18
F]fl uorobenzoate [27] , N - succimidyl - 4 - ([
18
F]fl uoromethyl)benzoate [28] , and 4 -
[
18
F]fl uorobenzaldehyde [29] , all prepared using aromatic nucleophilic
18
F - fl uorination as
the fi rst synthetic step. The single drawback to this approach is that all of these small
molecules are themselves synthesized using multiple steps and some purifi cation is needed
before they are usable in the fi nal desired reaction to label the target molecule.
Nucleophilic aromatic substitution of aryl rings followed by removal of the ring -
activating electron - withdrawing group offers a more lengthy but feasible route to the
18
F -
labeling of aromatic rings bearing no activating groups or, even more challenging, bearing
electron - donating groups that normally inhibit aromatic nucleophilic substitution reactions
[23, 30] . Although useful fl uorine - 18 radiopharmaceuticals have been prepared using these
methods [31, 32] , a certain loss of overall radiochemical yield is expected due to radio-
nuclide decay during these required extra steps after
18
F - fl uorination. As an alternative
there are more direct methods for radiofl uorination of electron - rich aryl rings, as discussed
in the next section.
14.3.4
18
F - Labeling of Electron - Rich Aryl Rings
As not all biological molecules or drug candidates present a suitable activated aryl ring
for simple nucleophilic aromatic substitutions, the direct
18
F - labeling of electron - rich aro-
matic rings represent a signifi cant challenge in the synthesis of radiolabeled pharmaceuti-
cals. Electrophilic reagents such as: [
18
F]F
2
and acetyl [
18
F]hypofl uorite (AcOF), have been
used to label electron - rich aromatics [28] . These electrophilic reagents are generally pro-
duced via carrier - added methods and thus provide fi nal products with low to at best mod-
erate specifi c activity. This has limited the use of electrophilic
18
F - fl uorination reactions
to the production of radiopharmaceuticals for which there is no need for high specifi c
activity and where the chemical species are not toxic [5] . In addition, these reagents are
very reactive and non - regioselective, often leading to a mixture of
18
F - labeled products
and a requirement for careful separation and purifi cation. This reduces the radiochemical
yield of any single desired product.
Recently, a few improved methods of direct
18
F - fl uorination on electron - rich aromat-
ics have been reported, using nucleophilic substitution [33 – 36] or electrophilic substitution
[37 – 39] . These improved methods are encouraging solutions to the complex problem of
18
F - fl uorination of electron - rich aryl rings.
14.3.4.1 Nucleophilic
18
F - Fluorinations Using Iodonium Salts
For years, diaryliodonium salts have been used for the direct
18
F - fl uorinations in nucleo-
philic substitution reaction on aromatic rings [34, 35, 40, 41] . The introduction of no -
carrier - added [
18
F]fl uoride into an aryl substituent of diaryliodonium salt results in a
[
18
F]fl uorinated arene and a corresponding iodoarene (Figure 14.4 ).
