Ochiai E. Chemicals for Life and Living
Подождите немного. Документ загружается.


32
3 Life Itself (A): How Do We Get Energy to Live by?
use Roman numerals to indicate the oxidation state of an atom in a chemical compound,
and Arabic numerals for the electric charge of a chemical entity. A convention used
in many textbooks is to use Arabic numerals to express both the oxidation state of
an atom and the electric charge of a chemical species; and this is confusing].
Another convention (rule 2) is that hydrogen atom in all compounds (except
when it binds to a metallic element) has +I oxidation state. So the oxidation state of
carbon in methane, CH
4
, must be −IV. How about the oxidation state of the carbon
in methanol, CH
3
OH? There are four hydrogen atoms, which contribute +4, and the
oxygen carries −II; therefore, the carbon must carry “−II.” How about the oxidation
state of carbons in ethanol C
2
H
5
OH? [the answer is “−II”]. The carbon atoms in
acetic acid, CH
3
COOH, the ingredient of vinegar, should be “0, zero” in oxidation
state [try it]. Carbon dioxide CO
2
has its carbon in +IV oxidation state. As you see
here, carbon atoms in compounds can take different oxidation states, from −IV to
+IV. “−IV” is the most reduced state and “+IV” is the most oxidized state of carbon.
So, for example, in the process of reaction:
4 23
CH (1 / 2 )O CH OH+→
, the carbon
atom can be regarded to have lost two electrons (try to see it) and hence to have been
oxidized. Addition of oxygen to a compound is usually regarded as “oxidation”
reaction. The chemical agent that oxidizes others is defined as “oxidizing agent.” Of
course, oxygen (O
2
), the oxidizing agent itself, is reduced, starting from “zero” and
becoming “−II”; so the other chemical that reacts with oxygen can be said to be a
“reducing agent.”
[Note: The oxidation state does not necessarily represent the actual electric
charge, though it does so in certain cases including that in ionic compounds. It is a
convenient way to keep track where and how electrons move in oxidation–reduction
reactions].
Let us look at nitrogen cases, using rules 1 and 2. N in ammonia NH
3
is −III, the
most reduced. N in N
2
is obviously zero. N in nitrate, NO
3
−
? Try it. [“+V” is the right
answer]. So the reaction to form ammonia:
22 3
N 3H 2NH+→
is a reduction as far
as nitrogen is concerned, and the reducing agent here is hydrogen which is formally
oxidized. To produce nitrate from ammonia you have to oxidize NH
3
; that is,
3 2 32
NH 2O HNO H O.+→ +
The oxidation state of N in NH
3
is −III and that in
HNO
3
is +V. Therefore, eight electrons have moved from the nitrogen atom to
oxygen atoms in this process.
Your car can rust; it is the oxidation of the iron of your car by oxygen in the air
to form iron oxide. It can be expressed formally as
2 23
4Fe 3O 2 Fe O+→
. In this
process, iron changes its oxidation state from “zero” to +III. Thus, iron loses elec-
trons (be oxidized) to oxygen. Iron can be also in +II oxidation state; that is,
2
2Fe O 2FeO+→
is also possible. As a matter of fact, iron can change very readily
its oxidation state between +II and +III. And this change is very widely used in
biological systems (see Chap. 6).
[Note: Rule 3 (of determining oxidation states) Halogen, F, Cl, Br, or I takes −I
oxidation states in compounds bound with other elements; for example, C is C
−II
,
and H
+I
and Cl
−I
in CH
3
Cl. Exception is when they bound with each others; in this
case, the lighter element carries −I oxidation state. For example, in FCl F is F
−I
and
Cl
+I
, whereas Cl
−I
and I
+III
in ICl
3
].

33
3.2 Reactions of Oxidation–Reduction Type
Oxidation–reduction reactions tend to be accompanied by larger free energy changes
than substitution reactions of acid–base type mentioned earlier. Oxidation reactions,
particularly reactions with oxygen, give off a lot of energy. Biological systems convert
this energy to that stored mainly in ATP, but also in carbohydrates in certain organisms.
The major energy-producing foodstuff is carbohydrates, which is exemplified by glu-
cose C
6
H
12
O
6
. What is the oxidation state of carbons in this compound? “Zero.”
