North, Gerald R., Erukhimova Tatiana L. Atmospheric Thermodynamics: Elementary Physics and Chemistry
Подождите немного. Документ загружается.

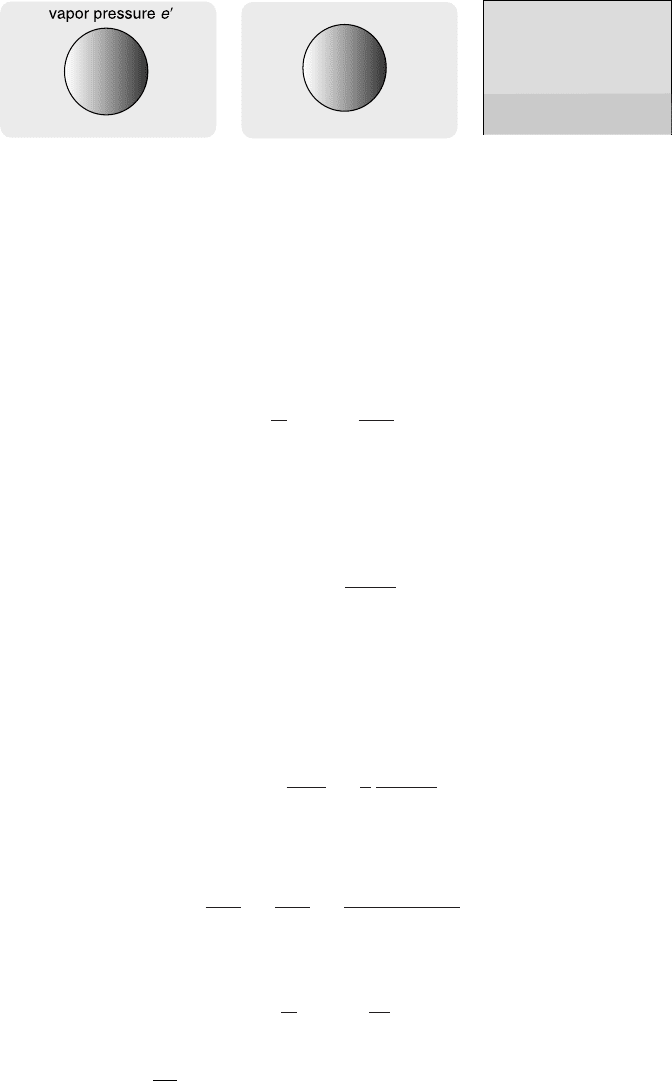
5.10 Equilibrium vapor pressure over a curved surface 127
vapor
oplet
liquid with flat surface
salt
solution
pure
water
droplet
vapor pressure
e
vapor
vapor pressure
e
s
vapor
Figure 5.16 Illustration of the notation. Left: saturation vapor pressure e
over a
solution droplet. Center: saturation vapor pressure e over a pure water droplet.
Right: saturation vapor pressure over a flat surface e
s
.
where e is the equilibrium vapor over a droplet composed of pure solvent. The
second expression comes after dividing numerator and denominator by n
w
.Ifwe
take into account that for a dilute solution n
salt
n
w
and expand the denominator
in (5.67) in a geometric series,
5
we find (retaining only the linear term),
e
e
= 1 −
n
salt
n
w
. (5.68)
In (5.68) the effect of dissociation of the ions is not yet taken into account. If the
salt dissociates into i ions, say Na
+
and Cl
−
giving i = 2, then the number of moles
of solute individuals in the droplet, ν
salt
,is
ν
salt
= i
M
salt
M
salt
(5.69)
where
M
salt
and M
salt
are the mass and molecular weight of the salt respectively
(be sure to use kg per mole here for the molecular weight). The degree of ionic
dissociation i ≈ 2 for sodium chloride and i ≈ 3 for ammonium sulfate. The
number of moles of water with molecular weight M
w
in the mass M
w
is
ν
w
=
M
w
M
w
=
4
3
πa
3
ρ
w
M
w
(5.70)
where a is the radius of the droplet.
6
The ratio of moles is equal to the ratio of
number densities:
n
salt
n
w
=
ν
salt
ν
w
=
3i
M
salt
M
w
4πa
3
ρ
w
M
salt
. (5.71)
After substituting (5.71) into (5.68) we obtain
e
e
= 1 −
c
a
3
(5.72)
5
The geometric series for
1
1+
= 1 − +
2
−···, where || < 1.
