Ochiai E. Chemicals for Life and Living
Подождите немного. Документ загружается.


62
5 Clothing and Shelters: Polymeric Material
staff. That is a kind of compound containing SH group (ammonium thioglycolate is
commonly used), which reduces the –S–S– bridges and breaks them down to –SHs.
Now the cross-linkages have been removed, and the hair has become more flexible.
You will have it shaped in any way you want. When this is done, the next step is to
recreate the –S–S– bridges in new locations (new setting). Now the hair shape has
been fixed. This last step is chemically speaking “oxidation” and is usually accom-
plished by oxygen coming from hydrogen peroxide; occasionally an oxidant such as
sodium bromate might be used.
5.2.4 Natural Rubber
Rubber can be obtained from a number of trees, but commercial production is
done with tropical plant Hevea brasiliensis. Rubber is a polymer of a single
monomer isoprene (2-methyl butadiene). Isoprene itself can chemically (i.e.,
artificially) be polymerized by various means to produce polyisoprene, but this is
not the way the natural rubber is produced in rubber trees. The biological process
to produce rubber (i.e., biological polymerization) is shown in Fig. 5.5. This
process is a kind of condensation with removal of a pyrophosphate group
(P
2
O
6
).
CH
3
-C
CH
3
CH
3
CH
3
CH
2
CH
3
-C=CH-CH
2
=C-CH
2
-CH
2
-O-PO
2
-PO
3
=CH-CH
2
-CH
2
-C=CH-CH
2
-O-PO
2
-PO
3
+ HO-PO
2
-PO
3
H-(-CH
2
-C=CH-CH
2
-)
3
-O-PO
2
-PO
3
CH
2
=C-CH
2
-CH
2
-O-PO
2
-PO
3
CH
2
-CH
2
CH
2
-CH
2
CH
2
-CH
2
CH
3
CH
3
CC
-O-PO
2
-PO
3
CH
3
CH
3
(isoprene unit)
(natural rubber)
CH
3
CH
3
HHH
CH
3
CC
CC
Fig. 5.5 The biological synthesis of polyisoprene

635.3 Synthetic Polymers
5.3 Synthetic Polymers
A large number and quantity of polymers are synthesized and used today. They are
used for a variety of purposes: clothing, wrapping film, plastics, bottle for soda and
others, water piping, all kinds of small parts for automobiles and other machinery,
and CD/DVD.
An earlier motivation to produce polymers artificially was to produce natural
fibers like silk and/or to modify the natural material to desirable fibers. Cotton is a
very good fiber, but silk has some appealing characters such as luster and its soft
feel upon touch. But silk was much more expensive. People tried to convert cellu-
lose which was much more abundantly available to silk-like fiber. From this effort
came the first synthetic fiber, “rayon.”
In 1845, a Swiss chemist tried to treat cotton with nitric acid (and sulfuric acid).
The resulting material, nitrocellulose, had several interesting properties. Cotton itself
does not dissolve in water nor alcohol (ethanol), but nitrocellulose does. Solutions of
nitrocellulose were used to turn it into fibrous or plastic form. Plastic form is called
“celluloid.” It is still used for some purposes. An interesting property of nitrocellulose
is its explosiveness. It turned out that if all the three OH groups of glucose units in
cellulose are nitrated, the resulting nitrocellulose is very explosive (see Chap. 8 for
the explanation of explosive). But it is not explosive, though still quite flammable, if
the degree of nitration is less than two. In 1884, nitrocellulose was made into fiber and
sold as Chardonnet. Unfortunately, it occasionally burst into flame or even exploded.
Three British chemists discovered that cellulose could be solubilized when it was
treated with sodium hydroxide and carbon disulfide. The product is called cellulose
xanthate. The viscous solution was then extruded through a nozzle into an acidic
solution, forming lustrous, silk-like fibers. They patented the process and commer-
cialized the product in 1894. This is “rayon.” It is chemically still cellulose, but its
texture is different from that of cotton. It can be produced from not only from cot-
ton, but also from any pulp.
Almost a century later, scientists at Courtaulds’, a rayon manufacturer, chanced
to discover that cellulose could be dissolved when heated in a solvent, N-methyl
morpholine oxide. A new fiber (of cellulose) was produced from this solution. Its
brand name is “Tencel.” It has a luxurious look and feel and yet strong to be made
into clothes like jeans.