It (carbon in this molecule) can be oxidized eventually to carbon dioxide CO
2
(+IV
oxidation state). This reaction is not a simple single step reaction. It can be defined as
“energy-yielding metabolism” (of carbohydrates) and consists of many steps.
The earlier part of this entire process does not actually require oxygen; this part
is called “glycolysis” (a fermentation). Oxidation in this part is done by removing
hydrogen atoms. That is, removal of hydrogen can also be regarded as “oxidation.”
Fermentation of glucose produces pyruvic acid CH
3
(CO)COOH. The reaction can
be represented formally as:
6 12 6 3 2
()C H O 2CH CO COOH 2H→+
. Let us use the
rules outlined earlier and figure out the oxidation state of carbons in this product.
It is +2/3 on each of carbon. Do not worry about the fractional value; the oxidation
state of an individual atom should be an integer, and different carbon atoms in this
molecule should be assigned different oxidation states. But all we need now is an
average value, which in this case is a fractional value. OK, you started with C
6
H
12
O
6
where the oxidation state of carbons is “zero,” and now ended up with CH
3
(CO)
COOH in which the oxidation state of carbons is +2/3. So the carbon atoms must
have lost electron(s); i.e., this is an oxidation. This reaction would give off energy
of about −76 kJ (per mole of glucose). The net yield of ATP in glycolysis is 2 mol
of ATP from 1 mol of glucose.
As the oxidation state of carbon in pyruvic acid is +2/3, it can be oxidized further
(up to +IV). This is done in the subsequent respiratory process using oxygen as the
ultimate oxidizing agent. First, it goes through a process called “TCA” cycle (tricar-
boxylic acid cycle) or “citric acid cycle,” which produces FADH
2
(through succinic
acid) and NADH in addition to some ATPs. FAD is flavin adenine dinucleotide and
similar to NAD; FADH
2
is the reduced form of FAD. The special hydrogen atoms in
FADH
2
and NADH are further oxidized in the final process of respiration (called
“electron transport/oxidative phosphorylation”) to be turned completely into water
(FADH
2
and NADH turns to FAD and NAD
+
, respectively). A number of ATPs are
produced in this last process. Overall, 38 mol of ATP are produced from 1 mol of
glucose. However, only 36 mol of ATP will be produced from 1 mol of glucose in
brain and muscle cells, because of a slightly different type of metabolism predomi-
nates in these cells.
Let us examine the relationship between energies gained in the respiratory pro-
cess and the simple combustion. Suppose that you conduct the following combustion
reaction in water solution:
6 12 6 2 2 2
()C H O glucose 6O 6CO 6H O+→ +
, the associ-
ated free energy change is −2,872 kJ per mole of glucose (180 g). As argued before,
1 mol of ATP will produce about −50 kJ of free energy; therefore, the total free
energy converted in the form of ATP amounts to 40 × (−50) = −2,000 kJ (2ATPs
produced in glycolysis is included). This is 69.6% of the simple combustion energy.

34
3 Life Itself (A): How Do We Get Energy to Live by?
In other words, the efficiency of energy conversion in the mitochondria is 70%,
more than two thirds, which is very good.
In another process similar to citric acid cycle, NADPH is produced instead
of NADH. NADPH is used not to produce ATPs but to reduce some important
biocompounds, such as sulfate and nitrate, and ribonucleotides (to produce the
raw material of DNA) and produce some important biological compounds such
as lipids.
Proteins and lipids are also degraded and partially used to make ATP. A lipid, for
example, fatty acid CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
COOH, has the carbons in
low oxidation state [try to calculate it; −16/10]. So when it is oxidized, it will give
off more energy (per carbon atom) than carbohydrates. That is why fat gives more
calories.
A small number of organisms use compounds other than oxygen, as the oxidiz-
ing agent. Nitrate (NO
3
−
) is a good oxidizing agent (see Chap. 8 on the fireworks)
and is used for example by E. coli when not enough oxygen is available.
The things are getting messier. So let us summarize here what we talked about so far.
The free energy in oxidizing glucose (net reaction:
6 12 6 2 2 2
C H O 6O 6CO 6H O+→ +
)
is utilized to convert ADP to ATP, the biological energy carrier. ATPase, one of the
crucial enzymes, that is involved in the process of converting ADP to ATP (the
reverse reaction in Fig. 3.1) was studied among others by Paul D. Boyer of UCLA,
and John E. Walker of Research Council of Molecular Biology in Cambridge, UK.