6
We assume that dissolving the salt particle does not change the volume of the droplet.

128 Air and water
where we have introduced a new parameter to simplify the notation
c = 3i
M
salt
M
w
/4πρ
w
M
salt
. (5.73)
After substituting ρ
w
= 1000 kg m
−3
, M
w
= 18 g mol
−1
we obtain
c ≈ 4.3 × 10
−6
i
M
salt
M
salt
(m
3
). (5.74)
Combining Kelvin’s formula (5.66) and (5.72) we obtain the formula for
equilibrium vapor pressure of a solution droplet:
e
e
s
=
1 −
c
a
3
e
b/a
. (5.75)
To better understand this formula consider a limiting case. We again let the radius
of the droplet go to infinity, a →∞. From (5.75) we obtain the known result: the
equilibrium vapor pressure over the plane surface of water (a =∞) is equal to the
saturation vapor pressure, e
= e
s
.
Consider now a droplet with a radius a b. Then one can expand the exponential
function in (5.75) in a Taylor series
7
and get
e
e
s
= 1 + b/a − c/a
3
. (5.76)
The second term on the right-hand side of (5.76) is responsible for the surface
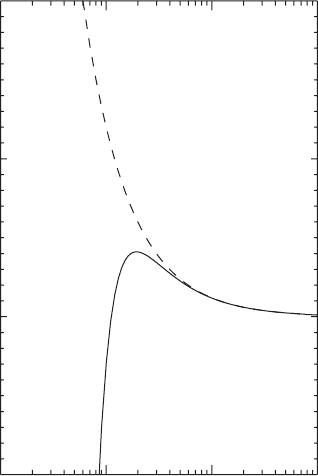
tension, the third term is due to the presence of the salt in the droplet. A graphical
illustration of formula (5.76) is given in Figure 5.17 for 10
−19
kg of sodium chloride.
The curve showing the dependence of relative humidity on radius of the solution
droplet is called the Köhler curve. The dashed curve corresponds to homogeneous
nucleation (5.63) with no salt present. These two curves differ considerably for
small values of the radius of the solution droplet. The most important result is that
with an embedded soluble salt nucleus in the droplet a much lower supersaturation
is required for the droplet to be in equilibrium with its environment than in the case
of a pure water droplet of the same size. For example, with supersaturation of just
0.1% a droplet with a radius slightly larger than 0.1 µm can be formed. With a very
small radius the droplet can be in equilibrium with the surrounding air even with a
relative humidity less than 100%. This is possible only because of the presence of
hygroscopic particles. With relative humidity 90% a solution droplet with radius
0.05 µm can form if there are 10
−19
kg of sodium chloride (not shown in the graph).
For a small droplet radius, the Köhler curve increases monotonically until it
reaches a maximum at radius a = a
, and then it decreases monotonically. Consider
7
e
x
≈ 1 + x, for |x|1.
5.10 Equilibrium vapor pressure over a curved surface 129
0.01
102
101
100
exp(b/a)
Relative humidity, %
99
0.10
Droplet radius, µm
1.00 10.00
Figure 5.17 Relative humidity with which a solution droplet formed on 10
−19
kg
of NaCl is in equilibrium with water vapor as a function of the droplet radius. The
temperature is 5
◦
C.
a droplet with a radius less than a
. If the relative humidity increases, such a particle
grows to adjust to the new equilibrium conditions. The equilibrium is stable, since
slight fluctuations in condensation or evaporation do not lead to further growing or
shrinking of the droplet. If the droplet grows slightly by condensation, the relative
humidity increases, the diffusive flux is directed away from the droplet and the
droplet evaporates to return to equilibrium. With slight evaporation, the relative
humidity in the vicinity decreases, which causes diffusive flux toward the droplet,
and the droplet again returns to equilibrium with the surrounding air. Such droplets
with radius less than a
are called haze particles.
The situation is different if the droplet reaches the radius a
. Now the equilibrium
is unstable. With any further growth of the droplet the relative humidity decreases
and the droplet continues to grow. This is one mechanism for cloud droplet
formation.