Rayon used to be called “art silk,” but the fibers mentioned above are not quite
artificial. They are modified products of natural material. Another effort to produce
silk-like fibers led to the invention of “nylon,” a completely synthetic (artificial) fiber.
5.3.1 Nylon
In 1928, an organic chemist at Harvard, Wallace Carothers, was appointed to the
director of organic research lab at the DuPont Chemical Company. He led a team to
study polymers, the chemistry of which was just emerging. In 1938, he and his team
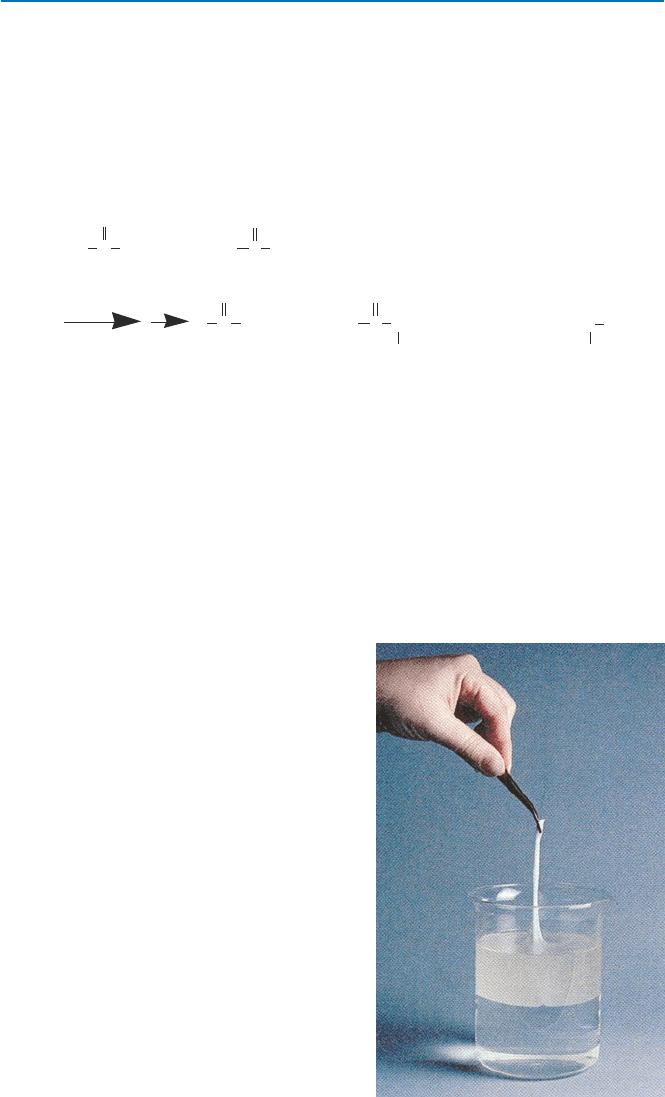
64
5 Clothing and Shelters: Polymeric Material
The repeating unit in nylon 66 is the combination of adipic acid and hexameth-
ylenediamine, both of which consist of six carbon atoms. Hence, the qualification
“66.” In each step as two molecules are connected, one molecule of water is removed.
This process is a typical condensation polymerization. Because an amide group on
one polymer chain can hydrogen-bond to that of the adjacent polymer chain as in
silk (see Fig. 5.4), nylon forms a structure similar to the b-pleated sheet. The forma-
tion of nylon 66 can readily be demonstrated as some of you might have seen
(Fig. 5.6). As you see, you can combine any compounds as long as one has amine
invented “nylon 66.” This is an entirely new, synthetic polymer, and the only
resemblance (in chemical terms) to a natural fiber, silk, is that the two monomers are
connected by a peptide bond as in proteins. The same type of bond, peptide, is
called “amide” in synthetic polymer chemistry, and hence nylon 66 is an example of
polyamides. Kevlar is another example of polyamide.
Nylon 66 is synthesized by the following condensation reaction between adipic
acid and hexamethylenediamine:
Fig. 5.6 Formation
of nylon 66 [from Atkins P
and Jones L, “Chemistry
Principles, 3rd ed.”
