Ochiai E. Chemicals for Life and Living
Подождите немного. Документ загружается.


262
21 Are Atoms and Molecules for Real? Can We See Them?
Fig. 21.2 An electronmicro-
gram of DNA molecules
Fig. 21.3
HRTEM image
of potassium iodide (K)
embedded in a carbon
nanotube. As the inset
indicates, the atoms of K
+
and
I
−
are clearly seen as separate
balls (from R. R. Myer et al.,
Science, 289 (2000),
1324–1327)
Fig. 21.4 HRTEM image of
strontium titanate (SrTiO
3
);
atoms of Sr, Ti, and O are
seen as separate balls (from
Z. Zhang et al., Science,
302 (2003), 846–849)
26321.2 Scanning Probe Microscopy
21.2 Scanning Probe Microscopy
Figures 21.2–21.4 illustrates the best you can do with the electron microscope.
In 1980s, two scientists, Heinrich Rohrer and Gerd Binnig at IBM’s Zürich Research
Center in Switzerland developed a revolutionary method of microscopy. They were
awarded a Nobel prize in 1986 for this invention. The principle is simple, though
technologies required to realize them are quite advanced.
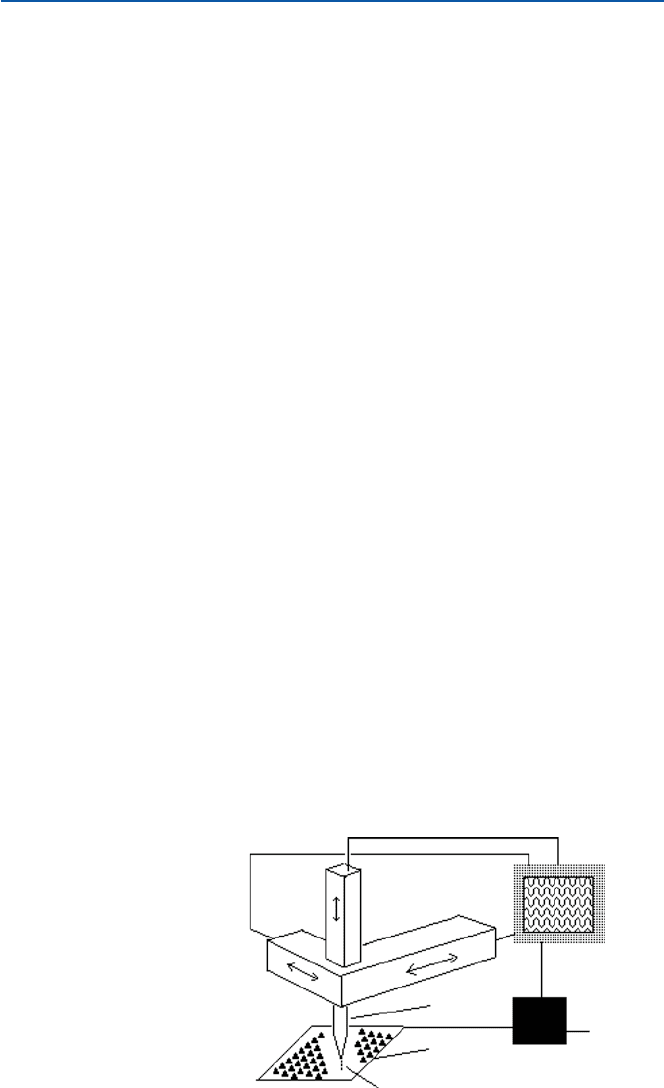
Figure 21.5 illustrates the principle of scanning tunneling microscope [STM,
a kind of scanning probe microscopy (SPM)]. The probe tip is sharpened so that it
is made of a single metallic atom (to make such a probe is not an easy task). A solid
sample (a metal or a semiconductor) is placed flat and the probe tip is brought very
close to the surface of the sample. If the probe comes within a few atomic distance
from the atom just below (of the sample), a strange thing happens. Electrons jump
over that distance from the atom of the probe to that of the sample. This phenome-
non is known as “tunneling” (of electron). This corresponds to a flow of electric
current between the probe and the sample, though they are not in contact. It is
known that the electric current flow due to tunneling is dependent on the distance
and the nature of atoms of probe and sample.