Example 5.9 How big is a salt particle whose mass is 10
−19
kg? Using the density
of 2165 kg m
−3
, we can calculate that a spherical particle of this mass would have
a radius of 0.022 µm. How many molecules are in this particle? The number

130 Air and water
of moles is M/M
NaCl
= 10
−19
× 1000/58.44 kg mol
−1
= 0.017 ×10
−16
mol.
The number of molecules is Avogadro’s number times the number of moles:
1.03×10
6
molecules.
5.11 Isobaric mixing of air parcels
When two parcels of dry air at the same pressure (altitude) are brought into contact
and mixed, the final temperature is
T
f
=
M
1
T
1
+ M
2
T
2
M
1
+ M
2
. (5.77)
If the mixing ratios of the parcels are
w
1
and w
2
, we can arrive at the same kind of
linear relationship
w
f
=
M
1
w
1
+ M
2
w
2
M
1
+ M
2
. (5.78)
It is possible that although the combinations (
w
1
, T
1
) and (w
2
, T
2
) are neither one
individually saturated, the mixed air parcel (
w
f
, T
f
) is saturated. Two clear moist
air parcels can come into contact with a foggy result.
Example 5.10 Suppose two parcels of equal volumes and pressures, but differing
temperatures (273 K and 293 K) come into contact near the ground (p = 1000 hPa).
Let each have relative humidity 90%. We can use
p =
M
1
V
RT
1
=
M
2
V
RT
2
. (5.79)
Then
M
1
M
2
=
T
2
T
1
. (5.80)
The final temperatures are:
T
f
=
2T
2
T
1
T
1
+ T
2
= 283 K. (5.81)
We can compute the initial mixing ratios:
w
1
= 0.622 × 0.9 × 6.11/1000 = 0.0034, w
2
= 0.0131. (5.82)
The final mixing ratio is
w
f
= 0.0083. (5.83)
But the saturation mixing ratio at T
f
is
2.497 × 10
8
e
−5417/283
× 0.622/1000 = 0.0076. (5.84)
Notation and abbreviations 131
We find that the final mixing ratio is above the saturation value for the final
temperature, hence we have supercooled water vapor and therefore fog.
The final temperature will actually be slightly above T
f
because some heating
will occur during the condensation.
Notes
More advanced treatments of water and air can be found in Bohren and Albrecht
(1998), Irebarne and Godson (1981), and Curry and Webster (1999). Discussions
of cloud drops, etc., can be found in Fleagle and Businger (1980), Rogers and Yau
(1989), Houze (1993) and Emanuel (1994).
Notation and abbreviations for Chapter 5
a droplet radius (m)
a
∗
critical droplet radius (m)
e, e
s
vapor pressure, saturation vapor pressure (Pa)
e
vapor pressure over a solution (Pa)
= M
w
/M
d
= 0.622 (dimensionless)
g
l
, g
g
specific Gibbs energy for liquid, gas (J kg
−1
)
g
v
, g
w
specific Gibbs energy for vapor, liquid water (J kg
−1
)
G Gibbs energy (J)
h specific enthalpy (J kg
−1
)
H (r) flux of heat crossing the surface of a sphere (J s
−1
)
κ
H
thermal conductivity coefficient (J m K
−1
s
−1
)
L = H
vap
the enthalpy (latent heat) of evaporation (J kg
−1
)
LCL lifting condensation level
M
v
, M
d
, M
e
gram molecular weight of vapor, dry air and effective
(g mol
−1
)
M
l
, M
g
bulk mass of liquid, gas (kg)
n
s
number density of vapor molecules at saturation
(molecules m
−3
)
n
sat
number density of vapor molecules at saturation
(molecules m
−3
)
n
w
number density of water molecules in vapor
(molecules m
−3
)
n
0
number density (molecules m
−3
)
N
A
Avogadro’s number (molecules mol
−1
)
q specific humidity (kg water vapor/kg of moist air)
r relative humidity
R
w
the gas constant for water vapor (Table 1.1)

132 Air and water
R
eff
effective gas constant for a mixture of species
(J kg
−1
K
−1
)
R
∗
universal gas constant (J mol
−1
K
−1
)
s
l
, s
g
specific entropy for liquid, gas (J K
−1
kg
−1
))
S entropy (J K
−1
)
σ surface tension (J m
−2
)
T Kelvin temperature (K)
T
D
dew point temperature (K)
T
v
virtual temperature (K)
T
w
wet-bulb temperature (K)
θ potential temperature (K)
v mean molecular speed (m s
−1
)
v
l
, v
g
specific volume for liquid, gas (m
3
kg
−1
)
w mixing ratio (kg water vapor per kg of dry air)
w
s
saturation mixing ratio (kg water vapor per kg of dry air)
Problems
5.1 A kilogram of water is vaporized at 0
◦
C and at 1000 hPa atmospheric pressure. (a)
Calculate the change in enthalpy of the water substance in the transition and (b) the
change of entropy for the process.
