NP 100 - Mariners Handbook
Подождите немного. Документ загружается.


CHAPTER 5
130
Surfaces
Homogeneous arc
Homogeneous band
Home
Contents
Index

CHAPTER 5
131
Corona
2 At the time of an intense solar flare or eruption, a flash
of ultra-violet light and a stream of charged particles are
emitted from the sun.
3 The flash of ultra-violet light takes only 8 minutes to
reach the Earth, where it produces great ionisation
(electrification) at abnormally low layers of the upper
atmosphere. Short radio waves which travel round the Earth
by being reflected from a higher layer of the upper
atmosphere cannot penetrate this barrier of ionisation and a
radio “fade-out” is experienced. Long radio waves however
may be reflected more strongly from the base of the lower
layer of ionisation. Since these short range radio fade-outs
and long wave enhancements are caused by the effects of
ultra-violet light from the sun, they are confined to the
sunlit side of the Earth and are almost simultaneous with
the flare, lasting on the average for about 20 minutes.
4 The stream of charged particles, travelling much more
slowly than light, arrives at the Earth, if it is suitably
directed, at from 1 to about 3 days after it leaves the sun; it
visibly signals its arrival by producing a bright and active
aurora. It too causes great ionisation in the upper
atmosphere, which is much more prolonged than that
Home
Contents
Index

CHAPTER 5
132
caused by the ultra-violet light. There is again deterioration
in short wave radio communications, which may be a
complete “black-out” in higher latitudes. At this time
currents of the order of a million amperes may circulate in
the upper atmosphere. The magnetic field of the fluctuating
currents is appreciable at the Earth’s surface and may
deflect a compass needle noticeably from its normal
position. The effects on these so-called magnetic and
ionospheric storms, which may persist with varying
intensity for several days, are usually greatest in higher
latitudes. Radio black-outs and simultaneous deviations of
the magnetic compass needle by several degrees are not
uncommon in and near auroral zones. When a great aurora
is seen in abnormally low latitudes, it is invariably
accompanied by a magnetic and ionospheric storm. Unlike
the fade-out which occurs only on the sunlit side of Earth,
the interference with radio communications which
accompanies an aurora and magnetic storm may occur by
day and at night.
5 All these effects occur most frequently, and in most
intense forms, at the time of sunspot maximum.
Increases in solar activity could affect the reliability of
GPS and other satellite systems; for further details see
Admiralty List of Radio Signals Volume 2.
CLOUD FORMATIONS
Classification
5.67
1 Clouds are continually changing and appear in a variety
of forms. It is possible however to define a limited number
of characteristic forms, observed all over world, into which
clouds can be broadly grouped.
Level
(Over UK)
Designation Type Abbreviation
High
(base usually
>20 000 ft)
C
H
Cirrus
Cirrocumulus
Cirrostratus
Ci
Cc
Cs
Medium
(base usually
>6500 and
<20 000 ft)
C
M
Altocumulus
Altostratus
Nimbostratus
Ac
As
Ns
Low
(base usually
<6500 ft)
C
L
Stratocumulus
Stratus
Cumulus
Cumulonimbus
Sc
St
Cu
Cb
2 See also pages 132 to 136.
ADDITIONAL INFORMATION
5.68
1 Additional information on maritime meteorology can be
found in Meteorology for Mariners (Met.0.895), Cloud
Types for Observers (Met 0.716 (1982 Edition)) and Marine
Observer’s Handbook (Met 0.1016) published by the
Meteorological Office.
