Ochiai E. Chemicals for Life and Living
Подождите немного. Документ загружается.


222
18 Domain of Chemistry
There is one other important sub-discipline of chemistry; that is, analytical
chemistry. You would need to identify and quantify the material you deal with; i.e.,
chemical analysis. Analytical chemistry borrows ideas from all branches of chemis-
try, particularly physical chemistry, and devises means to do analysis. It is an applied
field of chemistry. In real life, analytical chemistry is, perhaps, the most-often-used
chemistry. Depending on the material dealt with, you can have bioanalytical chem-
istry, environmental analytical chemistry and so forth. Another way of subdividing
analytical chemistry is based on the means used: “wet chemical analysis,” typically
dealing with solutions (hence wet) and using more traditional analytical methods,
and “instrumental analysis” which uses a number of different instruments to do
analysis: spectrophotometric, electrochemical, etc.
An entirely different kind of chemistry sub-discipline is nuclear chemistry.
It deals with chemicals all right, but its concern is quite different from all the
sub-disciplines mentioned above. It studies the nuclei of atoms in chemicals. The
nuclei obey quite different kinds of rules than the ordinary chemicals do, as the next
Chap. (19) explains. It treats the radioactivity, nucleosynthesis (how elements are
produced), nuclear fission and fusion, and extends to cosmochemistry.

223
E. Ochiai, Chemicals for Life and Living,
DOI 10.1007/978-3-642-20273-5_19, © Springer-Verlag Berlin Heidelberg 2011
This chapter is not meant to be a comprehensive, detailed, and systematic presentation
of all chemical concepts/principles and their applications. It tries to present a bare
minimum of the essential chemical concepts that may be necessary to appreciate the
content of this book.
One of the essentials discussed is the concept of Avogadro number, but its appli-
cation, i.e., the quantitative calculations involving “moles,” is hardly discussed in
the main body of the book. The author considers that grasping of such quantitative
calculations is unnecessary except for the practitioners of chemistry and the related
areas. The inclusion of this important concept is to only emphasize the nature of
material world; i.e., it consists of particles, atoms, ions, molecules, etc. And the
quantities of any material is fundamentally measured in terms of the number of the
particles, though in everyday life the quantities may be measured in terms of mass
(weight), volume, length, etc. Occasionally, such calculations do appear in the text
for sure, but it is only meant to be a reminder to those who understand or need
such a calculation. In general, quantitative calculations that appear in the text are
meant to give the readers a sort of feeling toward the quantities talked about. They
are not intended to be precise and detailed and often are not shown of the details of
assumptions, calculations, and data.
Other major omissions are the concept of atomic orbital hybridization vs shape
of molecule and the determination of the molecular structures. These topics would
require a lengthy discourse to do full justice to the subject. We entirely omit these
aspects and present all the chemical structures as given.
19.1 Atoms/Molecules/Mole/Avogadro Number
According to chemistry, a substance is an aggregate of a very large number of very
tiny particles that can be a kind of atom or a kind of molecule. A substance that is
made of a single kind of atom is called an “element,” while a substance consisting
19
Chemistry’s View of the Material World:
Basic Principles

224
19 Chemistry’s View of the Material World: Basic Principles
of a large number of a same kind of molecule or the same combination of different
atoms is a “compound.” We are using terms such as “atom,” “element,” and “mole-
cule” here without defining them. We are relying on your knowledge of these terms;
it does not matter at this stage whether your own definition is precise or not. We will
eventually find out exactly what they are.