(W. H. Freeman and Co.,
2005)]
HO C
O
C
O
CH
2
CH
2
CH
2
CH
2
C
O
OH
H
2
N-CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
-NH
2
+
adipic acid
hexamethylenediamine
- H
2
O
CH
2
CH
2
CH
2
CH
2
C
O
N-CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
-N
)
H
H
n
(
nylon 66
amide (bond)
655.3 Synthetic Polymers
groups (NH
2
) at both ends and the other carboxylic acid group (COOH). Indeed,
many types of nylon have been synthesized, but only a few are still produced in
quantities.
One of these is nylon 66, and the other is nylon 6, which can be synthesized by
polymerizing a compound called caprolactam. This compound is first hydrolyzed
by water to form amino caproic acid. It is a kind of amino acid. Whereas natural
amino acids should be characterized as a-amino acid, as the amine group and the
carboxylic acid group bind to the same carbon atom, the amino caproic acid is said
to be e-amino acid, because an amine group is situated at e (5th) position from the
carboxylic group. The amine and carboxylic groups in two molecules of e-amino
caproic acid can condense (with removal of water) to form a dimer. Its continua-
tion will result in the formation of nylon 6 (see the reaction equation below).
Nylons are cheaper and much stronger than the natural fiber silk and yet give the
feel and touch of silk. Nylon made an instant success, particularly as hosiery
material.
5.3.2 Polyesters
Polyesters are produced by a chemically similar means; i.e., condensation polymer-
ization. For example, methyl ester of terephthalic acid and ethylene glycol are
polymerized by removing methanol (instead of water), and the poly(ethylene
terephthalate) results. This polymer can be made into a unique fiber; crease-resistant
fiber. As the hexagonal unit (benzene ring) is rather rigid, the fiber made of this
H
2
C
H
2
C
H
2
C
CH
2
CH
2
C=O
N
H
CH
2
CH
2
CH
2
CH
2
CH
2
C
O
N
CH
2
CH
2
CH
2
CH
2
CH
2
C
O
H
()
n
nylon 6
caprolactam
+ H
2
O
NCH
2
CH
2
CH
2
CH
2
CH
2
C
O
H
HOH
-amino caproic acid
H
2
N
COOCH
3
H
3
COOC
+ HOCH
2
CH
2
OH
H
3
COOC
COO CH
2
CH
2
OH
- CH
3
OH
H
3
COOC COO COO
CH
2
CH
2
OOC(
)
n
CH
2
CH
2
OH
polyethylene terephthalate (PET)

66
5 Clothing and Shelters: Polymeric Material
polymer can resist shape change. Once it has been set in a certain shape, it can retain
its shape, no matter how you treat it. Hence, it has been used for shirts that require
no ironing after washing: a convenience for travelers.
Today, however, the polymer is more often used as the material to make PET
bottle. In this case, the polymer is produced by heating terephthalic acid and
ethylene glycol with removal of water molecule. The reaction is similar to that
shown above, except that the CH
3
groups in methyl terephthalate should be
replaced by Hs.
5.3.3 Polymers Obtained by Addition Polymerization
Carothers of DuPont company also invented a synthetic rubber, chloroprene. This
polymer is produced by addition-polymerization unlike the polymers mentioned so
far. Let us see how it is done with an example of polyethylene. Polyethylene is used
in everyday life as a wrap, the thin plastic film. It is a polymer of ethylene, ethene in
technical terms. Ethylene has the chemical formula of CH
2
=CH
2
. Addition polym-
erization can be conducted with a compound having one or two double bonds; such
a compound is often called “olefin,” Ethylene is the simplest olefin. One of the two
bonds in a double bond is a s-bond and the other is a p-bond, which is weaker and
more readily splits than the s-bond. Alternatively, the electron in the p-bond easily
binds to an entity called a “free radical.” A free radical has an unpaired (single)
electron, which seeks another electron to pair up. The electrons in the p-bond pro-
vide such an electron. So what happens is this:
( )
""
2 2 22
.
R free radical, indicates an electron CH CH R-CH -CH
..
+= →
In this reaction, the entity R˙ is artificially produced or added and is called an
“initiator” (of polymerization). The resulting entity RCH
2
–CH
2
˙ now has an
unpaired electron, which can react with another molecule of ethylene:
22 2 2 2222
R-CH -CH CH CH R-CH -CH -CH -CH
..
