Ochiai E. Chemicals for Life and Living
Подождите немного. Документ загружается.

162
13 Chemistry of the Universe: What Is It Made of?
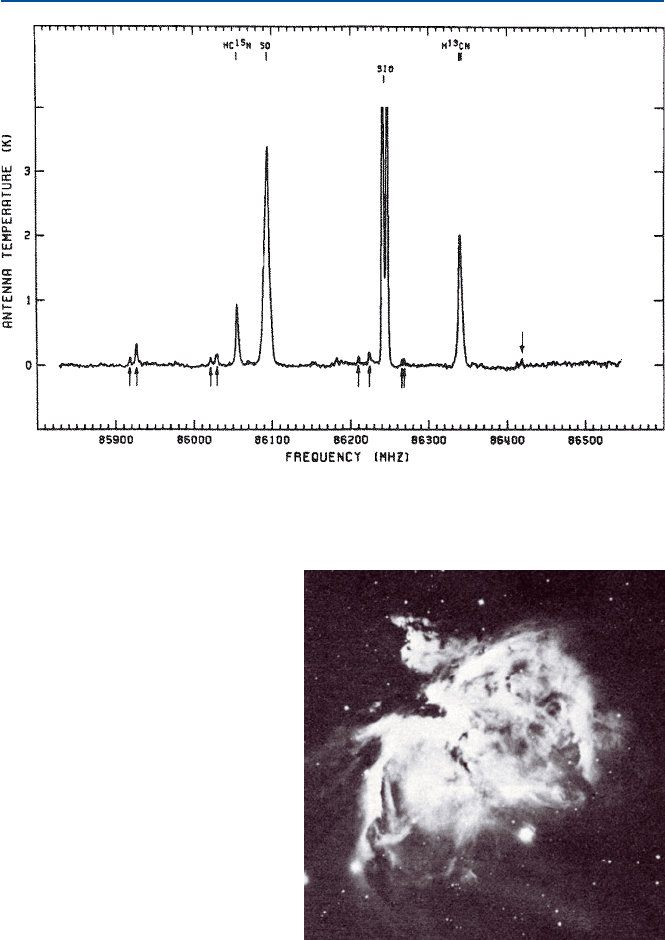
much of the chemical species is present in the view range of the telescope, and also
how strongly a chemical species emits that light. The latter factor can be determined
in a laboratory experiment, and hence the height (intensity) of a peak would give us
an estimate of the abundance of a chemical species].
Fig. 13.2 Spectrum of the Orion molecular cloud [from W. M. Irvine and Hjalmarson, in
“Cosmochemistry and the Origin of Life” (ed by C. Ponnamperuma, D. Reidel, 1983)]
Fig. 13.3 Orion Nebula
16313.3 Formation of Molecules and Dusts in Interstellar Space
A lot more chemical species (molecules) have been identified in the Orion
Molecular Cloud. They are listed in Table 13.1 (above). There seems to be a prepon-
derance of compounds that contain cyanide (CN) group: hydrogen cyanide HCN
and cyano acetylenes consisting of C≡C (acetylenic group) and CN. Larger cyano
acetylenes such as HC≡C–C≡C–C≡C–C≡C–C≡N have been identified in other
interstellar clouds such as that of Taurus. It is also worth noting that these com-
pounds are made of the biologically important elements, that is, carbon, hydrogen,
nitrogen, and oxygen. This suggests that life can be possible almost anywhere in the
universe at least in the sense that all elements essential for organisms of the type
found on the earth are found in the interstellar cloud.
How might have these compounds been produced in the interstellar medium?
This question has not yet been adequately answered. It seems, however, that the
presence of a kind of solid surface might be imperative, on which atoms and mole-
cules bind and chemical reactions take place.