Stratocumulus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Home
Contents
Index

CHAPTER 5
133
Stratus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Altostratus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Home
Contents
Index

CHAPTER 5
134
Altocumulus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Cirrostratus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Home
Contents
Index

CHAPTER 5
135
Cirrocumulus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Cumulus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Home
Contents
Index

CHAPTER 5
136
Cirrus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Cumulonimbus
(Photograph − P. K. Pilsbury, Courtesy of the Meteorological Office)
Home
Contents
Index
CHAPTER 5
137
5.69
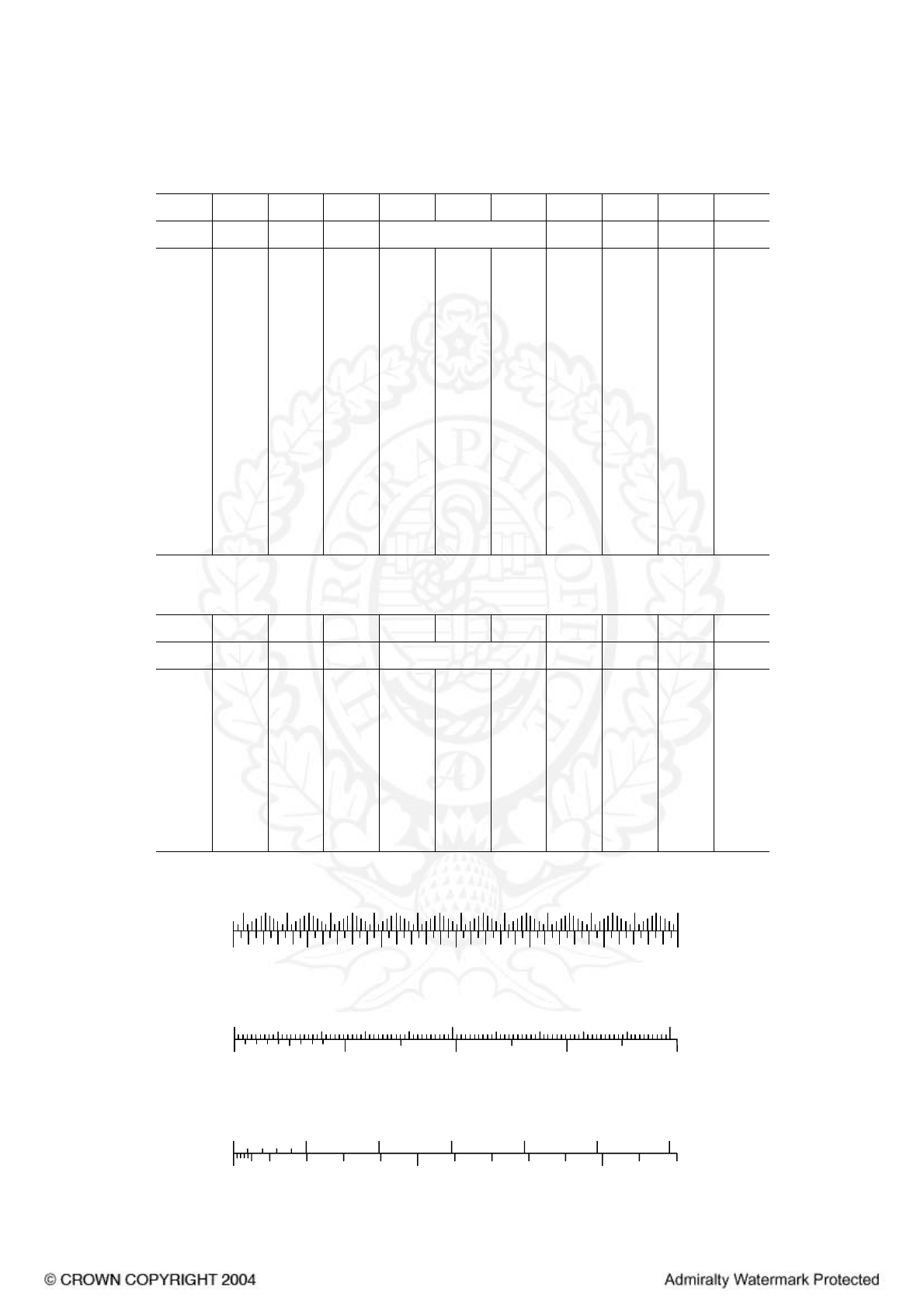
METEOROLOGICAL CONVERSION TABLE AND SCALES
Fahrenheit to Celsius
°Fahrenheit
0 1 2 3 4 5 6 7 8 9
°F Degrees Celsius
−100
−90
−80
−70
−60
−50
−40
−30
−20
−10
−0
+0
10
20
30
40
50
60
70
80
90
100
110
120
−73⋅3
−67⋅8
−62⋅2
−56⋅7
−51⋅1
−45⋅6
−40⋅0
−34⋅4
−28⋅9
−23⋅3
−17⋅8
−17⋅8
−12⋅2
−6⋅7
−1⋅1
+4⋅4
10⋅0
15⋅6
21⋅1
26⋅7
32⋅2
37⋅8
43⋅3
48⋅9
−73⋅9
−68⋅3
−62⋅8
−57⋅2
−51⋅7
−46⋅1
−40⋅6
−35⋅0
−29⋅4
−23⋅9
−18⋅3
−17⋅2
−11⋅7
−6⋅1
−0⋅6
+5⋅0
10⋅6
16⋅1
21⋅7
27⋅2
32⋅8
38⋅3
43⋅9
49⋅4
−74⋅4
−68⋅9
−63⋅3
−57⋅8
−52⋅2
−46⋅7
−41⋅1
−35⋅6
−30⋅0
−24⋅4
−18⋅9