Now take an ounce (28.35 g) of water. It turns out to consist of approximately
1000000000000000000000000 (there are 24 “0”s) molecules of water. [By the way,
300000 (5 “0”s) is often expressed as 3 × 10
5
; so the large number above with 24
“0”s can be written as 1 × 10
24
for short. This kind of expression is called “scientific
notation”]. A single molecule of water represents the smallest unit that has the char-
acter of water, and hence, weighs only about 3/10
23
g (i.e., 28.35 g (i.e.,1 oz)/10
24
)
[As 1/10
3
, i.e., 1/1000 one-thousandth, is written as 1 × 10
−3
, 3/10
23
can be written as
3 × 10
−23
and is a very tiny value]. When chemists look at water, they think in terms
of literally a sea of these very tiny molecules of water. They are moving around
randomly within a confine at ambient temperatures and pressures, and thus the
aggregate of water molecules show a character of liquid at ordinary temperatures.
Some of the water molecules go out of the liquid into the surrounding space and
come back. All of the molecules there always keep going out and coming back, but
overall, the majority of water remains in the liquid. However, if molecules that
escaped into the space would not come (as in the case where water is in an open
container), eventually all water molecules disappear into space.
Let us now go to a little deeper level. That is, the idea of “atom.” The atoms
constitute molecules and hence are the basis of all materials. For example, a water
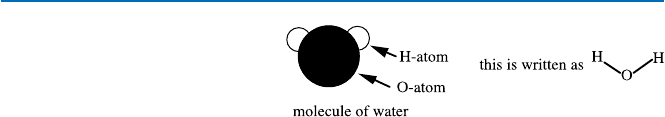
molecule has been shown to consist of two hydrogen atoms and an oxygen atom.
The idea of “atom” is as old as ancient Greece, where several philosophers sug-
gested that the material is made of atoms. Atoms are the smallest units of matter that
cannot be divided further. Democritus coined the word “atom” to mean what was
just said. He said: “The first principles of the universe are atoms and empty space;
everything else is merely thought to exist.” There are different kinds of atom. An
aggregate of atoms of the same nature is called an “element”; so atoms of different
natures belong to different elements. Some Greek philosophers thought that the
whole world was made of four elements: for example, air, water, fire, and soil. We
know now that these are not elements in the modern sense.
Today, science recognizes about 100 different elements in the whole universe.
“Elements” should be understood as “chemical elements” to distinguish it from
“elements” in ordinary usage. Everything that is present in this universe is made of
one or more atoms of these 100 or so elements. A water molecule is made of two
atoms of element hydrogen (symbol “H”) and one atom of element oxygen (symbol
“O”). And this fact is expressed as H
2
O. Chemists also have learned that in water
molecule, two hydrogen atoms are bound with the one oxygen atom as shown in
Fig. 19.1. This is the so-called structure of water molecule. In real life we would not
be able to deal with a single molecule, for it is too tiny. Instead we will be dealing
with, say, a gallon of water. A gallon of water contains a very large number of water
molecules as we told you earlier. But the properties of this large body of water or a
22519.1 Atoms/Molecules/Mole/Avogadro Number
small amount of water, say a drop of water, are determined by the molecule having
H
2
O composition; therefore, we will use “H
2
O” notation even when we talk about
this large number of water molecules, i.e., the substance or compound water. This is
the chemical formula of water (molecule).
Iron is another element (chemical symbol = Fe). Metal iron, say 100 g, is made of
a very large number (about 10
24
) of atoms of element iron. When iron rusts, it turns
into a substance called iron oxide, which may be described as consisting of two
atoms of element iron and three atoms of element oxygen (Fe
2
O
3
). (It might be
pointed out here, though it may be confusing at this stage, that there is no such
molecular entity having the composition Fe
2
O
3
. It shows only the composition).
Element carbon (chemical symbol = C) is made of atoms of a single element
carbon, but can take different shapes. All carbon atoms in these substances are
bound to each other, but their arrangements can be different. As a result, graphite
and diamond, though consisting of only carbon atoms, i.e., being elemental carbon,
have such disparate properties. Carbon atom(s) make million different compounds
(molecules), binding hydrogen atom(s), oxygen(s) and nitrogen(s, chemical
symbol = N); they are collectively called “organic compounds.” Some of them such
as proteins and DNA constitute our body.