These two scientists shared a Nobel Prize in 1997, with Jens C. Skou who studied a
related enzyme, Na(I),K(I)-ATPase. The details of how ATPases work are beyond
this discourse.
3.2.2 Reduction
We obtain carbohydrates, our energy source, from plants. Plants produce them
from carbon dioxide and water. The overall reaction can be expressed as
2 2 6 12 6 2
6CO 6H O C H O 6O .+→ +
This is opposite of the oxidative metabolism of
glucose mentioned above. The reaction can be regarded to be a reduction of
carbon dioxide by hydrogen that comes from water. Since oxidation of glucose is
energy releasing, the formation of glucose from CO
2
and H
2
O must require a lot
of energy (energy absorbing). Plants use “energy from sunlight” to accomplish this
feat, synthesis of carbohydrates. Hence, the process is called “photosynthesis.” The
details of photosynthesis are too much to talk about here. But essentially, sunlight
forces electrons out of the green pigment, chlorophylls in the first part (called
“photosystem I”) of the photosynthetic machinery in a green leaf of plants, and the
electrons are to be added to carbon dioxide (remember that an addition of electrons
to a compound is “reduction”). Another part (photosystem II) of the machinery
contains a mechanism to decompose water to produce oxygen O
2
and electrons.
This process also uses the energy from sunlight. And the electrons produced in the

35
3.3 Chemical Logic of Life on Earth
photosystem II are then transferred to photosystem I to replenish the consumed
electrons. The photosynthetic apparatus is contained in a vesicle called “chloro-
plast” in cells of green leaves and produces some ATP molecules in addition to
carbohydrates.
A small number of bacteria use hydrogen sulfide (H
2
S) instead of water (H
2
O) as
the electron source. These are called “sulfur photosynthetic bacteria” and produce
carbohydrates, as well as ATP, and deposit sulfur (S) in the surroundings. Organisms
that use sunlight to produce their food and energy, i.e., both water-decomposing
ones (cyanobacteria and plants) and sulfur photosynthetic bacteria, are called “pho-
toautotrophs.” Some special microorganisms conduct photosynthesis using Fe(II) as
the source of electrons.
Yet other organisms use chemical energy (instead of energy from sunlight) to
produce carbohydrates and ATP. For example, some microorganisms oxidize iron,
Fe(II) to Fe(III) by oxygen. This oxidation produces some energy, and the
organisms use this energy to synthesize carbohydrates from carbon dioxide and
water. Others oxidize ammonia (NH
3
) (to N
2
and NO
2
−
, NO
3
−
) or some organic
compounds using oxygen as the oxidizing agent. These organisms are called
“chemoautotrophs,” as they use the energy of chemical reactions to produce their
own food and ATP.
There are several other important reduction processes. One is the reduction of
sulfate (SO
4
2−
) to produce hydrogen sulfide (H
2
S). Hydrogen sulfide is then
incorporated into organic compounds such as cysteine (one of the amino acids) and
glutathione. Nitrate (NO
3
−
) likewise is reduced to ammonia in most organisms.
Ammonia is then incorporated into organic compounds such as proteins and DNAs.
Ammonia can also be produced by reducing the nitrogen molecule in the
atmosphere. The overall reaction
22 3
N 3H 2NH+→
does not require energy input;
i.e., the free energy change is slightly negative. However, both the industrial and
biological processes require a large amount of energy input to effect this reaction.
The industrial process operates at high temperature and high pressure, and it
requires energy. The biological process is catalyzed by an enzyme called nitroge-
nase, which operates under an ordinary condition, ambient temperature and
pressure, but requires a lot of energy in the form of ATP to carry out the reduction.
Nitrogenase (see Fig. 21.12 for the molecular structure) is found in a number of
microorganims such as Azotobacter, Chromatium (photosynthetic), anaerobic
Clostridium, and bacteria called Rhizobium found in the nodules on the roots of
plants such as alfalfa, clover, peas, and beans, as well as some fungi. These
processes contribute to the global cycling of nitrogen.