−16⋅7
−11⋅1
−5⋅6
0
+5⋅6
11⋅1
16⋅7
22⋅2
27⋅8
33⋅3
38⋅9
44⋅4
50⋅0
−75⋅0
−69⋅4
−63⋅9
−58⋅3
−52⋅8
−47⋅2
−41⋅7
−36⋅1
−30⋅6
−25⋅0
−19⋅4
−16⋅1
−10⋅6
−5⋅0
+0⋅6
6⋅1
11⋅7
17⋅2
22⋅8
28⋅3
33⋅9
39⋅4
45⋅0
50⋅6
−75⋅6
−70⋅0
−64⋅4
−58⋅9
−53⋅3
−47⋅8
−42⋅2
−36⋅7
−31⋅1
−25⋅6
−20⋅0
−15⋅6
−10⋅0
−4⋅4
+1⋅1
6⋅7
12⋅2
17⋅8
23⋅3
28⋅9
34⋅4
40⋅0
45⋅6
51⋅1
−76⋅1
−70⋅6
−65⋅0
−59⋅4
−53⋅9
−48⋅3
−42⋅8
−37⋅2
−31⋅7
−26⋅1
−20⋅6
−15⋅0
−9⋅4
−3⋅9
+1⋅7
7⋅2
12⋅8
18⋅3
23⋅9
29⋅4
35⋅0
40⋅6
46⋅1
51⋅7
−76⋅7
−71⋅1
−65⋅6
−60⋅0
−54⋅4
−48⋅9
−43⋅3
−37⋅8
−32⋅2
−26⋅7
−21⋅1
−14⋅4
−8⋅9
−3⋅3
+2⋅2
7⋅8
13⋅3
18⋅9
24⋅4
30⋅0
35⋅6
41⋅1
46⋅7
52⋅2
−77⋅2
−71⋅7
−66⋅1
−60⋅6
−55⋅0
−49⋅4
−43⋅9
−38⋅3
−32⋅8
−27⋅2
−21⋅7
−13⋅9
−8⋅3
−2⋅8
+2⋅8
8⋅3
13⋅9
19⋅4
25⋅0
30⋅6
36⋅1
41⋅7
47⋅2
52⋅8
−77⋅8
−72⋅2
−66⋅7
−61⋅1
−55⋅6
−50⋅0
−44⋅4
−38⋅9
−33⋅3
−27⋅8
−22⋅2
−13⋅3
−7⋅8
−2⋅2
+3⋅3
8⋅9
14⋅4
20⋅0
25⋅6
31⋅1
36⋅7
42⋅2
47⋅8
53⋅3
−78⋅3
−72⋅8
−67⋅2
−61⋅7
−56⋅1
−50⋅6
−45⋅0
−39⋅4
−33⋅9
−28⋅3
−22⋅8
−12⋅8
−7⋅2
−1⋅7
+3⋅9
9⋅4
15⋅0
20⋅6
26⋅1
31⋅7
37⋅2
42⋅8
48⋅3
53⋅9
Celsius to Fahrenheit
°Celsius
0 1 2 3 4 5 6 7 8 9
°C Degrees Fahrenheit
−70
−60
−50
−40
−30
−20
−10
−0
+0
10
20
30
40
50
−94⋅0
−76⋅0
−58⋅0
−40⋅0
−22⋅0
−4⋅0
+14⋅0
32⋅0
32⋅0
50⋅0
68⋅0
86⋅0
104⋅0
122⋅0
−95⋅8
−77⋅8
−59⋅8
−41⋅8
−23⋅8
−5⋅8
+12⋅2
30⋅2
33⋅8
51⋅8
69⋅8
87⋅8
105⋅8
123⋅8
−97⋅6
−79⋅6
−61⋅6
−43⋅6
−25⋅6
−7⋅6
+10⋅4
28⋅4
35⋅6
53⋅6
71⋅6
89⋅6
107⋅6
125⋅6