Are there such solids in the interstellar space? Yes, there are a lot of solids out
there; they are called “dust” or “grain.” What are they? A big problem with these
kinds of material is again that we cannot hold them in our hands to study, as in the
case of the molecular species in the space mentioned earlier. The study of the dust
or grain in the space is more difficult than that of simpler molecular species.
Sometimes, you may be able to obtain some spectra (in the infrared region) that give
some indications as to the identities of compounds. In this way, some interstellar
grains have been discovered to consist of silicates and frozen water. It is believed
that such grains provide cores onto which other atoms and molecules are condensed.
Those atoms and molecules condensed on the core react further under the influence
of ultraviolet (and also other energetic particles available).
Another way to study this kind of situation is to recreate the situation in a lab.
From a limited knowledge of the nature of grain such as above, we create such
grains in a laboratory on this Earth and place it under such a condition as might
prevail in the interstellar space. You need a lot of guessing, but science makes pro-
gresses essentially by guessing and testing. M. Greenberg of Leiden University tried
such experiments. Simple compounds such as H
2
O, NH
3
, and CH
4
were condensed
onto small (of 0.12 m diameter) silicate particles, and they were subjected to ultra-
violet radiation. The researchers noted formation of complex organic molecules
including such complex compounds as amino acids, in addition to some of the com-
pounds listed in Table 13.1 above.
Table 13.1 Compounds
identified in the Orion
mole cular cloud (Nebula)
H
2
, OH, CH, H
2
O, H
2
S, NH
3
CO, CS, OCS, SiO, SO, SO
2
, O
3
(?), CO
+
CN, HCN, H
2
N–CN, H
3
C–C≡CH, HC≡C–CN, HC≡C-C≡C–CN
C≡CH, H
3
C–C≡CH, HN=C=O, HNC
H
2
C=O, H–C
+
=O, H–C
+
=S, H
2
C=S
H
3
C–O–CH=O, H
3
COH, H
3
C–O–CH
3

165
E. Ochiai, Chemicals for Life and Living,
DOI 10.1007/978-3-642-20273-5_14, © Springer-Verlag Berlin Heidelberg 2011
14.1 How Was the Earth Formed?
The solar system formed about 4.6 billion years ago. The Earth and other terrestrial
planets are believed to have formed by gathering together the so-called planetesi-
mals. Planetesimals are formed by the coalescence of fine- or coarse-grained min-
eral matters, metals, and gases of various kinds (such as mentioned in the previous
chapter). As planetesimals stuck together mostly by gravity and the body thus
formed grew larger, it became a precursor of terrestrial planet. Some of these bodies
were smashed by other bodies, and their fragments became meteorites. Hence, stud-
ies of meteorites would provide a lot of insight into the formation and the earlier
state of the Earth.
The planet Earth was thus formed. Heat was created as the coalescence (of plan-
etesimals) proceeded due to gravity, and heat also came from radioactivity of several
radioactive elements such as aluminum-26. So the newly formed body was heated
and the core was melted. As the material becomes liquid (as a result of melting), the
materials contained in the liquid separate out according to their densities. The more
dense material would sink closer to the bottom (core). Thus, the present layer struc-
ture of the Earth formed. The innermost core is a dense solid of about 1,200 km
radius, whose density is about 12.6 g per cubic centimeter (12.6 × 10
6
kg/m
3
). It is
made of mostly iron metal and a small amount of nickel. By the way, the density of
iron metal is only 7.8 × 10
6
kg/m
3
under the ordinary pressure. The next layer is the
outer core (up to 3,500 km from the center of the Earth), which is liquid and has a
density of 9.5–12 × 10
6
kg/m
3
. The chemical composition seems to be about the
same as that of the inner core. There is an abrupt change in density in the next layer,
mantle. The width of mantle is about 2,900 km (3,500–6,380 km from the center).
Its density ranges from 4 to 5.5 × 10
6
kg/m
3
. The mantle is made of mostly
magnesium–iron silicates (silicon oxides). The outermost layer is the thin crust of
about 35–45 km on the land portion, and about 6 km under the ocean portion.