5.2 What is the virtual temperature for a kilogram of air at T = 283 K, relative humidity
50% at 1000 hPa?
5.3 The normal temperature of human blood is 37.2
◦
C. If a person is lifted in a balloon
the air pressure decreases. There will be a pressure (altitude) where the blood begins
to boil. What is that pressure in hPa? At about how many meters is that above sea
level?
5.4 It is a muggy night at the old ball park. The temperature is 30
◦
C and the humidity
is 85%. What is the change in density (%) of the air from a dry night at the same
temperature and pressure (1000 hPa)?
5.5 Assume the atmosphere is isothermal at 303 K (very tropical), which gives a scale
height of H = 8.87 km. The surface humidity is 80%. The vapor is distributed
uniformly in the first 1.5 km, and the air is dry above that. For simplicity take the
air pressure to be uniform in this lowest 1.5 km. How much water (in vapor form) lies
above a given square meter of surface (kg m
−2
)? Express the result in mm equivalent
of liquid water. Compare to the situation when the temperature is 273 K.
5.6 A system consists of dry air mixed with water vapor at a temperature of 20
◦
C. The
pressure of the mixture is 990 hPa. The relative humidity is 50%.
(a) What is the saturation vapor pressure?
(b) What is the partial pressure of the water vapor?
(c) What is the density of the mixture. Compare it with the density of dry air at the
same T and p.
Problems 133
(d) What are w and w
s
?
(e) If the system (parcel) is lifted adiabatically to 500 hPa, which is conserved
e or w?
5.7 Calculate the equilibrium vapor pressure over spherical droplets with radii 0.01, 0.1,
1, 10 µm at temperature 273 K. Plot the relative humidity (with respect to a flat water
surface) as a function of radius.
5.8 What supersaturation is needed for the droplets with radius 0.5 µmtobeinthe
equilibrium with water vapor at temperature of 10
◦
C?
5.9 An ammonium sulfate ((NH
4
)
2
SO
4
) particle of mass 10
−20
kg of radius 0.07 µm
is present in the air at temperature 0
◦
C. Find the relative humidity necessary for
heterogeneous nucleation.
5.10 Find the expression for the critical value (maximum of the Köhler curve) of the droplet
radius and relative humidity. Calculate these values for a droplet containing 10
−16
kg
of NaCl at 0
◦
C.
6
Profiles of the atmosphere
The properties of the atmosphere that vary with altitude include the pressure,
temperature and the composition of constituents such as water vapor. This chapter
provides some insight into these dependencies with simple derivations that hold
under idealized conditions. The stage will be set for the following chapter which
provides methods for analyzing the conditions at the time of observation.
6.1 Pressure versus height
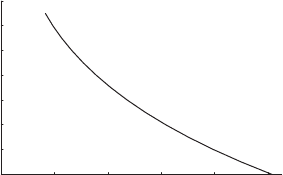
Atmospheric pressure drops off dramatically with height above the surface. This is
indicated by the graph in Figure 6.1 which shows the dependence of pressure on
altitude for the US Standard Atmosphere.
1
By balancing the vertical components of force on a slab of air at an arbitrary
height z it is possible to derive a formula for the average pressure as a function
of height, p(z). Consider a column of air with cross-section 1 m
2
. In the column
200 400 600 800 1000
p(hPa)
2
4
6
8
10
12
14
z(km)
US Standard Atmosphere
Figure 6.1 Dependence of pressure p (hPa) on altitude z (km) for the US Standard
Atmosphere.
1
The US Standard Atmosphere is a model of the atmospheric profile of various properties. It is used primarily in
aviation and satellite drag calculations. It attempts to give a global average of conditions. More can be learned
about it on the internet.