−99⋅4
−81⋅4
−63⋅4
−45⋅4
−27⋅4
−9⋅4
+8⋅6
26⋅6
37⋅4
55⋅4
73⋅4
91⋅4
109⋅4
127⋅4
−101⋅2
−83⋅2
−65⋅2
−47⋅2
−29⋅2
−11⋅2
+6⋅8
24⋅8
39⋅2
57⋅2
75⋅2
93⋅2
111⋅2
129⋅2
−103⋅0
−85⋅0
−67⋅0
−49⋅0
−31⋅0
−13⋅0
+5⋅0
23⋅0
41⋅0
59⋅0
77⋅0
95⋅0
113⋅0
131⋅0
−104⋅8
−86⋅8
−68⋅8
−50⋅8
−32⋅8
−14⋅8
+3⋅2
21⋅2
42⋅8
60⋅8
78⋅8
96⋅8
114⋅8
132⋅8
−106⋅6
−88⋅6
−70⋅6
−52⋅6
−34⋅6
−16⋅6
+1⋅4
19⋅4
44⋅6
62⋅6
80⋅6
98⋅6
116⋅6
134⋅6
−108⋅4
−90⋅4
−72⋅4
−54⋅4
−36⋅4
18⋅4
−0⋅4
+17⋅6
46⋅4
64⋅4
82⋅4
100⋅4
118⋅4
136⋅4
−110⋅2
−92⋅2
−74⋅2
−56⋅2
−38⋅2
−20⋅2
−2⋅2
+15⋅8
48⋅2
66⋅2
84⋅2
102⋅2
120⋅2
138⋅2
HECTOPASCALS TO INCHES
950 960 970
980 990
1000 1010 1020
1030 1040
1050
28 29
30 31
INCHES
millimetres
50
0
10 20 30
40
60 70 80 90
100
(1) (for small values)
0
0⋅51⋅5
3⋅52⋅5
1
3
4
500 1000
1500 2000
2500 3000
millimetres
(2) (for large values)
0
510
20 30 40
50
60 70
80 90
100
110 120
inches
HECTOPASCALS
MILLIMETRES TO INCHES
2
0
inches
Home
Contents
Index

138
NOTES
Home
Contents
Index
139
CHAPTER 6
ICE
SEA ICE
Arctic and Antarctic regions
6.1
1 Due to the physical dissimilarities of the Arctic and
Antarctic regions their climates and ice regimes differ
greatly. The Arctic region contains a basin about 3000 m
deep which is covered by a thin shell of ice about 4 m
thick. The Antarctic, similar in extent, is a continent
covered by an ice cap which is up to 3000 m thick.