14
Chemistry of the Earth

166
14 Chemistry of the Earth
14.2 Chemistry of the Earth: Its Mantle and Upper Crust
The inner core of the earth is made of very much compressed metallic iron and a
little bit of nickel; the outer core is not much different, only it is not as compressed
as the inner core. These are essentially metals. However, the rest of the solid Earth
is made of various minerals based on silicates.
The lower portion of the mantle (3,500–5,400 km from the center of the earth) is
considered to be more or less homogeneous and made of magnesium–iron metasili-
cate (generally called “pyroxene”) and magnesium–iron oxide (the mineral name is
periclase). Pyroxene has the composition (Mg,Fe)SiO
3
. The iron content is about
10–20% of the total of magnesium and iron. We will talk about the structures of
silicates later on. The next 600 km is a sort of transition zone and the last 400 km
or so is the upper mantle. The main minerals in the transition and upper mantle
zone are olivine and amphibole-derived minerals. Olivine is basically magnesium
silicate Mg
2
SiO
4
, and an example of amphiboles is calcium–magnesium silicate
(Ca
2
(Mg,Fe)
3
Si
8
O
22
(OH)
2
).
The crust is the only layer we can have a direct access to. The upper portion of
the crust consists of various constituents, granite igneous, metamorphosed, and
sedimentary rocks, though the last two are secondary rocks. The granite igneous
rocks come from the magmatic activities (volcanism). The lower portion of the crust
is mainly basaltic and does also come from the molten material of the upper mantle.
The igneous rock is made of quartz, feldspar, pyroxene, hornblende, biotite, tita-
nium oxide, apatite, and others. The main components are the first three. Do not
worry too much about these geochemical terms.
These rocks are weathered/eroded by air (air oxidation), water (rain/river), and
temperature fluctuation, carried by river and wind, and deposited at the ocean bot-
tom. Alternatively, dissolved substances may precipitate as the condition changes.
These deposited substances eventually turn into sedimentary rocks.
Rocks can change their compositions and crystal form within the solid mass
under the influence of the surrounding chemical environments (water and others),
high pressure, and/or high temperature. This process is metamorphosis, and the
resulting rocks are metamorphic rocks.
14.3 Igneous Rocks
14.3.1 Silicates
The rocks in the crust are predominantly (more than 90%) made of silicates (includ-
ing silica SiO
2
) and aluminosilicates. A question may be asked: why are silicates
and aluminum derivatives the predominant components of rocks on the Earth? There
are two reasons. One is that silicon and aluminum are among the most abundant
elements on the Earth (and also in the universe). The second is that silicates form a
variety of stable solid structures.
16714.3 Igneous Rocks
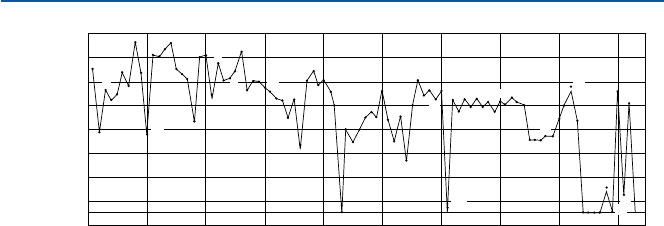
Figure 14.1 shows the distribution of elements in the Earth’s crust. You may note
that it is significantly different from Fig. 13.1 (the cosmic element distribution) of
the previous chapter. Lighter elements such as hydrogen, helium, carbon, and nitro-
gen are relatively less abundant on the Earth than in the Universe as a whole. The
lighter elements seem to have been lost to the space when the Earth was forming,
perhaps because the relatively small size of the Earth could not hold the lighter ele-
ments due to its relatively weak gravity force.