134
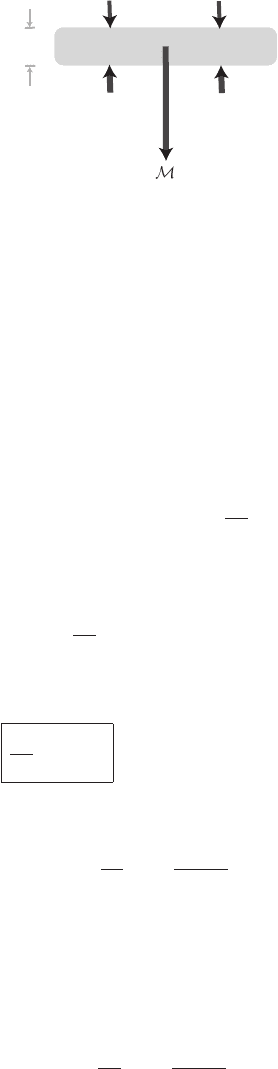
6.1 Pressure versus height 135
(d )g
p(z +dz)
A
p(z)A
dz
Figure 6.2 Diagram of a column of air of cross-section area A with a slab of
thickness dz at height z. The pressure and gravitational forces on the slab are
indicated.
we picture a thin horizontal slab of air whose bottom surface is located at height z
above sea level and whose thickness is dz (see Figure 6.2). The mass of material in
the slab is (density times volume): d
M = ρAdz, where A = 1m
2
is the horizontal
cross-sectional area of the slab. The weight of the slab of gas is (d
M)g = (ρAdz)g.
Beneath the slab is a pressure force pushing upwards: p(z)A. Above the slab is a
pressure force pressing downwards,
p(z + dz)A ≈
p(z) +
dp
dz
dz
A. (6.1)
Equating the net pressure force on the slab to the gravitational force,
dp
dz
dzA=−ρg dzA. (6.2)
After cancellations we obtain the hydrostatic equation:
dp
dz
=−ρg
[hydrostatic equation]. (6.3)
We can use the Ideal Gas Law to write:
dp
dz
=−
p(z)g
RT (z)
(6.4)
where we indicate explicitly that both temperature and pressure are functions of
altitude z. In the last step we used the ideal gas equation of state. The hydrostatic
equation has many uses, but it is particularly useful if the dependence of T on z is
known. This may often be the case, at least approximately. If it is true we can write:
dp
p
=−
g dz
RT (z)
. (6.5)

136 Profiles of the atmosphere
Then integrating each side from the surface up to level z leads to:
p(z)
p
0
dp
p
=−
g
R
z
0
dz
T (z
)
(6.6)
ln
p
p
0
=−
g
R
z
0
dz
T (z
)
(6.7)
where we have indicated the “dummy” integration variable by z
to distinguish it
from the upper limit of the integral. Finally,
p(z) = p
0
exp
−
g
R
z
0
dz
T (z
)
. (6.8)
If the integrals can be performed, we have an analytical expression for p(z). Even
if the z dependence of T is known only graphically or in tabular form, the integral
can be performed numerically.
Example 6.1 In Example 2.16 we found that a ball of mass m bouncing elastically
on the floor gives an average force on the floor of mg. We can also derive the
hydrostatic equation for a ball bouncing on the floor, but reflecting back elastically
on a ceiling only a short distance above. Let the ball leave the floor with vertical
velocity
v
0
and when it gets to the ceiling h, its velocity will be v
1
. The rate of
momentum transfer to the ceiling is 2m
v
1
/T , where T is the time for a round trip
ceiling to floor and back. We can show that T = 4h/(
v
0
+v
1
). The average pressures
exerted by the reflecting ball at the ceiling p
h
and at the floor p
0
are
p
h
=
m
v
1
(v
0
+ v
1
)
2hA
, p
0
=
m
v
0
(v
0
+ v
1
)
2hA
, (6.9)
where A is the area on the surface of the floor or ceiling, and the difference in the
pressures is
p
0
− p
h
=
m
2hA
(
v
0
− v
1
)(v
0
+ v
1
) =
m
2hA
(
v
2
0
− v
2
1
) =
m
2hA
2gh. (6.10)
We then have
p
0
− p
h
=
mgh
Ah
=
mgh
volume
= ρgh. (6.11)
Finally, p/z =−ρg. Of course, we are to picture a large number of balls
(molecules) bouncing up and down so as to make a steady pressure.
Example 6.2: constant density case Consider the case of constant density as a
function of height. This profile is more like the ocean than the atmosphere. Taking
