North, Gerald R., Erukhimova Tatiana L. Atmospheric Thermodynamics: Elementary Physics and Chemistry
Подождите немного. Документ загружается.

5.9 Wet-bulb temperature, LCL 117
Pressure
T
D
T
w
T
Temperature
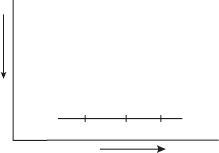
Figure 5.9 Relative positions of the temperature T , dew point T
D
, and wet-bulb
temperature T
w
of an air parcel.
5.9 Wet-bulb temperature, LCL
The thermodynamic state of an air parcel is determined by its temperature, pressure
and mixing ratio. However, in some applications it is not convenient to use the
mixing ratio. For example, the mixing ratio is not easily measured directly. There
is another indicator of atmospheric moisture called the wet-bulb temperature (T
w
)
which can be measured more directly. The wet-bulb temperature lies between the
dew point temperature and the air temperature (see Figure 5.9).
Before introducing the wet-bulb temperature, let us turn again to the dew point
temperature which is also a measure of moisture in the air. Recall that it is the
temperature to which air must be cooled at constant pressure in order to become
saturated with respect to a plane surface of water. As we perform the cooling we
must keep the mixing ratio of the air fixed. At this temperature the actual mixing
ratio becomes equal to the saturation mixing ratio:
w(T
D
) = 0.622e
s
(T
D
)/p. (5.42)
At temperatures higher than the dew point the air contains some moisture, but
less than the saturation value. As the temperature is decreased to the dew point
condensation occurs. Since at the dew point the mixing ratio is equal to the saturation
mixing ratio, it is evident that the dew point temperature is always lower than or
equal to the air temperature. If the two are close together, the relative humidity is
high. If the dew point is far below the air temperature, the relative humidity is low.
The dew point is a good indicator of human discomfort. When temperatures are high
it is more comfortable with a low T
D
rather than with a high dew point temperature
because of the higher relative humidity. When the relative humidity is high the
rate of evaporation from a moist surface is low (it is actually proportional to 100
minus the relative humidity in percent). Meteorologists often refer to the difference
between temperature and dew point, which is called the dew point depression.
The frost point is defined similarly to the dew point. The frost point is the
temperature to which air must be cooled at constant pressure in order to become
118 Air and water
saturated with respect to a plane surface of ice. Note that the mixing ratio is constant
during a constant pressure cooling of a parcel.
The dew point temperature is difficult to measure directly. It is easy to measure
the wet-bulb temperature. This is the temperature of a wet surface (nominally a wet
cloth wrapped around the bulb of a thermometer) that is immersed in the ambient
air. The wet surface evaporates moisture into the surrounding (typically less than
saturated) air and in so doing the temperature of the wet surface is lowered (as
perspiration into dry air cools the skin). The wet surface will come to an equilibrium
temperature after a short time if the air near the wet surface is continually ventilated
with the dryer ambient air. This equilibrium temperature of the wet cloth is called
the wet-bulb temperature. If the surrounding air is fully saturated, there will be no
net cooling since the rate of evaporation will just equal the rate of condensation onto
the wet surface, leaving the wet-bulb temperature to be the same as the dew point
temperature. For unsaturated air the wet-bulb temperature always falls between the
dew point temperature and the dry-bulb temperature (the difference between the
dry-bulb and wet-bulb temperatures is called the wet-bulb depression). Note that an
evaporating cloud droplet or raindrop has a temperature at the wet-bulb temperature.
The wet-bulb temperature can be measured with a sling psychrometer, which
consists of a thermometer with wet gauze covering its bulb. This hand-held device is
swung around from a short chain to maintain the proximity of fresh ambient air at the
wet surface. Without the swinging, stagnant saturated air would accumulate around
the wet bulb and raise its temperature to an erroneous level. Water molecules leaving
the wet surface diffuse away from the bulb through the thin boundary layer of air
surrounding it. At the same time heat is being conducted from the warmer ambient
air towards the cooler wet surface through the same thin boundary layer. Equilibrium
is established between the enthalpy flux due to evaporation carried by out-flowing
molecules and the in-flowing enthalpy flux. A formula can be derived for the relative
humidity given the wet-bulb and dry-bulb temperatures (see Wet-bulb derivation in
the box below). For practical use the relationship is commonly expressed in tables.