The most abundant element in the crust is oxygen (O), followed by silicon (Si),
aluminum (Al), iron (Fe), and then calcium (Ca), magnesium (Mg), potassium (K),
and sodium (Na). If silicon and aluminum, the two most abundant metallic elements,
can do well in forming solid material, they would be the dominating elements in the
crust. And, as it turns out, they are chemically very much suitable for making solid
material, rocks.
First of all, they are situated in the middle of the periodic chart side by side, and
they bind very strongly with oxygen. They form very stable oxides, SiO
2
and Al
2
O
3
.
The former is quartz. Quartz is found in many different forms; transparent crystals,
amethyst, smoky quartz, and agate (the colors in these minerals are due to impuri-
ties), and sand is mostly quartz. It consists of a structural unit of SiO
4
, in which the
silicon atom is nominally in the oxidation state of Si(IV) and the four oxygen atoms
(O
2−
each) are bound to the silicon in a tetrahedral manner. The tetrahedrons are
bound to each other by sharing the corner (oxygen atoms) in a continuous three-
dimensional manner. The most stable form of SiO
2
at room temperature and an
ordinary pressure is a-quartz, the transparent and typical crystal familiar to many of
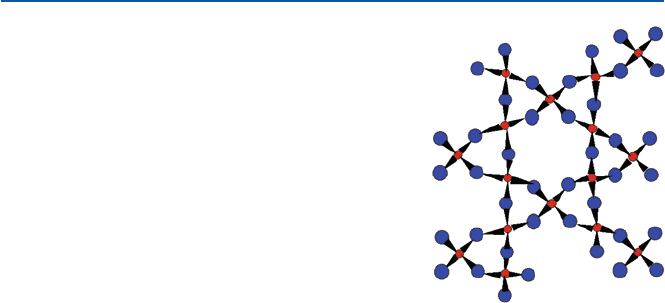
you. In this crystal form, the tetrahedral units interlink three-dimensionally in a
helical chain (Fig. 14.2). At 573°C, a-quartz changes to b-quartz crystal form.
At higher temperatures, it will change to other forms, tridymite (at 867°C) and cris-
tobalite (1,470°C), and it will melt at 1,713°C.
Many of the igneous rocks found in the Earth’s crust are derivatives of silica,
called metal silicates, in which the units SiO
4
bind in various manners. The unit
SiO
4
has a tetrahedral shape and is nominally represented as [SiO
4
]
4−
; that is,
each unit carries four negative charges. Among the simplest silicates is M
2
II
SiO
4
.
H
100
01020304050
Atomic number
Abundamce, weight per cent
60 70 80 90
1
10
-2
10
-4
10
-6
10
-8
10
-10
10
-12
<10
-12
c
Li
He
Ne
A
Tc
Pm
Po
Rn
Ra
Pa
At
Fr
Ac
Np
Be
B
N
F
AI
Na
Si
K
C1
Ca
Fe
Ti
Mn
Ni
V
Sc
Cr
Co
Cu
Zn
Ge
Se
Kr
Rh
Ru
Cd
Ag
Pd
In
Te
Xe
Eu
Sm
Gd
Dy
Er
Yb
Hf
Ta
W
Lu
Tm
Ho
Tb
Re
Au
Pt
Hg
Pb
Bi
Th
U
T1
Ir
Os
Sb
I
La
Pr
Ce
Nd
Ga
As
Br
Rb
Sr
Zr
Y
Nb
Mo
Sn
Ba
Cs
Mg
P
S
O
Fig. 14.1 The elemental distribution in the Earth’s crust [from B. Mason, “Principles of
Geochemistry, 2nd, ed.”, (Wiley and Sons, 1966)]
168
14 Chemistry of the Earth
The most common M(II) is Mg(II), that is Mg
2
SiO
4
, and the mineral of this
composition is called forsterite. Since Fe(II) has a size (radius = 92 pm) similar to
Mg(II) (86 pm), the magnesium in forsterite can be replaced by Fe(II) in an arbi-
trary proportion. The resulting mineral is then represented by (Mg,Fe)
2
SiO
4
, and is
known as olivine, one of the most common rock minerals. The structure consists of
independent SiO
4
units interspersed by Mg(II)/Fe(II), which in turn is bound to six
oxygen anions of SiO
4
units. Silicate of this type is called “orthosilicate.”