+= →
This process can go on and on, making a long chain of –CH
2
–CH
2
– units; hence,
the product of such a polymerization is called “polyethylene.” Polyethylene is
essentially the same as the molecules in gasoline or wax; only the chain length is
enormous, often tens of thousands of ethylene units. [You might have noticed that
you smell wax when you burn polyethylene plastic wrap]. In this polymerization
process, you will note, no small molecule like water is removed as in condensation
polymerization, and a monomer is simply added to the growing end of a polymer.
Hence, polymerization of this type is called “addition” polymerization.
Derivatives of ethylene in which one of the hydrogen atoms is replaced by
another entity are called “vinyls.” For example, vinyl chloride is H
2
C=CHCl,
and vinyl acetate is H
2
C=CH(OCOCH
3
). The polymer of vinyl chloride is
poly(vinylchloride), PVC, which has been widely used for plastic cover and water pipe.
675.3 Synthetic Polymers
Other compounds of vinyl type that are used for polymers include acrylonitrile
(vinyl cyanide), H
2
C=CH(CN), and styrene, H
2
C=CH(C
6
H
5
). Vinyl compounds are
all polymerized by addition process. Tetrafluoroethylene, F
2
C=CF
2
, in which all the
hydrogen atoms of ethylene are replaced by fluorine atoms, polymerizes similarly
to form a polymer of unique character, teflon.
Another kind of monomer (repeating units) of interest is what is called “diene.”
Ethylene and vinyl compounds are so-called monoene, compounds that have in them
only one double bond, as you saw. Diene molecules have two double bonds in them.
Butadiene CH
2
=CH–CH=CH
2
, isoprene CH
2
=C(CH
3
)–CH=CH
2,
and chloroprene
CH
2
=CCl–CH=CH
2
are important examples of diene. We have seen isoprene before;
it is the repeating unit of natural rubber latex.
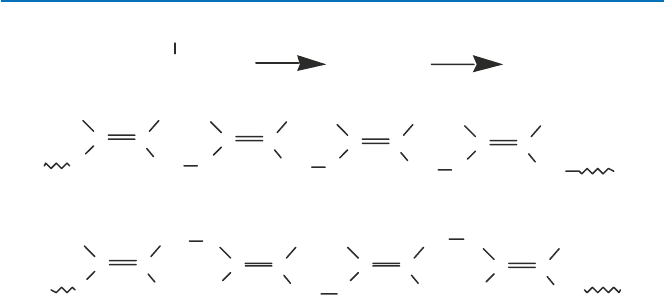
A monomer such as XCH=CH
2
can polymerize in different ways as illustrated in
Fig. 5.7. In the first example, the groups Xs are arranged in the same direction when
the monomers polymerize. In the second case, the directions of Xs alternate, whereas
they are random in the third case. The resulting polymers of different specific struc-
tures (stereospecific) may have different properties. For example, the first two types
of structure tend to crystallize well, as compared with the random one; hence, they
are more suitable for use as a solid plastic. The modern process of polymerization
can control the stereospecificity of polymer to a large extent.
In the case of a diene CH
2
=CX–CH=CH
2
polymerization, there are two orienta-
tions of polymer chain across the central C=C double bond of each monomer unit,
as seen in Fig. 5.8. In one, the polymer chain orients itself in the cis-direction (the
same direction) across the C=C double bond; this is called cis-polymer. In the other,
it orients in the trans-direction (the opposite direction); trans-polymer. The cis-
polymer usually behaves like a rubber, while the trans-polymer is more like
plastic.
X
CC
CC
C
C
CCC
+
+
C
CC CCCC
CCCC
CC C
CC CC CC CC C
CCC
C
CCCCCCCCCC
X
X
X
X
X
XX
X
X
XX
X
X
X
XX
X
XXXXX
H
H
H
H
HHHHH
H
HH
H
(head-to-tail connection)
(head-to-head connection)
(occasionally
(X’s are in the same direction)
(X’s in the aiternate direction)
(X’s direction: random)
HHHH H
HH
H
H
HH HHH
H
HH
HHH
HH
HH
H
H
HH
HH
H
HH HH
HH HH HH
HH
H
HH HH HH HH H
H
H
(X=CH
3
C1 etc)
or
or
H
Fig. 5.7 Stereospecific polymerization of a vinyl compound
68
5 Clothing and Shelters: Polymeric Material
Addition polymerization can be accomplished not only through a free radical
initiator as mentioned above, but also by some other means. The most important
polymerization catalyst is of the type known as Ziegler–Natta catalyst. These two
chemists discovered that a combination of chemicals titanium tetrachloride and tri-
ethyl aluminum is an excellent catalyst for polymerizing a number of olefins. They
were awarded Nobel Prize in 1963 for this discovery. Subsequent research by others
found that similar combinations of chemicals: a transition element compound and
triethyl aluminum or similar alkylating agent do catalyze polymerization of olefins.