The probe is scanned across the surface of the sample, and the height of the probe
is adjusted so that the tunneling current flow is kept constant as it is being scanned,
and the height can then be recorded as the probe is scanned all over the surface of
sample. This movement of the probe must necessarily be minute and precise and is
accomplished by a devise based on piezoelectricity. The trace of height of the probe
may represent the shape and arrangement of atoms and vacancies in between. The
scanning result can then be treated by a computer and be visualized.
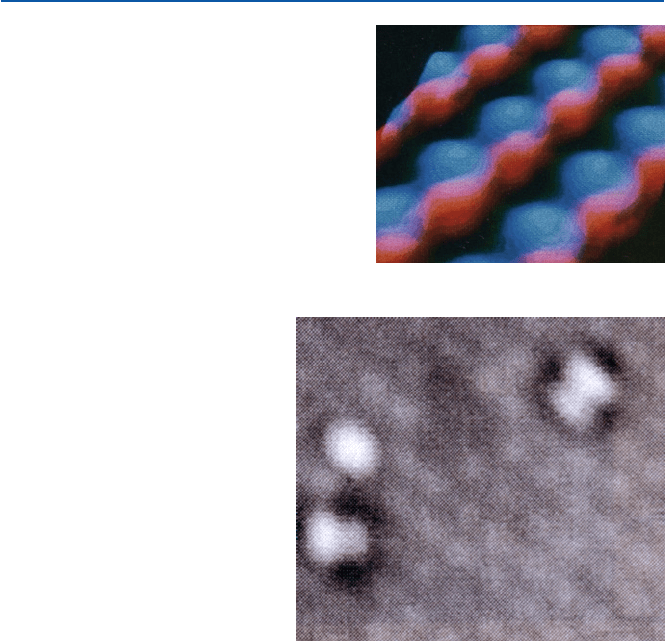
Such an example as shown in Fig. 21.6 then can be regarded as that of the
atomic arrangement on the surface. This is gallium arsenide (GaAs); the blue rep-
resents arsenic atoms and the red gallium. Each hump represents a single atom.
This is the way chemists had pictured the structure of this compound (and others)
long before a devise like STM gave them the picture of atoms in a substance.
In other words, the chemists’ depiction of atoms and molecules was not very bad.
Piezoelectric
control
Probe
Sample
Tunneling current
Feedback
generator
Reference
signal
Display
Fig. 21.5 A schematic
presentation of STM
264
21 Are Atoms and Molecules for Real? Can We See Them?
By the way, gallium arsenide is expected to function as a semiconductor like
silicon, which is the basis of the today’s high-technology industry. The diameters
of gallium and arsenide can then be estimated from this result. They are about 0.2
and 0.4 nm (2 and 4 Å), respectively, and are in agreement with the data obtained
by other methods. These results suggest that the resolution of STM is of the order
of 0.01 nm (=0.1 Å = 10 pm).
Astonishingly, a small molecule like dioxygen O
2
, which is the form of oxygen
found in the air, has also been imaged by STM. Figure 21.7 shows two O
2
molecules
bound (adsorbed) on the surface of silicon. The extra blob just above the oxygen
molecule at the left-hand corner is a defect in the underlying silicon crystal. As you
see, the dioxygen molecule looks like a dumbbell; i.e., each dioxygen molecule is
made of two spheres bound together. These molecules do not look like being bound
flat to the surface, but being bound slanted. The sphere that represents an atom is
actually the shape of the electron cloud around the nucleus. Again this is the same
as the picture chemists have been using to represent the oxygen molecule O–O.