The SiO
4
units can bind each other by sharing the oxygen atoms in various man-
ners. They bind, for example, in a linear fashion sharing a single oxygen atom
(see Fig. 14.3a); the repeating unit is then [SiO
3
]
2−
, which is called “metasilicate.”
The most common mineral with such a structure is pyroxene type M
II
SiO
3
. The pure
MgSiO
3
is a mineral enstatite, and (Mg,Fe)SiO
3
is hypersthene. This is merely the
simplest linear structure. There is a wide variety of ways to connect the [SiO
4
] units
in chain fashion (as illustrated in Fig. 14.3). Two linear chains may form a double
chain as shown in Fig. 14.3. This is the basic structure of amphiboles, where the
repeating unit is [Si
4
O
11
]
6−
. Tremolite with the composition of Ca
2
Mg
5
(OH)
2
(Si
4
O
11
)
2
is a typical amphibole. These are examples of long chains of the silicate units.
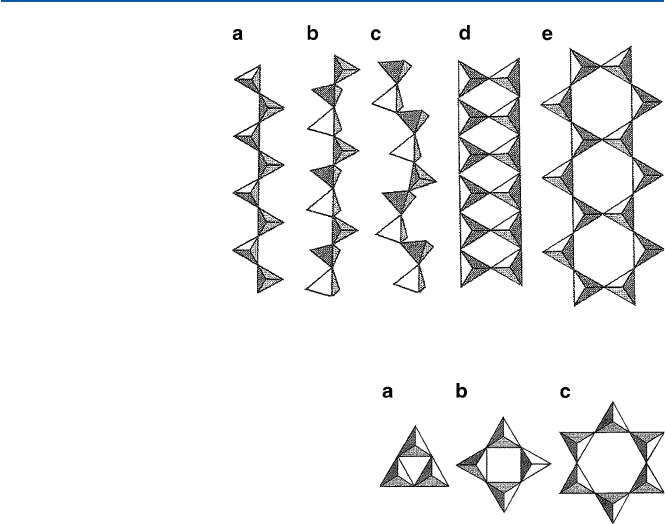
The linear structure of the basic units, single or double-stranded, suggests that
some of these minerals under certain conditions may form fibrous material. Such
material with flexibility, high tensile strength, and heat resistance is called “asbestos”
in general. The most common asbestos is chrysotile, commonly known as “white
asbestos,” which has a composition of Mg
3
Si
2
O
5
(OH)
4
. It has the double-stranded
structure similar to Fig. 14.3d, where Mg(II) ions bridge the two strands. A less
common asbestos is the mineral crocidolite: Na
3
Fe
4
Si
8
O
22
(OH)
2
, and is known as
“blue asbestos.” The repeating unit of this fibrous mineral has the structure shown
in Fig. 14.3e. This asbestos’ fiber is shorter than that of crysolite and is believed to
be mostly responsible for the environmental hazard.
SiO
4
units can also condense themselves to form discrete polysilicate anions;
examples are shown in Fig. 14.4. These possibilities suggest the wide variety in
which silicates form minerals.
Fig. 14.2 The structure of
a-quartz
16914.3 Igneous Rocks
14.3.2 Aluminosilicates
The other interesting chemical character is that the silicon atom in [SiO
4
]
4−
may be
replaced by cations of similar sizes without significant modification of the structure.
The most important one is aluminum Al(III). However, when this happens, the unit
[AlO
4
] will now carry “5−” electric charge instead of “4−.” That means that cations
(M(I) or M(II)) must be added to compensate the electric charge difference when
Si(IV) is substituted by Al(III), or that an O
2−
unit is replaced by an OH
−
unit.