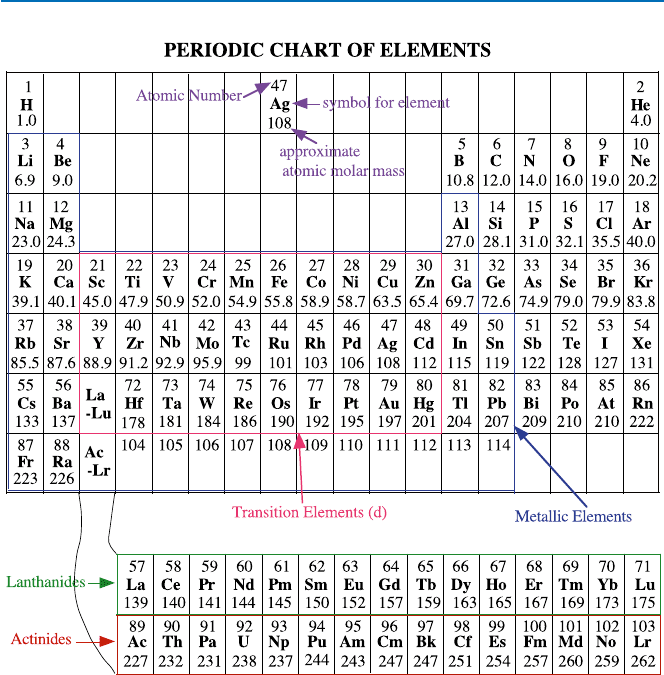
All the elements are now arranged neatly in the so-called periodic table, which
consists of 18 columns and 7 rows plus 2 extra segments (Fig. 19.2). Each element
is given a number (atomic number) and then pigeonholed in each of the boxes.
Chemistry deals with all these elements, their compounds, and how they behave.
Remember that all the materials that exist are made of these elements.
As we said before, all the atoms and molecules are miniscule and it is very hard
to deal with a single atom or a single molecule. Therefore, chemists invented a unit
of measurable quantity of elements or compounds (substances).
It is called “mole.” One mole of a molecular substance is defined to be a collec-
tion of approximately 6 × 10
23
molecules. This huge number 6 × 10
23
(23 0s after 6)
is called “Avogadro Number.” Avogadro is an Italian chemist who attempted to
determine this number first. The number is frighteningly large, but the idea of
“mole” is the same as, say, “dozen,” which consists of 12 bodies. So 1 mol of water
consists of 6 × 10
23
particles of water molecule H
2
O. As quarter a dozen is equal to
12/4 = 3, quarter a mole is 6 × 10
23
/4 = 1.5 × 10
23
. By the way, no significance should
be attached to the fact that these two quantities 12 and 6 (×10
23
) appear somehow
related. This is a pure coincidence. “6” in the quoted Avogadro number is an approx-
imation; it has been estimated to be “6.022,” to be more precise. This value itself is
still an approximation, but practically accurate enough to be useful.