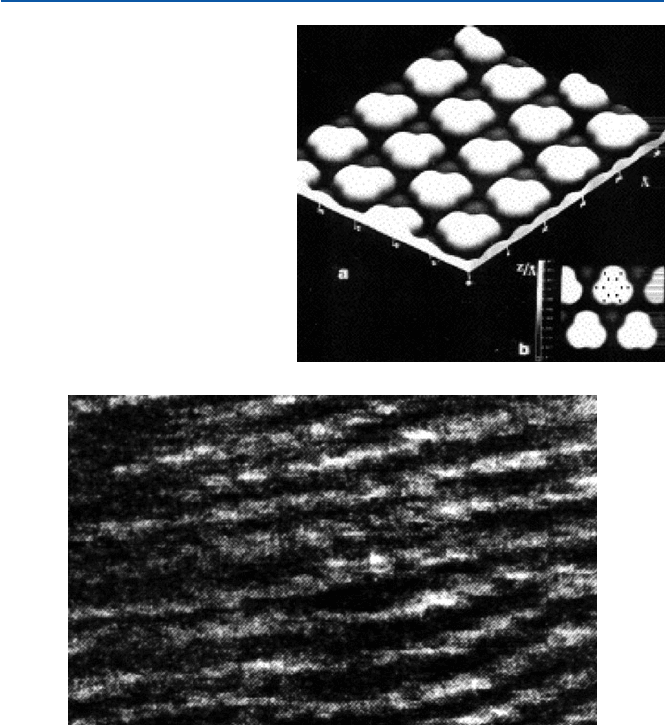
Figure 21.8 gives an STM image of benzene molecule adsorbed on a rhodium metal
surface. The positions of six carbon atoms and six hydrogen atoms are shown by
Fig. 21.7 STM image of
dioxygen molecule (from B. C.
Stipe et al., Science, 279
(1998), 1907–1909)
Fig. 21.6
STM image of
gallium arsenide (GaAs) (from
D. P. Kern et al., Science, 241
(1988), 936–944)
26521.2 Scanning Probe Microscopy
small dots in the inset portion. Because it is adsorbed on a metal, it is slightly dis-
torted from the free benzene molecule. However, the overall picture corresponds
well to the structure that chemists have been imagining based on the theories.
STM is but an example of scanning probe microscopes. Another important scan-
ning probe microscope is called atomic force microscope (AFM). When a probe is
brought close to a sample, a force will be exerted between the probe and the sample.
If this force (atomic force) is of a general character, i.e., London dispersion force,
then the force will be dependent on the distance and the nature of the probe and the
atomic nature of the sample. Hence, a geometrical structure of a sample will be
imaged at atomic level by scanning such a probe that responds to atomic force.
The double helix structure of a DNA has been imaged by AFM. That is shown in
Fig. 21.9. A DNA molecule looks like a right-handedly wound ropes. The ropes are
the (alpha) helically coiled double strand. These images (Figs. 21.6–21.9) give us
Fig. 21.8 STM image of
benzene molecule (from P.
Saulet and C. Joachim, Chem.
Phys. Lett., 185 (1991), 23)
Fig. 21.9 AFM image of DNA (from J. Mou et al., FEBS Lett., 371 (1995), 279–282)

266
21 Are Atoms and Molecules for Real? Can We See Them?
very good pictures of molecules with atomic level resolution in some cases, and
really convince us that, yes, atoms and molecules do exist, and moreover, that they
do look like how chemists have been saying they do. What more do you need to be
convinced?
21.3 X-Ray Diffraction: Atomic Structure of Large Molecular
Compounds and Ionic Compounds
The scanning probe microscopes are still unable to resolve atomic structures of such
complicated large molecules as proteins and DNAs. The atomic structures of these
large molecules (as well as smaller molecules and ionic compounds) are now almost
routinely determined by X-ray crystallography. This technique is much older than
SPM. However, the way this technique produces the atomic structure of a molecule is
not as straightforward as electron microscopy or scanning probe microscopy. Yet, the
structures obtained by this techniques, particularly those of smaller molecules and ionic
compounds, have well been verified by many other methods and experience. Therefore,
the atomic structure of a large molecule obtained by this method is also now well
believed to represent the correct structure of the compound. It is true, though, that the
structure is valid only for the solid crystal state to which this method is applied.