Al(III)-substitution occurs often in amphibole-type minerals. The amphiboles con-
tain [Si
4
O
11
] repeating units as mentioned earlier (Fig. 14.3), and Al can replace Si
up to the extent of [AlSi
3
O
11
]. The extent of Al-substitution depends on the condi-
tion of formation of such minerals. Minerals of hornblende has the composition of
Ca
2
Na
0−1
(Mg,Fe,Al)
5
[(Al, Si)
4
O
11
]
2
(OH)
2
.
By far the most important rock minerals are feldspars, which constitute about
60% of the igneous rock. They are essentially made of three fundamental types of
minerals: albite NaAlSi
3
O
8
, orthoclase KAlSi
3
O
8
, and anorthite CaAl
2
SiO
8
. Their
structures are continuous three-dimensional network of [SiO
4
] and [AlO
4
] tetrahe-
drons, interspersed by positively charged sodium (Na(I)), potassium (K(I)), or
calcium (Ca(II)). Albite and anorthite mix in arbitrary proportions; such mixed min-
erals are known as plagioclase feldspars. Albite and orthoclase also make mixtures;
they are alkali feldspars.
Fig. 14.3 The structures of
some linear polysilicates;
(a) Na
3
SiO
3
; (b) Ca
3
Si
3
O
9
;
(c) (Mn, Ca)
5
Si
5
O1
5
;
(d) [Si
2
O
5
2−
]; (e) [Si
4
O
11
6−
]
[from N.N. Greenwood and
A. Earnshaw, “Chemistry
of the Elements, 2nd ed”
(1997, Elsevier)]
Fig. 14.4 The structure
of discrete polysilicates:
(a) [Si
3
O
9
]
6−
; (b) [Si
4
O
12
]
8−
;
(c) [Si
6
O
18
]
12−
[from N.N.
Greenwood and A. Earnshaw,
“Chemistry of the Elements,
2nd ed” (1997, Elsevier)]
170
14 Chemistry of the Earth
Another type of widely occurring aluminosilicates is classified as zeolite. Zeolites
have much more open structures than feldspars, and, as a result, they can take up
loosely bound water or other small molecules into their cavity. Now zeolites are
artificially created, which have specific chemical characteristic and holes that
accommodate only a specific substance.
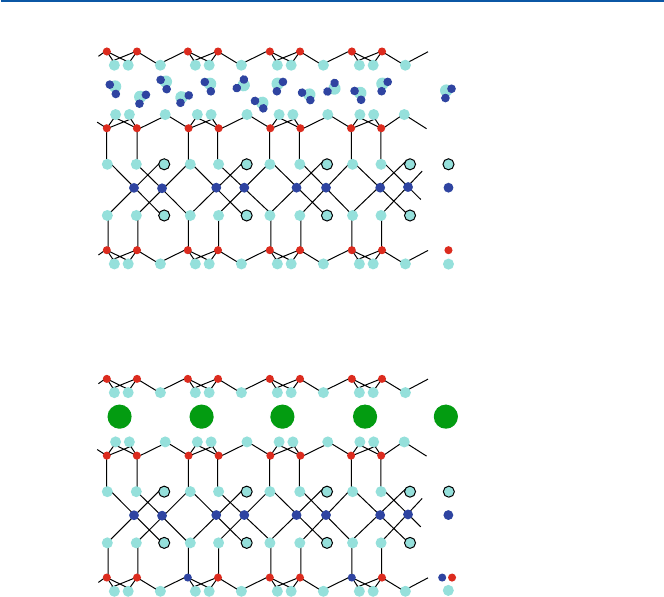
Aluminosilicates of mica group have layer structures (Fig. 14.5). The [SiO
4
]
units connect through three of the oxygen atoms and form a sheet structure.