3.3 Chemical Logic of Life on Earth
Let us now see the overall picture of the chemistry involved in the life process
discussed in the last two sections. Figure 3.2 provides such a picture. The ulti-
mate source of energy of our lives and all our activities is Sun. It drives the
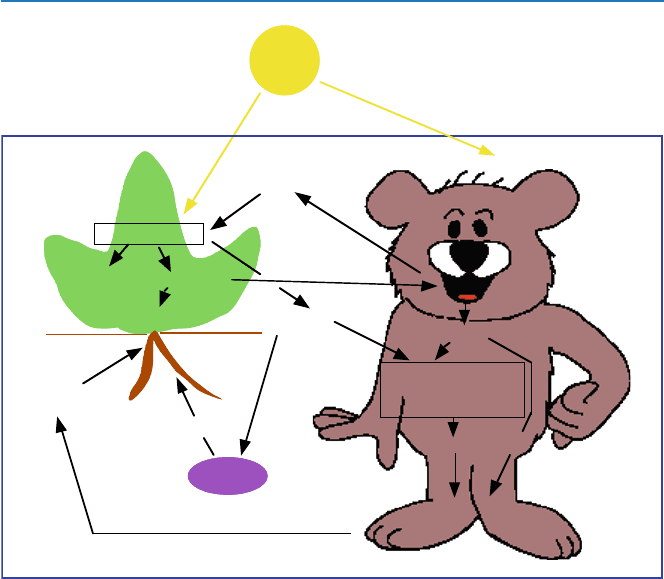
36
3 Life Itself (A): How Do We Get Energy to Live by?
energy-consuming photosynthesis, which produces ATP, the chemical energy
carrier, and carbohydrates. Carbohydrates are the energy source for animals.
Carbohydrates are degraded with use of oxygen (oxidation), and the energy
released in the process is converted into the chemical energy in the form of ATP.
Other accessory reactions are also indicated.
Figure 3.3 is a diagram with essentially the same information, but gives a little
more details and is intended for audience more chemically inclined. It shows the
overall life process on the earth. Three types of organisms are indicated: autotrophs,
heterotrophs, and some special types of bacteria and fungi as shown in different
colors. Major inorganic compounds are shown as cycled throughout this system.
Biological functions of some inorganic compounds are talked about in Chapter 6.
photosynthesis
ATP carbohydrates
proteins, dna's
light
NO , SO , PO
344
-
2-
3-
NH
3
digestion
glycolysis-citric acid
cycle-oxidative
phosphorylation
proteins, lipids
rna's and dna's
ATP, NADPH
CO , H O
22
EARTH
Energy input
energy-
consuming
energy-
releasing
SUN
O
2
bacteria
N
2
energy-
consuming
Fig. 3.2 Chemical logic of life on earth
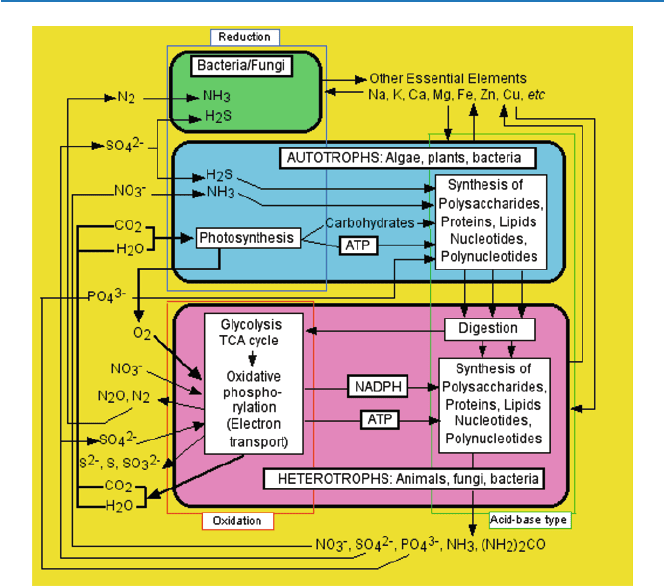
37
3.3 Chemical Logic of Life on Earth
Fig. 3.3 Types of chemical reactions involved in life process on Earth (chemical logic of life on
Earth)
The main biological processes are shown in boxes of thin line. The three categories
of biochemical reactions are indicated as boxed in different colors. The yellow
colored background represents solar energy.