The nucleophilic attack by [
18
F]fl uoride ion on the diaryliodonium salt occurs prefer-
ably at the more electron - defi cient ring [42] . Therefore, a more electron - rich ring such as
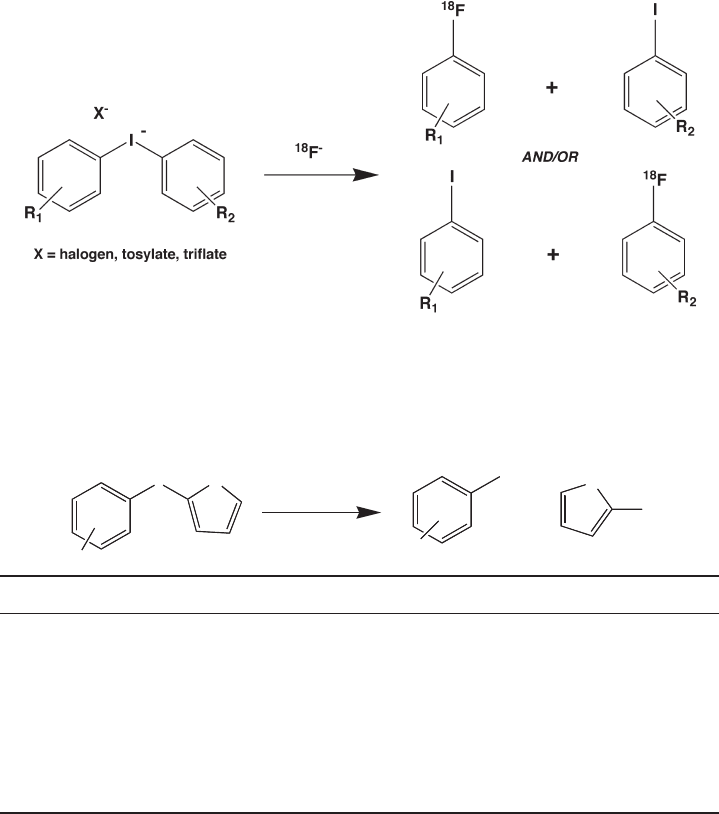
370 Fluorine in Medicinal Chemistry and Chemical Biology
a heteroaromatic moiety in the iodonium salt should allow the direct nucleophilic
18
F -
labeling of a second aryl group that is comparatively less but still electron - rich [36] . It has
been reported by Ross et al. using the 2 - thienyl group as a highly electron - rich heteroaro-
matic ring to direct the nucleophilic
18
F - fl uorination to the second, less electron - rich aryl
ring [34] . This heteroaromatic system provides a regiospecifi c single radioactive product
and yields of the corresponding aryl substituents via this method are shown in Table 14.2 .
As expected, the radiochemical yields were generally high with a decrease of the electron
density, except for the ortho - methoxy derivative (61%). The huge difference between
ortho - and para - substituted rings is not explainable by the regular character of the aromatic
nucleophilic substitution reaction mechanism, and the meta - derivative shows a slightly
higher initial reaction rate than the ortho - and para - derivatives but ends in a lower
Figure 14.4 Reaction of [
18
F]fl uoride ion with diaryliodonium salts. Proportions of the four
possible products ([
18
F]fl uoroarenes vs. iodoarenes) is dependent on the ring substituents.
Table 14.2 Radiochemical yields ( RCY ) for nucleophilic
18
F - fl uorination of
aryl(2 - thienyl)iodonium bromides
I
S
R
S
R
I
18
F
-
18
F
+
R RCY (%)
4 - OBn
36 ± 3
4 - OMe
29 ± 3
4 - Me
32 ± 2
H
64 ± 4
3 - OMe
20 ± 3
4 - Cl
62 ± 4
4 - Br
70 ± 5
4 - I
60 ± 8
2 - OMe
61 ± 5
Fluorine-18 Radiopharmaceuticals 371
radiochemical yield. These might be due to a so - called Meisenheimer complex formed as
an intermediate in this reaction, which is stabilized by resonance structures [34] .
14.3.5 Prosthetic Groups: Fluoroalkyl and Fluoroacyl Substituents
In the design of radiopharmaceuticals labeled with fl uorine - 18, it has become fashionable
to simply alter the structure of a known parent drug or biochemical substrate by the sub-
stitution of a fl uoroalkyl group for a methyl group. This often provides minimal disruption
to the physiochemical properties of a molecule (lipophilicity, molecular weight, p K
a
)
and has been found in many cases to provide molecules with equivalent or even
enhanced biological activity in vivo . Although in many cases appropriate precursors can
be prepared with leaving groups pre - positioned at the end of short alkyl chains (see
[
18
F]fl uoropropyl - DTBZ, above), the development of simple
18
F - fl uoroalkylating [43] and
18
F - fl uoroacylating agents [44, 45] also allows the application of this approach to families
of compounds without the need to synthesize for each one an appropriate precursor for a
single - step nucleophilic fl uorination. Most useful radiotracers have incorporated
[
18
F]fl uoromethyl, [
18
F]fl uoroethyl or [
18
F]fl uoropropyl alkyl groups, or [
18
F]fl uoroacetyl
and [
18
F]fl uoropropionyl acyl groups: larger groups begin to introduce considerable size
and lipophilicity to the original molecule.