Fig. 19.1 Structure of water
molecule (often this is further
simplified as H–O–H)
226
19 Chemistry’s View of the Material World: Basic Principles
The Avogadro number has been decided in such a way so that 1 mol of a
substance, say, water, weighs an ordinary everyday number, such as 18 g in this
case. It turned out that 1 mol of many elements comes close to an integer number
if we use the Avogadro number of about 6 × 10
23
; for example, 1 mol of hydrogen
Fig. 19.2 Periodic Chart. (Ac, actinium; Ag, silver; Al, aluminum; Am, americium; Ar, argon;
As, arsenic; At, astatine; Au, gold; B, boron; Ba, barium; Be, beryllium; Bi, bismuth; Bk, berke-
lium; Br, bromine; C, carbon; Ca, calcium; Cd, cadmium; Ce, cerium; Cf, californium; Cl, chlo-
rine; Cm, curium; Co, cobalt; Cr, chromium; Cs, cesium; Cu, copper; Dy, dysprosium; Er, erbium;
Es, einsteinium; Eu, europium; F, fluorine; Fe, iron; Fm, fermium; Fr, francium; Ga, gallium; Gd,
gadolinium; Ge, germanium; H, hydrogen; He, helium; Hf, hafnium; Hg, mercury; Ho, holmium;
I, iodine; In, indium; Ir, iridium; K, potassium; Kr, krypton; La, lanthanum; Li, lithium; Lr, law-
rencium; Lu, lutetium; Md, mendelevium; Mg, magnesium; Mn, manganese; Mo, molybdenum;
N, nitrogen; Na, sodium; Nb, niobium; Nd, neodymium; Ne, neon; Ni, nickel; No, nobelium; Np,
neptunium; O, oxygen; Os, osmium; P, phosphorus; Pa, protactinium; Pb, lead; Pd, palladium; Pm,
promethium; Po, polonium; Pr, praseodymium; Pt, platinum; Pu, plutonium; Ra, radium; Rb,
rubidium; Re, rhenium; Rh, rhodium; Rn, radon; Ru, ruthenium; S, sulfur; Sb, antimony; Sc, scan-
dium; Se, selenium; Si, silicon; Sm, samarium; Sn, tin; Sr, strontium; Ta, tantalum; Tb, terbium;
Tc, technetium; Te, tellurium; Th, thorium; Ti, titanium; Tl, thallium; Tm, thulium; U, uranium;
V, vanadium; W, tungsten; Xe, xenon; Y, yttrium; Yb, ytterbium; Zn, zinc; Zr, zirconium)

22719.2 Structures of Atoms
(symbol H) is about 1 g, carbon (C) 12, oxygen (O) 16, iron (Fe) 56, and so on.
Note that when you combine two hydrogen atoms and one oxygen, you will
come up with 2 × 1(H) + 16(O) = 18 g for 1 mol of water as indicated above. This
mass is called the “molar mass” of a substance. For example, the molar mass of
the rust Fe
2
O
3
would be approximately 2 × 56 + 3 × 16 = 160 g. There is a deeper
meaning to this fact; i.e., that the mass of 1 mol of an element comes close to an
integer number. In order to understand this, we need to look into the structure of
an atom.
19.2 Structures of Atoms
A lot of research on the structures of atoms has come up with a picture shown in
Fig. 19.3. An atom consists of a nucleus at the center and electrons surrounding it.
The nucleus carries a positive electric charge and is very minute, while electrons are
negatively charged. And the magnitudes of the positive charge on the nucleus and
of the negative charge on electrons are the same (in a neutral atom). Since an elec-
tron is regarded as a particle (one of the fundamental particles), we can assign an
electric charge to a single electron and use that charge as a unit of electric charge;
let’s say it is “e.” Suppose that a neutral atom has “n” electrons, then the nucleus
must have “n” of something that carries a positive electric charge of magnitude “e.”
This something is called “proton.” A proton, though carrying the same magnitude of
electric charge positive (instead of negative), turned out to be about 1,800 times as
heavy as an electron.