39
E. Ochiai, Chemicals for Life and Living,
DOI 10.1007/978-3-642-20273-5_4, © Springer-Verlag Berlin Heidelberg 2011
Besides “being alive,” the characteristic of living organisms is to produce progenies. And
the progenies are like their parents. A human being begets human beings; a frog
begets tadpole(s), which eventually become frog(s). A single E. coli bacterium cell
divides into two identical E. coli bacterial cells. Something must be transmitted
from a parental organism(s) to its progeny to direct the progeny’s cells to become
similar to its parent(s). This something is a “gene,” and the gene will govern the
characters of the organisms. This is the central dogma of “Life on the Earth.” There
are two things that a gene must do. One is that it should be able to duplicate itself or
rather should be able to dictate its own duplication (replication). The second is that
it must contain enough information to replicate the whole organism of which the
gene is a part. Let us see how this is done in terms of chemistry.
It turned out that the chemical principle behind these functions of a gene is very
simple and of a very fundamental chemistry. The gene had been known to be a
chemical molecule called DNA. DNA is an acronym for DeoxyriboNucleic Acid.
However, it took a long time and an enormous amount of human endeavors to
unravel the secrets of how DNA works as a gene. The efforts by scientists in this
respect had culminated in a discovery of the so-called double helix structure of
DNA by two (then) young scientists, James Watson and Francis Crick in 1950s.
They were awarded a Nobel Prize for the discovery in 1962 (along with M. H. F.
Wilkins). The story of this discovery is very well known and has been told over and
over again by many people including Watson himself. So we will not repeat it. We
will look at the very fundamentals.
4.1 Structure of DNA
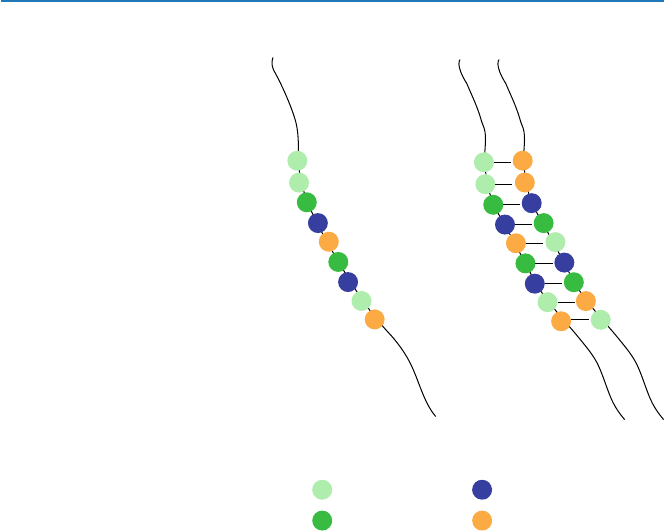
A DNA molecule is a long chain-like chemical consisting of four units called
“nucleotides” labeled as A, C, G, and T. You may visualize it as, say, a necklace
made of beads of four different colors, aquamarine (A), green (G), cobalt blue (C),
and tan (T) (Fig. 4.1a). A necklace may consist of at most hundreds of beads, but a
DNA molecule may be made of hundreds of thousands or even millions of these
4
Life Itself (B): Why Are We Like
Our Parents?
40
4 Life Itself (B): Why Are We Like Our Parents?
beads. It is a very, very long strand. Remember that the actual DNA molecule is
made of atoms that are very, very, very small. For example, the gene (a chromosome)
of a bacterium E. coli is made of about five million beads (nucleotides), which
is about 1.6 mm (one sixteenth of an inch) long when stretched. This is visible to
human eyes. You may consider this to be short in view of everyday things, but it
is enormously long in the world of individual molecules. This strand of beads
functions as a sort of recording tape, and the information on the tape is thus written
in those four letters, A, C, G, and T. In contrast, the information on a digital
recording tape, CD, or computer diskette is written by two letters, i.e., 0 and 1.
It turned out that the functioning DNA is a double strand. That is, it consists of
two complementary strands, and this is the basis of the functions of DNA. Bead A
specifically binds laterally with bead T, and G with C. Suppose that beads (a portion
of a DNA) are arranged in the order of AAGCTGCAT on a strand, then on the other
will be the complementary sequence TTCGACGTA, as seen in Fig. 4.1b.