14.4 Electrophilic
18
F - fl uorination: General Aspects
Although the majority of radiopharmaceuticals labeled with fl uorine - 18 have been
prepared using nucleophilic fl uorination reactions, in a few instances the application
of electrophilic fl uorination reactions has proved quite suitable. The most signifi cant
limitation of electrophilic
18
F - fl uorinations is the relatively low specifi c activities (less than
10 Ci/mmol) commonly obtained for fi nal products. This is a result of the fact that
electrophilic
18
F - fl uorination reagents (perchloryl fl uoride, acetyl hypofl uorite, xenon
difl uoride, N - fl uoro - N - alkylsulfonamides, diethylaminosulfur trifl uoride) are prepared in
low specifi c activity from
18
F - labeled fl uorine gas, which in itself produced in a carrier -
added fashion. A second drawback of electrophilic fl uorination is that the maximum
radiochemical yield obtainable is 50%, as only one of the two fl uorine atoms in fl uorine
gas can end up in the product (or, for preparation of electrophilic reagents such as acetyl
[
18
F]hypofl uorite, the maximum yield of preparing the reagent from [
18
F]F
2
is 50%).
Production of [
18
F]F
2
, from which numerous subsequent electrophilic fl uorination
reagents have been prepared, can be accomplished in two fashions. The fi rst is the irradia-
tion of cyclotron targets containing small amounts of carrier fl uorine gas in neon [46] ;
fl uorine - 18 is produced by the nuclear reaction
20
Ne(d, α )
18
F, with essentially exchange of
the fl uorine - 18 and fl uorine - 19 atoms to produce carrier - added [
18
F]F
2
. As an alternative
to direct production of fl uorine - 18 gas in a neon target, it is also possible to form [
18
F]F
2
using a two - step procedure of fi rst irradiation of oxygen - 18 (the high - yield method for
fl uorine - 18 production), followed by a second irradiation of a gas mixture containing
carrier fl uorine gas to initiate exchange of the fl uorine atoms, producing carrier - added
fl uorine gas [47, 48] .
372 Fluorine in Medicinal Chemistry and Chemical Biology
Despite the low specifi c activity obtained with electrophilic
18
F - fl uorination reagents,
important radiopharmaceuticals have been prepared in this fashion.
14.4.1 [
18
F ]Fluoro DOPA : One - step Electrophilic Fluorination
l - 3,4 - dihydroxy - 6 - [
18
F]fl uorophenylalanine ([
18
F]FDOPA) is a amino acid derivative pri-
marily utilized to follow the biosynthesis of dopamine in the brain, as it is taken up by
dopaminergic neurons and decarboxylated to 6 - [
18
F]fl uorodopamine and stored in vesicles
alongside endogenous dopamine. It was one of the fi rst fl uorine - 18 radiopharmaceuticals
developed for clinical investigations of movement disorders (e.g., Parkinson ’ s disease) and
its synthesis has drawn much attention and been discussed numerous times [4, 49] . The
synthesis of [
18
F]FDOPA can be achieved using both electrophilic and nucleophilic routes,
and provides a good example of how these different methods each have advantages and
drawbacks. The synthesis of FDOPA is also a general example of methods for preparation
of
18
F - labeled aromatic amino acids and derivatives, and thus has been extended to desired
molecules such as fl uorinated phenylalanines and fl uorotyrosines.
The initial syntheses of [
18
F]FDOPA were done using direct electrophilic fl uorination
of DOPA [49] , which was soon replaced with methods for regioselective
18
F - fl uorination
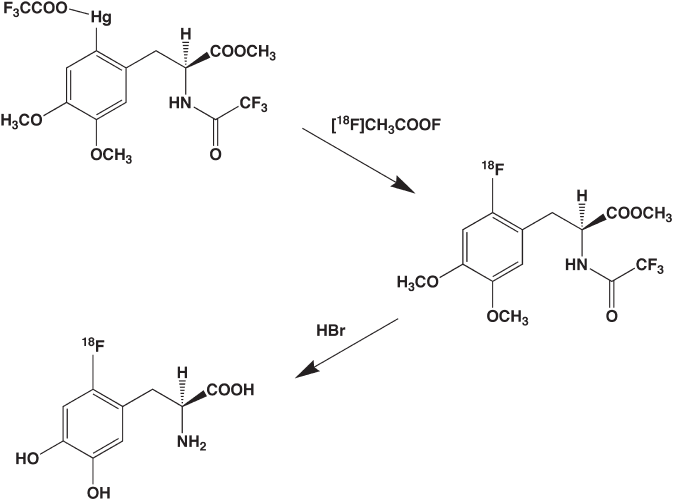
of DOPA using organomercuric [50] or organostannane precursors [51] (Figure 14.5 ).