The simplest atom consists of a single proton in the nucleus and an electron sur-
rounding it. This is hydrogen, symbolized as “
1
H
1
.” It turned out that another atom
exists that has one proton and one electron, but, in addition, has one more particle
called “neutron” in the nucleus. “Neutron” is approximately the same in mass as
proton but carries no electric charge. This atom is about twice as heavy as the regu-
lar hydrogen atom and is called “heavy hydrogen”
1
H
2
(or often called “deuterium D”
(
1
D
2
)). In this notation, the superscript is the total number of protons and neutrons
in the nucleus and the subscript is the number of protons (or the positive electric
charge in units of “e”). The former is called “atomic mass number” and the latter
“atomic number,” or more generally electric charge number. The reason that this is
Fig. 19.3 The atomic
orbitals that the electrons
will occupy. These are
actually more like fuzzy
clouds of different densities
and shapes

228
19 Chemistry’s View of the Material World: Basic Principles
still regarded as a kind of hydrogen is that deuterium (D) behaves almost like hydrogen,
as the chemical behaviors of an atom are determined mostly by the number of
electrons and the positive charge of the nucleus. Scientists invented a word “iso-
tope” to designate two or more atoms that have the same number of protons but
different number of neutrons in the nucleus. The isotopes with an atomic number
(i.e., the same number of protons) are chemically similar and hence designated as
belonging to the same element. Thus, both “
1
H
1
” and “
1
H
2
” (or
1
D
2
) are isotopes and
belong to the element “hydrogen.” A third hydrogen isotope
1
H
3
[how many protons
and neutrons here?] is also known. This is often called “tritium, T.” Not all the
isotopes of an element exist in an equal quantity in nature (on the Earth). In hydro-
gen case, the predominant isotope is
1
H
1
, whose natural abundance on the Earth is
99.985%, while the heavy hydrogen is present only 0.015% in nature (on the Earth).
Tritium can be produced only artificially.
Now let us move on. The second simplest element is helium, He or rather
2
He
4
;
that is, its nucleus contains two protons and two neutrons. The third element is
3
Li
7
(lithium, how many neutrons are here?). There is also an isotope
3
Li
6
. The natural
abundance of the former is 92.58% and the latter is 7.42%. Element iron, Fe, con-
sists of isotopes of
26
Fe
54
(natural abundance = 5.82%),
26
Fe
56
(91.66%),
26
Fe
57
(2.19%), and
26
Fe
58
(0.33%) (how many protons and neutrons here?). Since a proton
and a neutron weigh about the same, the mass of an isotope, say
26
Fe
56
would weigh
56 g (per mole), if we assign 1 g for 1 mol of hydrogen (i.e.,
1
H
1
). It usually comes
close to that, i.e., an integer number, but it is not quite an integer number. This is
because some mass (weight) is lost when protons and neutrons gather together to
form a nucleus. This weight lost is turned into an energy, through the famous
Einstein’s equation E = mc
2
, where E is the energy, m is the mass, and c is the speed
of light.
Nevertheless, this fact that a nucleus is made of an integer number of particles
(protons and neutrons) is the basis for the choice of the Avogadro number. Avogadro
number has been chosen in such a way that the molar quantity of an element (an
isotope to be more precise) comes out as a number very close to an integer [because
the mass loss in the form of mc
2
is relatively small]. Historically, the Avogadro
number has been reworked several times; currently the Avogadro number (i.e.,
6.022 × 10
23
) is chosen so that 1 mol of the isotope
6
C
12
weighs exactly 12.000 g. The
mass of 1 mol of an atom is called “(molar) atomic mass” (given in units of g/mol).
It should be and is close to an integer number (i.e., the mass number, the superscript
figure of isotope), if it is defined for a single isotope. However, the “atomic mass” is
usually defined for an element. Hence the term “(molar) atomic mass” is a misno-
mer and should be called “(molar) elemental mass.” The atomic mass for an element
can differ significantly from an integer number, because the atomic mass is the aver-
age of 1 mol of all the naturally occurring isotopes of an element. [Try to calculate
the (molar) atomic mass of iron based on the isotopic distribution data mentioned
above for a fun, assuming that each isotope has an integer number for its atomic
mass, though this assumption is not quite right. Compare your result with the
approximated value of molar atomic (elemental) mass given in the periodic chart,
Fig. 19.2. If you find a discrepancy, think why].