We will explain very briefly how it is done with a few examples. X-ray is a light
of very short wavelength. For example, when an electron beam is directed to a copper
metal, the copper metal produces an X-ray of 154 pm (1.54 Å). This is the source of
light (X-ray, that is). The light (any light) shone on a compound will be scattered by
the electron clouds (of atoms) of the compound. When the atoms are arranged regu-
larly as in a crystal, this scattered light will show a pattern. This is called diffraction
pattern, and is related to the crystal structure. The diffraction pattern can be photo-
graphed or now digitally recorded. The pattern consists of spots (where the diffracted
X-ray hits) of differing intensities. The intensity of a spot is related to the electron
cloud density that scatters X-ray. (The more dense electron cloud scatters X-ray more
strongly; thus the spot will be more intense). Therefore, we can reconstruct how the
electron clouds are distributed throughout space (in a crystal) by analyzing the dif-
fraction pattern including intensities. The result can be expressed as an electron den-
sity map (in three dimensions), and that can then be reinterpreted as an aggregate of
atoms. Softwares have now been developed to convert the diffraction pattern to the
atomic arrangements in a molecule. The resolution obtained is now routinely about
1.5–2 Å (150–200 pm = 0.15–0.2 nm). Hence, we can distinguish atoms in molecules,
as the inter-atomic distances in molecules are around 1–2 Å (0.1–0.2 nm).
The actual process is now fairly automated, but one of the basic problems is to
obtain a single crystal of an appropriate size. Many biologically interesting samples
such as proteins and DNAs are difficult to crystallize, and researchers would spend
days, months, and even years to get single crystals suitable for X-ray crystallographic
studies. Many other problems prevent X-ray studies of crystal structures from becom-
ing a truly routine technique. But it is not appropriate to pursue this issue further here.
Below we display a few protein structures (Figs. 21.10–21.12) determined by
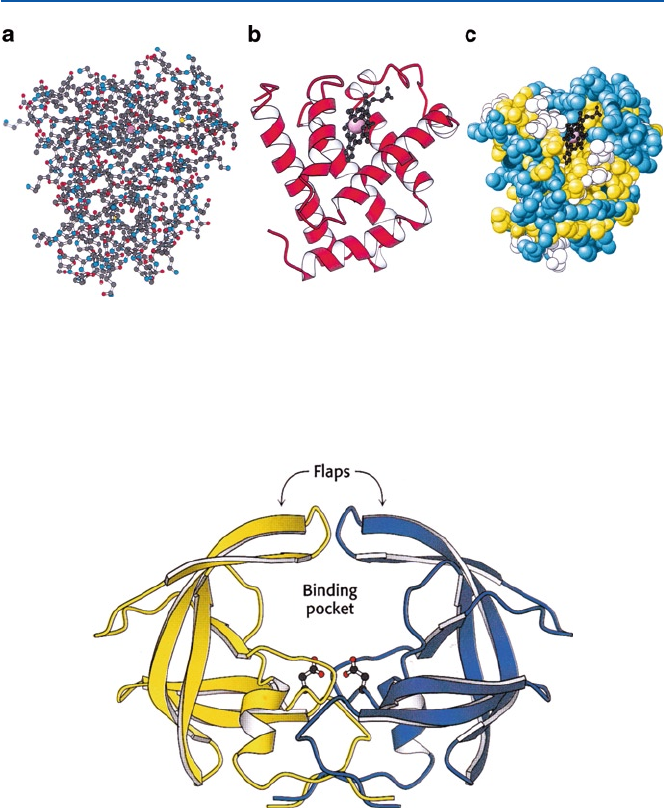
X-ray crystallography. Figure 21.10 shows three different modes of expressing
267
21.3 X-Ray Diffraction: Atomic Structure of Large Molecular Compounds…
a protein structure. Mode (a) is called “ball-and-stick” representation, and uses colored
dots to indicate the position of an atom where different colors represent different ele-
ments, and the bonds are shown as sticks. In mode (b) a helical strip tape is used to
schematically represent a helical structure and a string is a random coil portion.
Another major secondary structure is b-strand, and it is represented by flattened tape
as seen in Fig. 21.11. In mode (c), amino acids of different characters are represented
by colored balls; in this case yellow: hydrophobic amino acid, blue: hydrophilic
(electrically charged) amino acid and white: others. An account of the protein struc-
ture, including the secondary structures a-helix and b-strand, is found in Fig. 5.4.