Figure 14.5 Electrophilic
18
F - fl uorination of an organomercurial derivative of dihydroxyphe-
nylalanine as a route to synthesis of 6 - [
18
F]fl uoro - 3,4 - dihydroxyphenylalanine (6 - [
18
F]fl uoroDOPA,
FDOPA). Reaction is stereospecifi c and regioselective but requires a step for deprotection of
functional groups.
Fluorine-18 Radiopharmaceuticals 373
The methods using organometallics are relatively simple and can provide enantiomerically
pure [
18
F]FDOPA in yields as high as 25% (decay - corrected). These methods have even
been automated [51] . The use of the organometallic approach exemplifi es a simplifi cation
of the radiochemistry, which, however, complicates the quality control analysis, as in
addition to the normal requirements for a radiopharmaceutical (radiochemical purity,
radionuclidic purity, chemical purity), there is also the need to develop and implement
sensitive analytical techniques to ensure that there are no residual amounts of the metals
(mercury or tin) in the fi nal product. The advantage of the electrophilic approach to
[
18
F]FDOPA is that the product is obtained in two steps in enantiomerically pure form; the
disadvantages are the limited radiochemical yield, the low specifi c activity inherent in
electrophilic reactions, and the need for extensive quality control analysis. Fortunately,
[
18
F]FDOPA is one of the radiochemicals for which high specifi c activity is not required,
as the transporters and enzymes involved in [
18
F]FDOPA uptake and retention in neurons
are not saturated by the chemical amounts of FDOPA associated with the radiochemical
product, and it has also been demonstrated there is no toxicity associated with the
chemical. Efforts continue for improvement and simplifi cation of methods for electrophilic
fl uorination to yield FDOPA [52] .
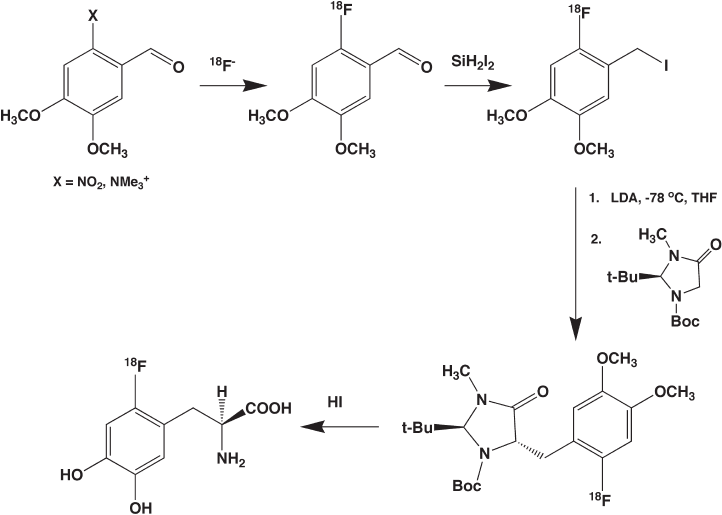
In contrast to the electrophilic approach, the nucleophilic methods [53 – 55] for syn-
thesis of [
18
F]FDOPA involve four synthetic steps (Figure 14.6 ) starting with [
18
F]fl uoride
ion, require a signifi cantly longer total synthesis time, and in the end probably do not result
Figure 14.6 Nucleophilic approach to synthesis of 6 - [
18
F]fl uoroDOPA. The multistep synthe-
sis introduces fl uorine - 18 in an initial nucleophilic aromatic substitution reaction.