22919.4 Nuclear Chemistry
19.3 Ordinary Chemistry and Nuclear Chemistry
An atom consists of a nucleus that is made of positively charged protons and neutral
neutrons, and electrons surrounding it, as we outlined above. Two entirely different
types of chemistry stem from this structure. One is concerned with the nucleus and
the other with how electrons behave. The former is “nuclear chemistry,” with
“radiochemistry” as its important sub-discipline. The latter is the ordinary “chemis-
try.” The basic reason for this division is that the nuclear forces binding protons and
neutrons in the nucleus are enormously stronger than the electrostatic force binding
the electrons to the nucleus. When one applies a force to a substance and induces a
change, a certain amount of energy may be expended or gained. Hence, an energy
change always accompanies a change in substance. “Energy” is often used as a
measure of a change in science, particularly in chemistry (see Sect. 19.8). In terms
of energy, then, a nuclear reaction (change in general) is greater by several orders of
magnitude (typically a million times) than a typical chemical reaction, as the nuclear
reaction involves changes in protons/neutrons in the nucleus while chemical reac-
tions involve changes in electrons. Therefore, ordinary chemical reactions would
not be able to cause a change in nucleus (i.e., nuclear reaction). As a result, it is
quite safe to deal with nuclear chemistry as separate from “ordinary” chemistry. As
a corollary, all isotopes that belong to an element, though they have different atomic
masses, can be assumed to behave (approximately) the same chemically. However,
isotopes behave very differently in terms of nuclear reactions. It is now obvious that
principles governing nuclear reactions are quite different from those operating in
the ordinary chemical reac-tions.
19.4 Nuclear Chemistry
19.4.1 Radioactivity: Spontaneous Nuclear Reactions
A nucleus that consists of neutrons and protons can be stable or unstable.
Understanding of the factors that affect the nuclear stability is beyond the scope of
this discourse, and it is not necessary to really understand for our purpose here.
However, we have the facts; i.e., which nuclei (isotopes) are stable and which are
unstable. The unstable isotopes would not remain as such and spontaneously change
into stable isotopes. In this process, the unstable nucleus emits particles and/or
energy in the form of radiation (gamma (g)-ray). Particles that are emitted include
electron (called b-particle), and helium nucleus (a-particle). Hence, these unstable
isotopes are called “radioactive,” emitting a, b, and/or g radiation. There are other
kinds of radiation, as well, but these three are the major ones.
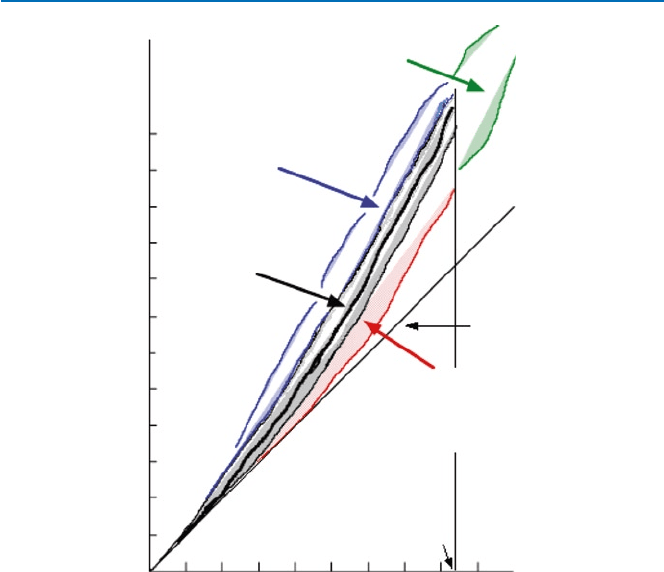
There are exactly 264 stable isotopes known on the Earth. They lie on or slightly
above a line that corresponds to the composition of equal numbers of neutrons and
protons, as shown in Fig. 19.4. The area that is occupied by stable isotopes is coined
as “stability peninsula” (the darkened area; the solid black curve represents a sort of
central area where most of stable isotopes are distributed). In the figure, each of the
230
19 Chemistry’s View of the Material World: Basic Principles
colored areas consist actually separate dots, each of which represents an isotope
(stable or unstable), and it represents an approximate area where these isotopes
are distributed.