2 The annual mean temperature at the South Pole is –49°C
(the lowest temperature yet recorded in Antarctica is
–88·3°C), whereas at the North Pole the annual mean
temperature is estimated to be –20°C (the lowest
temperature yet recorded in the Arctic is only a little below
–50°C).
3 The ice cap covering the Antarctic continent accounts
for more than 90% of the Earth’s permanent ice. The ice
constituting the ice cap is constantly moving outward
towards the coasts where many thousands of icebergs are
calved each year from glaciers and ice shelves which reach
out over the sea. As a consequence large numbers of
icebergs are to be found in a wide belt which completely
surrounds the continent. In contrast, the icebergs of the
Arctic region are almost entirely confined to the sea areas
off the E and W coasts of Greenland and off the E
seaboard of Canada. The Arctic Ocean remains almost
completely covered by drift ice throughout the year,
whereas the greater part of the drift ice surrounding
Antarctica melts each summer.
Forms of ice
6.2
1 Several forms of ice may be encountered at sea. By far
the most common type is that which results from the
freezing of the sea surface, namely sea ice. The other
forms are icebergs (6.17) and river ice. River ice is
sometimes encountered in harbours and off estuaries during
the spring break-up, but it is then in a state of decay so
generally presents only a temporary hindrance to shipping.
Formation, deformation and
movement of sea ice
Freezing of saline water
6.3
1 The freezing of fresh and salt water does not occur in
the same manner. This is due to the presence of dissolved
salts in sea water. The salinity of water is usually expressed
in International Standard Units: sea water typically has a
salinity of 35, though in some areas, especially where there
is a considerable discharge of river water, the salinity is
much less. In the Baltic, for example, the salinity is less
than 10 throughout the year.
2 When considering the freezing process, the importance
of salinity lies not only in its direct effect in lowering the
freezing temperature, but also in its effect on the density of
the water. The loss of heat from a body of water takes
place principally from its surface to the air. As the surface
water cools it becomes more dense and sinks, to be
replaced by warmer, less dense water from below in a
continuous convection cycle.
3 Fresh water reaches its maximum density at a
temperature of 4°C; thus when a body of fresh water is
cooled to this temperature throughout its depth convection
ceases, since further cooling results in a slight decrease in
density. Once this stable condition has been reached,
cooling of the surface water leads to a rapid drop in
temperature and ice begins to form when the temperature
falls to 0°C.
4 With salt water the delay due to convection in the
lowering of the temperature of the water to its freezing
point is much more prolonged. In some areas where there
is an abundant supply of relatively warm water at depth,
such as SW of Spitsbergen, convection may normally
prevent the formation of ice throughout the entire winter
despite the very low air temperatures. This delay is, in part,
due to the great depths of water found in the oceans, but is
mainly due to the fact that the density of salt water
continues to increase with cooling until the surface water
freezes. In fact the theoretical maximum density of sea
water of average salinity (which can be achieved by
super-cooling in controlled laboratory conditions) is well
below its freezing temperature.