374 Fluorine in Medicinal Chemistry and Chemical Biology
in a much higher yield than that obtained by electrophilic routes. The syntheses require
the use of chiral auxiliaries or chiral phase - transfer reagents to produce [
18
F]FDOPA in
appropriate enantiomeric purity [54] ; otherwise a chiral column chromatography step is
necessary to separate the two isomers formed in the alkylation of a simple glycine synthon,
which of course results in loss of some product as the wrong isomer. Use of the nucleo-
philic route does produce higher specifi c activities, but this is not a clear advantage for a
radiopharmaceutical that does not require such. In contrast, [
18
F]fl uorodopamine serves as
an excellent example where the choice of a synthetic strategy is very important; the syn-
thesis via nucleophilic aromatic
18
F - fl uorination [56] provides a high specifi c activity
product without in vivo hemodynamic effects, whereas the same compound made by an
electrophilic route [57] yields a carrier - added radiopharmaceutical that is not suitable for
radiotracer studies of in vivo biochemistry.
14.4.2 Fluoro - destannylation
Trimethyltin substituents are known to react with [
18
F]fl uorine in a regioselective manner
[58] . Several important radiopharmaceuticals such as [
18
F]fl uoro - l - DOPA [39] [
18
F]fl uoro -
l - tyrosine [37] , and [
18
F]fl uorometaraminol [59] have been synthesized using this method.
The limitation of this method is again the low specifi c activity from [
18
F]fl uorine gas.
Hopefully, with development of advanced technology, a better electrophilic [
18
F]fl uorine
source might be available. Another concern of this method is the residue of potentially
toxic metals, but tin has less toxicity than mercury. Recently, resin - bonded aryltin precur-
sors have been reported for
125
I - labeling by destannylation [60, 61] . The desired radioactive
product will only be released from resin via destannylation, while unreacted precursor and
tin - containing side - product remains bonded to the resin which is easily removed by fi ltra-
tion. This method may have great potential for use in
18
F - fl uorination in a similar
fashion.
14.5 New Directions in
18
F - labeling
Labeling of biologically active molecules with fl uorine - 18 has taken great strides in the
last two decades, largely due to the successful application of well - known reaction methods
– nucleophilic and electrophilic fl uorinations – to an increasingly diverse assortment of
chemical structures. Two recent developments, the application of “ click ” chemistry and
the use of protic solvents in nucleophilic fl uorinations, deserve mention here as new
directions in
18
F - labeling that have the potential to signifi cantly impact both the types of
compounds labeled and the yields obtained.
14.5.1 Application of “ Click ” Chemistry to
18
F - labeling
“ Click ” chemistry is a generic term that describes chemical reactions that are easy to
perform and work up, are high yielding, and are insensitive to oxygen or water [62, 63] .
However, there has arisen one particular reaction that has become the leader of the fi eld
Fluorine-18 Radiopharmaceuticals 375
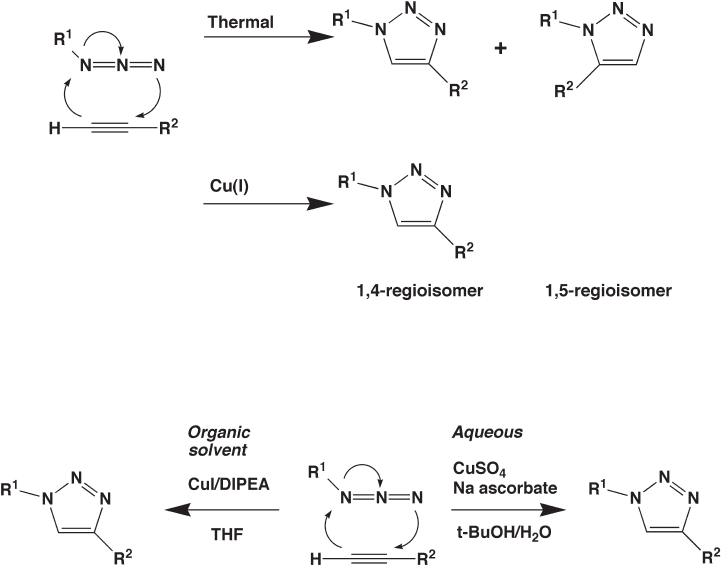
and that is the Huisgen 1,3 - dipolar cycloaddition of terminal alkynes with azides to give
1,2,3 - triazoles (Figure 14.7 ) [64] .