In understanding the radioactivity, we need to recognize some changes that can
occur with neutron and proton. That is, they can interchange in the following
manner:
1 10
0 11
n neutron p pro( )( ) (ton e elect )ron
-
®+
(19.1)
101
11 0
pen
-
+®
(19.2)
(
)
1 10
1 01
p n e positron
+
®+
(19.3)
[In these equations we omitted another particle “neutrino”].
Unstable isotopes that lie above the stability peninsula have too many neutrons.
Hence they become stable by reducing the relative number of neutrons. This can be accom-
plished by emitting an electron (i.e., converting a neutron to a proton through (19.1)).
Radioisotopes
Radioisotopes
Stable Isotopes
Atomic Number (Number of Protons)
Number of Neutrons
100
90
80
70
60
60 70 80 90
83
50
50
40
40
30
30
20
20
10
10
120
110
b
+
–emission
neutron–emission
Radioisotopes
b-emission
neutron-emission
mostly a-emission
Line of equal
number of
neutrons and
protons
Fig. 19.4 Stable isotopes and unstable (radioactive) isotopes

23119.4 Nuclear Chemistry
Therefore, most of these neutron-rich unstable isotopes are b-emitters. What would
be an alternative way to reduce the neutron/proton ratio?
On the other hand, the unstable isotopes that lie below the stability peninsula would
want to reduce the excess protons, by e.g., either (19.2) or (19.3). Process (19.2) is
called electron capture and would not emit a particle, but usually emit g-radiation.
Process (19.3) would emit a positron, which has the same character as an electron
except for the positive electric charge (instead of negative); this is called b
+
-radiation.
As you see in Fig. 19.4, there are no stable isotopes for elements whose atomic
numbers are 84 or higher. These heavy elements usually decay by emitting a-parti-
cles. An example is
92
U
235
, which is used for the nuclear power generation.
The speed of the radioactive decay (disintegration) is governed by only the nature
of the nucleus, and often is characterized by the time that it takes for one half of a
sample of a radioactive isotope to disintegrate. This “time” is called “half-life.”
Some isotopes have very short half-life: e.g., 2.4 s in the case of
6
C
15
. They disinte-
grate very quickly. Others have very long half-life: e.g. 4.5 × 10
9
years in the case of
92
U
238
. This means that it takes 4.5 billion years (approximately the life span of the
Earth) for 1 g of radioactive
92
U
238
to become half a gram of radioactive
92
U
238
; in this
process, the end product is lead
82
Pb
206
, through a multistep process.
19.4.2 Induced Nuclear Reactions: Modern Alchemy
Nuclei, stable or unstable, behave on their own. It is difficult to make them change
their courses of destiny. This is because the energies involved in the change are
quite large. And this is the reason that alchemists had never succeeded in converting
lead into gold. However, if large enough energy is provided, one can cause changes
in a nucleus. Such nuclear reactions are taking place in nature (in the universe) and
also can now be induced by artificial means.
In the upper portion of the atmosphere on the Earth, nitrogen isotope
7
N
14
is con-
stantly bombarded by (energetic) neutrons of the cosmic ray and is undergoing the
following nuclear reaction:
14 1 14 1
7 06 1
NnCp+® +
[Note that the sum of the superscripts on the left-hand side (14 + 1 = 15) is equal
to that on the right-hand side and also that the sum of the subscripts on the left-hand
side (7 + 0 = 7) is equal to that on the right-hand side (6 + 1 = 7). This conservation of
mass number and electric charge always holds true for nuclear reactions. Check this
with (19.1)–(19.3) above as well]. By the way, the resultant nucleus
6
C
14
is radioac-
tive, though
7
N
14
is not, and the radioactivity of
6
C
14
is used to determine the age of
the archaeological artifacts.
British physicist Rutherford succeeded in 1919 for the first time in human history
in changing an element into another. He bombarded
7
N
14
with a-particles and
converted it into oxygen. That is:
14 4 17 1
7 2 81
N He O p Check the balance of super/subscr( .[]t) ip sa+ ®+