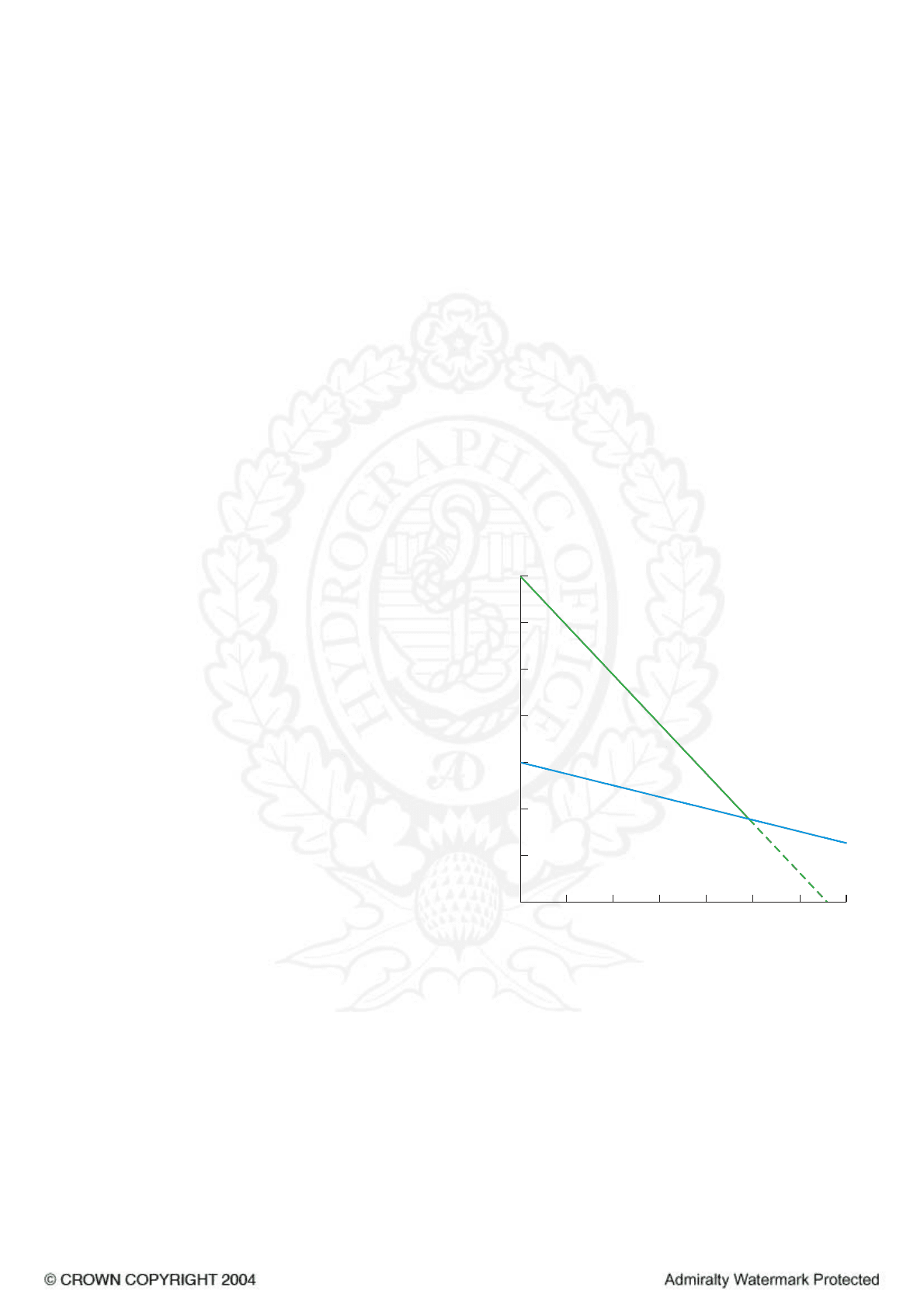
Maximum Density and Freezing Point related to
Temperature and Salinity (6.3)
5 The diagram shows the relationship between
temperature, salinity and maximum density. It can be seen
that in water with salinity of less than 24·7 the maximum
density is reached before the freezing temperature and
where the salinity is greater than 24·7 the freezing point is
reached before the density attains its theoretical maximum
value.
6 The greatest delay in reaching the freezing temperature
occurs when the sea water, throughout its depth, is initially
at an almost uniform density. In some areas, however, the
density profile is not uniform. In these cases, discontinuities
occur where a layer of lower salinity overlies a layer of
higher salinity. (At temperatures between about 3°C and
M
A
X
IM
U
M
D
E
N
S
IT
Y
FREEZING POINT
TEMPERATURE (°C)
SALINITY
(24.7-1.3°C)
-3
0 5 10 15
20 25 30 35
-2
-1
0
+1
+2
+3
+4
Home
Contents
Index