Representative minerals of mica group are muscovite KAl
2
(AlSi
3
O
10
)(OH)
2
and bio-
tite K(Mg,Fe)
3
(AlSi
3
O
10
)(OH)
2
. The sheet structure leaves one oxygen free. Two
sheets made of tetrahedral [SiO
4
] are placed together with the free oxygen (vertex)’s
pointing inward. These oxygen ions are then cross-linked by Al(III) in muscovite or
by Mg(II) and Fe(II) in biotite. Then such sets of two sheets are held together by
potassium ion (K(I)). This binding by potassium is relatively week, accounting for
the characteristic basal cleavage of mica.
Fig. 14.5 Clay minerals: (a) Montmorillonite, (b) Muscovite
H
2
O molecules
Montmorillonite
Al
4
(Si
4
O
10
)
2
(OH)
4
+ nH
2
O
OH
Al
Si
O
K
Muscovite
K
2
Al
4
(Si
3
AlO
10
)
2
(OH)
4
OH
Al
3Si + 1Al
O
a
b

17114.5 Sedimentary Rocks and Metamorphic Rocks
14.4 Clay and Soil
Igneous rocks that formed under high temperature and high pressure are unstable
under the conditions prevailing on the surface of the Earth. Rocks are thus subject
to various forces, physical, chemical, and biological, and will be slowly changed
and eroded. This process is known as weathering. Only quartz is highly resistant to
weathering processes, and all the other minerals tend to change.
The temperature on the Earth changes significantly by day and night, seasons,
and over the long range of geological time. Temperature changes will expand and
contract the rocks and eventually crack them. More significant is the effect of water.
Water seeps into the cracks of rocks and it expands when it is frozen. This will
facilitate the cracking. Rolling of hard rock upon softer rocks helps disintegrate the
softer rocks. Wind and river then will carry smaller rock fragments away. These are
merely physical effects.
Some components, for example, Fe(II), are oxidized by air to Fe(III). Water will
dissolve the chemical substances in rocks. The presence of carbon dioxide usually
helps dissolution process, because it makes water acidic. The fragmented and/or
dissolved material may be redeposited on land, which forms soil. The fragmented
and dissolved material may be carried to the ocean and eventually deposited and
compacted at the bottom, which forms sedimentary rocks.
Soil consists of weathered material (clay) of the bedrock and other material. The
main clay minerals are silicates and aluminosilicates derived from muscovite, bio-
tite, olivine, and pyroxene. Iron oxide and aluminum hydroxide also constitute clay
minerals. For example, olivine will be slowly eroded by the effect of air and water.
The chemical reactions involved are summarized as follows:
4 2 2 23 2 2 4 4
2( Mg,Fe) SiO olivine (1 / 2) O 5H O Fe O 3H O Mg() SiO Si(OH)+ +→ + +
i
The resulting Mg(II) ion (shown as Mg
2
SiO
4
) and Si(OH)
4
are relatively soluble
in water and can be washed away. The iron oxide Fe
2
O
3
colors the clay brown. Other
silicates, pyroxene and others, are less soluble and more resistant to erosion, but will
be weathered slowly. The only exception is quartz; it may be pulverized mechani-
cally (to become sand), but is resistant to chemical weathering.
The major clay minerals are muscovite, montmorillonite, pyrophilite, and kao-
linite. Kaolinite is the major ingredient of the clay used for making ceramics and is
talked about in Chap. 10. These minerals all have layered structures (Fig. 14.5) like
kaolinite (Fig. 10.1).
14.5 Sedimentary Rocks and Metamorphic Rocks
Sandstone, shale, and limestone are typical sedimentary rocks. The sedimentary
rock that forms from finely fragmented mineral, mostly of silica and some silicates,
is sandstone. It will be called “conglomerate” if it contains larger fragments and the
texture is coarse. Mud (clay and others) leads to the formation of shale. Calcium