Prior to the use of copper(I), the cycloaddition required elevated temperatures for
prolonged periods. Typically, reactions required refl uxing in toluene or carbon tetrachlo-
ride for 10 – 48 h. Under these conditions, the cycloaddition was non - regiospecifi c with two
possible regioisomers (1,4 and 1,5) formed. Some control of regiospecifi city was obtain-
able when electron - withdrawing groups were substituted on the alkyne, favoring produc-
tion of 1,4 - regioisomer. Meanwhile, the addition of electron - withdrawing groups to the
azide favors production of the 1,5 - regioisomer. In practice, the cycloaddition yielded
mixtures of product and exclusive production of one regioisomer remained elusive [65] .
Thus, the cycloaddition under these conditions failed to fulfi ll the requirement of a “ click ”
chemistry [64] .
The utility of the Huisgen 1,3 - dipolar cycloaddition was discovered when it was
realized that copper(I) not only catalyzes the reaction but also promotes the regiospecifi c-
ity, with exclusive production of the 1,4 - triazole regioisomer (Figure 14.8 ) [64] . The fi rst
publication by Meldal et al. , in 2002, outlined the use of copper(I) in the cycloaddition
reaction for triazole synthesis on a solid phase [66] . This was an organic - solvent - based
Figure 14.7 The 1,3 - cycloaddition of an azide and a terminal alkene to form a 1,2,3 - triazole.
The thermal reaction produces both the possible regioisomers, whereas the copper - catalyzed
reaction yields the single 1,4 - regioisomer.
Figure 14.8 Use of the copper(I) - mediated alkene - azide 1,3 - cycloaddition ( “ click ” chemis-
try) in both organic solvent and aqueous solvent systems. The aqueous system, with in situ
generation of copper(I) from copper(II), is less sensitive to oxygen.
376 Fluorine in Medicinal Chemistry and Chemical Biology
procedure that used copper(I) iodide with N,N - diisopropylethylamine (DIPEA) in
various solvent such as acetonitrile, dichloromethane, tetrahydrofuran, toluene, or N,N -
dimethylformamide, with the terminal alkyne immobilized on a swollen solid support.
This was closely followed by the report of Folkin and Sharpless and colleagues showing
that the reaction could be carried out in water using copper sulfate and sodium ascorbate
[67] . Both methods have recently become very popular and have made the Huisgen 1,3 -
dipolar cycloaddition the essential “ click ” chemistry.
The facility of this water - based method earned it many applications. The azide and
terminal alkyne are mixed in a mixture of tert - butanol and water (1:1 or 2:1). Then sodium
ascorbate (5 – 10% mol) is added followed by a copper(II) sulfate solution (1 – 5% mol),
and the fl ask is sealed and stirred vigorously at ambient temperature [56] . Copper(I) is
generated in situ by reduction of the copper(II) with an excess of sodium ascorbate and
under these condition the normally oxygen - sensitive copper(I) survives. These reactions
are typically run overnight, but a mild thermal or microwave - assisted heating shortens
reaction time to 10 – 15 minutes [68] . A number of modifi ed reaction conditions have been
reported, using copper(I) species directly, as CuI, CuOTf · C
6
H
6
or [Cu(CH
3
CN)
4
PF
6
], with
a nitrogen base such as triethylamine or pyridine [69 – 71] . The reaction mechanism is still
under investigation and appears quite complex [53, 64 72] . A recent analysis suggests that
both azide and alkyne are activated by copper, possibly within a multinuclear copper -
acetylide species, supporting earlier reports of two copper centers participating in the
catalysis [72, 73] .
Organic solvent - based procedures have been used in situations when the reactants
are not soluble in aqueous media. Copper(I) is supplied directly to the reaction in form of
CuI with DIPEA, and co - solvents such as acetonitrile, dichloromethane, tetrahydrofuran,
toluene, or N,N - dimethylformamide are used. Some alternative protocols, using THF with
Cu(PPh
3
)Br and DIPEA, or CuBr in DMF with bipyridine, have also been reported
[74, 76] .