The well-known structure of a DNA is double helix (there are other types of
structures known.). The “double” portion means “double-stranded” as shown in
Fig. 4.1b and is essential to the functioning of DNA. The “helix” portion is nones-
sential to the functioning of DNA, but is a result of chemical nature of the molecule
and is also important in storing DNA. A DNA in the nucleus of eukaryotic cells
coils up and then coils up further (supercoiled). As a result, its overall volume is
reduced so that it can be confined in a small volume of nucleus. DNA cannot do this
by itself. Some kinds of protein assist DNA in coiling up.
A Single Strand of
DNA (bead model)
ab
A Double Strand
of DNA
A (=adenosine)
G (=quanosine) T (=thymidine)
C (=cytidine)
Fig. 4.1 DNA model
414.1 Structure of DNA
So the crucial question is why those beads (nucleotides) bind specifically. This is
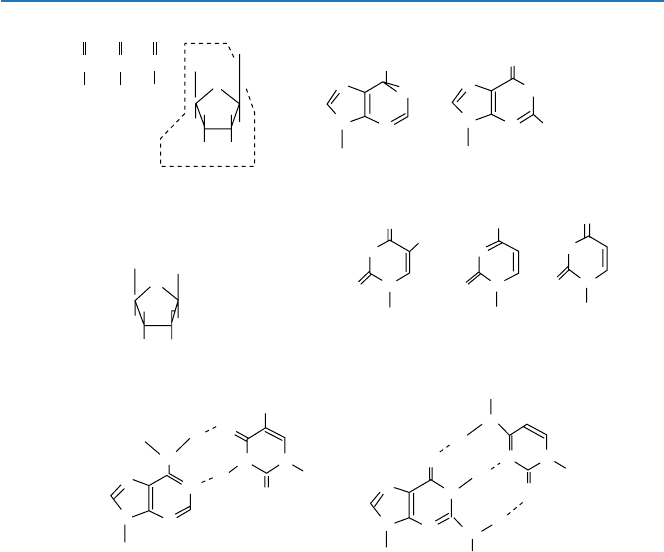
a straight chemistry. We have to see the chemical structures of those components
(called deoxyribonucleotides; nucleotides for short): A, C, G, and T of DNA. Each
of these consists of three parts: (deoxy) ribose, phosphate, and base (Fig. 4.2a). The
first two are common to all nucleotides and make up the backbone of DNA. There are
four different bases used in DNA. They are A = adenine, C = cytosine, G = guanine,
and T = thymine. They have chemical structures as shown in Fig. 4.2b. Adenine and
guanine belong to a class of compounds called purine base and have similar struc-
tures. Cytosine and thymine are pyrimidine bases. These compounds use nitrogen
and oxygen, in addition to carbon and hydrogen. Oxygen and/or nitrogen atoms in a
compound can bind relatively strongly to oxygen and/or nitrogen atoms in another
compound through “hydrogen bond.” “Hydrogen bond” is typically formed between
water molecules (H
2
O), as was talked about in Chaps. 1 and 19. It turned out that
adenine binds with thymine most comfortably through hydrogen bonds and that
guanine does so with cytosine, as shown in Fig. 4.2(b). The other combinations such
A–C or G–T are possible, but these combinations are not as strong as the standard
combinations A–T and G–C.
Hydrogen bonds (...) formation between A and T (U), and between G and C
Adenine
Deoxyribonucleotide (of DNA)
Thymine
(Uracil)
Cytosine
Guanine
Thymine
CH
3
NH
2
CH
3
O
N
N
H
H
ribose (in RNA)
OH
HO
H
H
H
H
O
-CH
2
(phosphate-deoxyribose-base)
H
N
N
N
N
N
O
NH
2
NH
2
O
N
N
N
N
N
N
N
N
NH
O
O
N
N
N
HN
H
N
H
O
O
N
N
N
N
N
NN
H
H
H
O
O
O
HN
used in DNA
used in RNA
Guanine
Adenine
BASE
Base (purine)
Base (pyrimidine)
Cytosine
Uracil
deoxyribose
(tri)phosphate
HO H
H
H
HH
O
O
-
O
-
O
-
O
--
P
-
P
-
P
-
O
-
O
-
O
-
CH
2
O
O
O
Fig. 4.2 Structures of nucleotides