Fig. 21.10 Structures of myoglobin as determined by X-ray crystallography; myoglobin is the
oxygen-binding protein in muscle. (a) shows all the atoms except for hydrogen as small balls;
(b) ribbon presentation of secondary structure – the spiral is helix; (c) space-filling representation
of constituting amino acids (from J. M. Berg, J. L. Tymoczko and L. Stryer, “Biochemistry 5th ed”
(W. H. Freeman and Co, 2002))
Fig. 21.11
HIV-protease that consists mostly of b-strand (from J. M. Berg, J. L. Tymoczko
and L. Stryer, “Biochemistry 5th ed” (W. H. Freeman and Co, 2002))
268
21 Are Atoms and Molecules for Real? Can We See Them?
HIV-protease (Fig. 21.11) is the target of one kind of AIDS drugs, as discussed
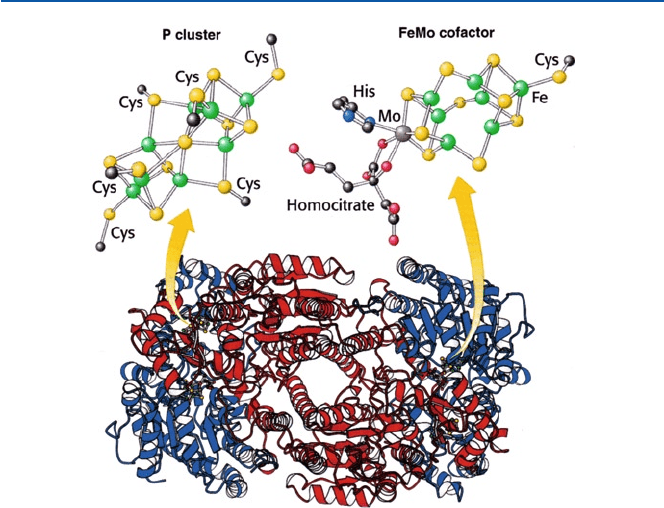
in Chap. 17. Figure 21.12 is nitrogenase, one of the most complex enzymes, and is
talked about in Chap. 6. Determination of protein (enzyme) structures as illustrated
here and others help enormously in understanding their functions and the mecha-
nisms in which they play crucial roles.
Fig. 21.12 Nitrogenase; its active sites P-cluster and FeMo cofactor are shown schematically (from
J. M. Berg, J. L. Tymoczko and L. Stryer, “Biochemistry 5th ed” (W. H. Freeman and Co, 2002))

Part VII
Postscript

271
E. Ochiai, Chemicals for Life and Living,
DOI 10.1007/978-3-642-20273-5_22, © Springer-Verlag Berlin Heidelberg 2011
The readers have hopefully been convinced by now that our material world is indeed
made of chemicals, and that our entire universe, including us human beings, would
not exist without chemicals. Chemistry as a scientific discipline has been fairly well
advanced to give us a nice consistent picture of the material world. But this is a
chemical view of the world; i.e., the world is viewed only through “chemistry” eye-
glasses and in terms of chemical concepts and theories advanced so far.
Chemistry as a discipline is an analytical (reductionist) endeavor; i.e., it tries to
understand things by dissecting them into compounds (molecules and ionic ensem-
bles) and then taking account of interactions among atoms, ions, and molecules.
The reality of the world consists of a whole set of these compounds interacting with
each other in diverse ways. Compounds and molecules involved may or may not
have been known to chemistry (i.e., Homo sapiens), and chemistry may or may not
have fully recognized or understood the diverse ways they interact. In other words,
we should recognize that chemical understanding of the world as practiced by
today’s chemists is far from perfect, both in the analytical sense (i.e., we do not yet
know all the compounds existent or emerging in the whole universe and all their
interactions) and also the“synthetic” sense. Well, language here is inadequate
because chemical synthesis as a technique is widely practiced by chemists, but
“synthesis” as a philosophical concept is alien to the discipline of chemistry.
In other words, chemistry may not concern itself with synthetic understanding of
things taking into account all the material presents (known and unknown) and all the
interactions at chemical, physical, biological, and (maybe geological) levels.
This kind of limitation exists implicitly in all the so-called disciplinary sciences
or modern (western) science, but particularly acute in chemical science (and also in
many of the so-called “social science” disciplines including economics and political
sciences). The entirety of a phenomenon (material world, society, world economy,
etc.) is there as a whole, but each disciplinary science dissects it and recognizes only
an aspect (or portion) of the whole. Understanding of the world then requires more
than disciplinary understandings, and technology based on disciplinary thinking has
its limitation.
22
Holistic Chemical View of the World