The simplicity and effi ciency of the “ click ” chemistry is attractive to fl uorine - 18
chemistry, where time plays an important role in synthesis due to the relative short half -
life of fl uorine - 18. This one - pot reaction provides a versatile tool for coupling drug - like
fragments in high yield and under mild conditions. The product 1,2,3 - triazole formed from
cycloaddition is biologically stable with polarity and size similar to an amide group that
is a common functional group in many radiopharmaceuticals [77] .
18
F - labeled peptides are a rapidly emerging fi eld for targeted PET imaging probes.
Although a variety of
18
F - labeled prosthetic groups have been developed in the past decade,
only a limited number of chemical reactions have been utilized to incorporate the pros-
thetic groups into peptides, including acylation [78 – 83] , alkylation [84] , and oxime forma-
tion [85, 86] . Acylation is the most commonly used approach and requires multistep
protection and deprotection of other functional groups within the peptide sequence, which
otherwise would be acylated. For both the alkylation and oxime formation reactions, the
reagents used have potential to react with other functional groups within the peptides as
well. The products formed by acylation, alkylation and oxidation are often species that
are susceptible to hydrolysis or oxidation. Therefore the “ click ” chemistry of azides and
terminal alkynes is expected to be a superior method for the preparation of
18
F - labeled
peptides because of the following advantages: (i) the reaction can be performed in an
aqueous media using readily accessible reagents and without exclusion of atmospheric
Fluorine-18 Radiopharmaceuticals 377
oxygen; (ii) the relatively mild reaction conditions are tolerant of peptide bonds, and neu-
tralization of the reaction media is not required before or after reaction; (iii) since the
reaction between alkyne and azide is orthogonal to any functional groups [87 – 91] it is not
necessary to protect other functional groups within the peptide sequence; (iv) the reaction
is highly regioselective, leading to 1,4 - disubstituted 1,2,3 - triazole in high yield with simple
work - up and purifi cation; and (v) the product is relatively stable. The large dipole moment
and the nitrogen atoms in positions 2 and 3 of the triazole serve as weak hydrogen bond
acceptors and improve the solubility of the product in water [63] .
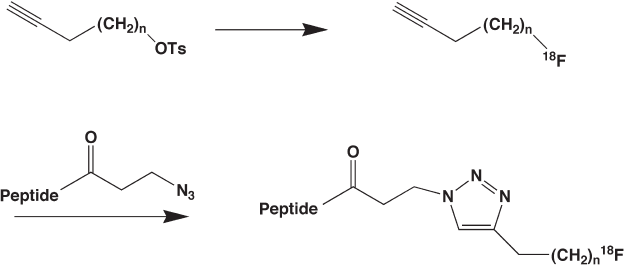
The fi rst application of copper(I) - catalyzed 1,3 - dipolar cycloaddition in preparation
of [
18
F]fl uoropeptides was reported by Marik and Sutcliffe in 2006 (Figure 14.9 ) [92] .
Three [
18
F]fl uoroalkynes ( n = 1, 2, and 3) were prepared in yields ranging from 36% to
80% by nucleophilic substitution of a p - toluenesulfonyl moiety with [
18
F]fl uoride ion.
Reaction of these [
18
F]fl uoroalkynes with various peptides (previously derivatized with
3 - azidopropionic acid) via the Cu(I) - mediated 1,3 - dipolar cycloaddition provided the
desired
18
F - labeled peptides in 10 minutes at room temperature with yields of 54 – 99% and
great radiochemical purity (81 – 99%) [82] .
The kinetically driven copper(I) - catalyzed cycloaddition of azides and alkynes
requires hours of reaction time to obtain quantitative yields [63] . However, in the case of
no - carrier added radiochemical synthesis the ratio of reactants and catalysts differs con-
siderably from that in traditional chemistry. In particular, the azide component and catalyst
are in huge excess compared with the [
18
F]fl uoroalkyne. The quantitative incorporation of
[
18
F]fl uoroalkyne could be achieved in 10 minutes when an optimized catalytic system
was used [81] . Reversed - phase HPLC analysis of all
18
F - labeled peptides showed only
a single product, indicating that the reaction proceeded regioselectively to yield 1,4 -
disubstituted 1,2,3 - triazoles as previously reported [64] .