Specific combination of such chemicals allow formation of polymers of specific
stereochemistry.
Ethylene is the simplest olefin. Propylene (propene) is the next simplest. The
polypropylene produced by a Ziegler–Natta catalyst turned out to be of the structure
of a special stereochemistry, and to form a fairly crystalline plastic. It was too hard
for most of applications and people who developed this polymer had to invent some
use for it. One of the uses they came up with was “hoola hoop.” This is an example
in which a product had been produced before they knew what they could use it for.
Is this a rare exception? Would not people know the uses for a product before they
decide to produce it? It is usually the case. However, there have been a number of
cases in which a product had been produced first for no good reason or other pur-
poses and only afterward they discovered its good application.
5.3.4 A Story of Vinyl Chloride
Let us talk about such an example. Paper industry needs a chemical called sodium
hydroxide (caustic soda) to clean the pulp. The most convenient way to produce
sodium hydroxide of a good quality is to use “electrolysis” of a brine solution con-
taining sodium chloride. That is, if you put electric current through the solution, you
can get a sodium metal which can easily be converted to sodium hydroxide by react-
ing the metal with a steam. The other product of the electrolysis of the brine solution
Fig. 5.8 Cis- or trans-polymerization
H
2
C=C-CH=CH
2
X
polymerize
X
CH
2
CH
2
H
X
CH
2
CH
2
H
X
CH
2
CH
2
HX
CH
2
CH
2
HX
CH
2
CH
2
H
X
CH
2
CH
2
H
X
CH
2
CH
2
H
C
C
CC CC
C
C
C
C
CC
C
C
C
X
CH
2
CH
2
H
Cis-polymer
Trans-polymer
C

695.3 Synthetic Polymers
is “chlorine.” Chlorine (Cl
2
) is used for bleaching the pulp and to disinfect drinking
water. It is also used for the production of chlorine-containing organic compounds
such as described in Chap. 16, but the production of these compounds has declined
for the reason of environmental concerns in recent decades. People did not know
what to do with the excess chlorine. One of the uses they came up with was to pro-
duce vinyl chloride. Hydrogen chloride that can be produced from chlorine easily is
made to react with acetylene; the resulting compound is vinyl chloride. Today, how-
ever, vinyl chloride is made from ethylene and chlorine.
Vinyl chloride is polymerized to produce polyvinyl chloride (PVC) as mentioned
earlier. It is cheap, and fairly stable and sturdy, and hence has been used very widely
for such purposes as plastic film, plastic cover, fake surface material (in any shade
like walnut) for wall and furniture, and water pipe.
It has some problems. The plastic loses plasticity and becomes brittle as it is
exposed to sunlight. This is due to a complicated series of chemical reactions ini-
tially caused by sunlight. Sunlight breaks one of the bonds, likely C–Cl, forming a
Cl-free radical, which abstracts hydrogen atom on the nearby carbon atom, likely
resulting in the formation of C=C double bond. The double bond then breaks, prob-
ably reacting with oxygen (O
2
) in the air under the influence of sunlight. Otherwise,
PVC is fairly stable and persists long in the environment.
Another problem is the formation of HCl when it is burned. It is argued that it
even forms the toxic dioxin when it is burned at high temperatures in the incinerator.
The biggest problem, however, seems to be that some toxic substances including
dioxin are produced as by-products in the process of producing vinyl chloride itself.
How to treat them and contain them is the problem.

Essential chemicals outlined in Part I are not sufficient to maintain health of living
organisms. A number of the so-called inorganic elements (chemicals) are indeed
required for proper workings of living systems; they are often called “mineral nutri-
tion.” Human bodies are often subject to health-disrupting causes, and remedying
such ill conditions of health requires often chemicals (as medicine) in addition to a
number of endogenous mechanisms to combat such disruptions present in the
human body.
Part II
Enhancing Human Health