In another study, coupling of [
18
F]fl uoroethylazide with various alkyne substrates that
included a peptide to form the corresponding 2 - [
18
F]fl uoroethyl - 1, 2, 3 - triazoles has been
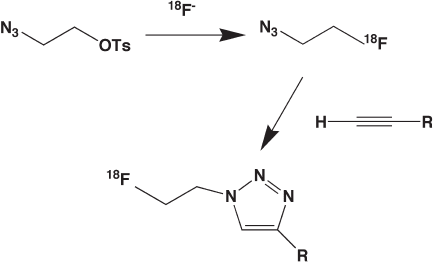
recently reported by Glaser and Arstad (Figure 14.10 ) [93] .
After nucleophilic fl uorination of 2 - azidoethyl - 4 - tolunenesulfonate, 2 -
[
18
F]fl uoroethylazide was reacted with a small library of terminal alkynes in the presence
of excess Cu(II) or copper powder (method A or B). The radiochemical yields were
Figure 14.9 Use of “ click ” chemistry of 1,3 - dipolar cycloadditions of [
18
F]fl uoroalkynes to
radiolabel N - (3 - azidopropionyl)peptides.
378 Fluorine in Medicinal Chemistry and Chemical Biology
measured by analytical HPLC 15 minutes post reaction at ambient temperature and also
after subsequent heating to 80 ° C for 15 minutes (Table 14.3 ) [93, 94] . At ambient tem-
perature, the degree of incorporation of 2 - [
18
F]fl uoroethylazide varied from no product to
greater than 98% yield of product, depending on the alkyne substrate and the catalytic
system. Following heating to 80 ° C for 15 minutes, nearly complete conversions of
2 - [
18
F]fl uoroethylazide to corresponding 1,2,3 - triazoles were observed for a majority of
the substrates. It should be noted that the “ click ” reaction has a high tolerance for other
functional groups in the terminal alkynes, including N - and C - terminal amides, which is
very attractive for labeling of peptides. With a suitable match of catalytic system and
alkyne substrate, high product yields can be obtained within a short reaction time under
mild conditions, which opens up the possibility of labeling fragile biomolecules that
otherwise cannot be labeled with fl uorine - 18 [95] .
The “ click ” chemistry has been rapidly adopted by radiochemists in
18
F - labeling.
There is a wide selection of
18
F - labeled prosthetic alkynes and azides, as well as com-
mercially available alkynes and azides, providing numerous possible combinations of this
azide – alkyne cycloaddition to form various
18
F - labeled biomolecules with numerous func-
tional groups. In addition to the continuously growing
18
F - labeled peptide synthesis [24,
81 – 84] ,
18
F - labeled folic acid, folates [96, 97] , glucose analogues [98] , oligonucleotides
[99] , and lipids [99] have been prepared using “ click ” chemistry. In all cases, 1,2,3 - triazole
formation was completed in less than 30 minutes at room temperature in aqueous media
in good yields. The products could be purifi ed using simple HPLC or solid - phase extrac-
tion with no multistep purifi cation method needed.
In conclusion, the Huisgen 1,3 - dipolar cycloaddition of terminal alkynes with azides
to form 1,2,3 - triazoles, referred to as “ click ” chemistry, provides a simple, fl exible, and
highly effi cient method for
18
F - labeling. This powerful linking reaction opens numerous
possibilities for combining alkynes with azides, forming all varieties of
18
F - labeled, highly
functionalized biomolecules. The improvement of peptide and lipid labeling using “ click ”
chemistry will further benefi t the
18
F - labeling of a variety of possible drug carries such as
dendrimers, micelles, microbubbles, liposomes, and cells. Nuclear medicine imaging will
be able to take advantages of the novel
18
F - labeling methodologies, where fl uorine - 18
could be simply clicked on these multifunctional vehicles without damaging other
Figure 14.10 Application of “ click ” chemistry of 1,3 - dipolar cycloaddition of [
18
F]fl uroethylazide
to a terminal alkene as a route to one - step radiolabeling of larger molecules.