It is interesting that the geometrical configuration of the wet cloth surrounding the
bulb does not matter because those factors cancel. More discussion of the wet-bulb
temperature can be found in Exercises 7.8 and 7.9 in Chapter 7.
The saturation mixing ratio depends on temperature and air pressure, thus it
is a function of height. When a parcel is lifted adiabatically, its temperature and
pressure both decrease. The temperature dependence of the parcel is linear with
height (we will see in the next chapter that it is 10 K km
−1
). Hence, as the air rises
1 km the temperature will fall about 10 K. The saturation vapor pressure becomes
half its surface value because of this decrease (remember the rule of thumb about
the doubling of vapor pressure for every 10
◦
C). The mixing ratio w
0
stays the same
for this ascent, while the air pressure p(z) and the vapor pressure e(z) in the parcel
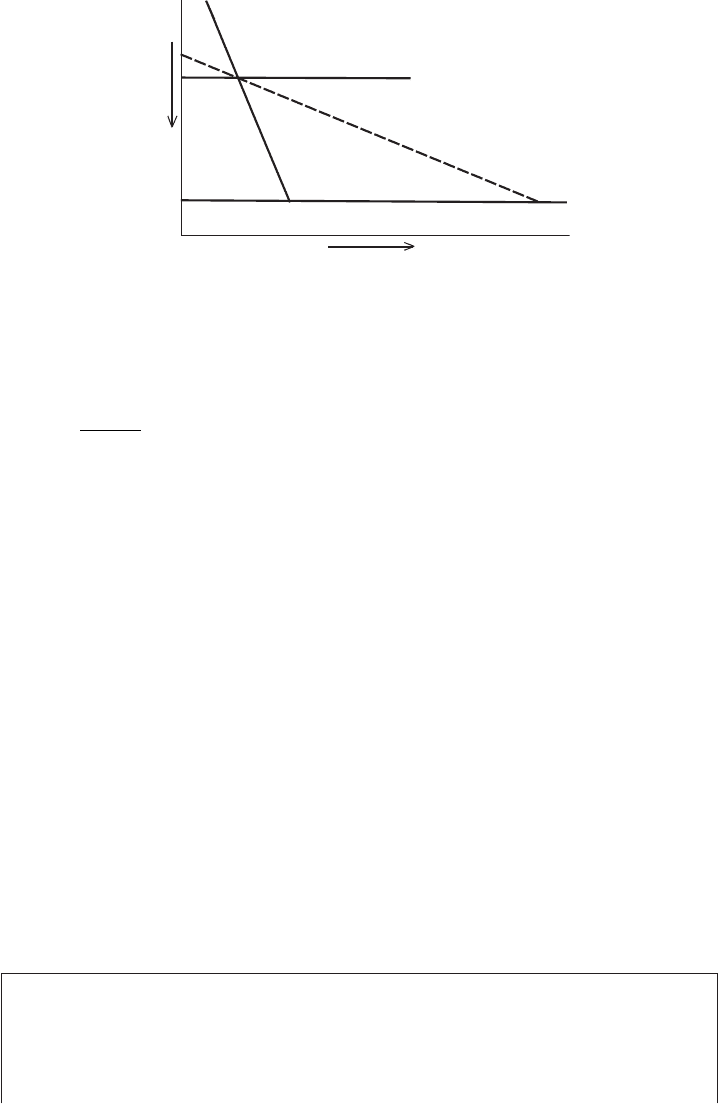
5.9 Wet-bulb temperature, LCL 119
Temperature
Pressure
T
T
D
w = const
s
Θ = const,
w = const
Lifting Condensation
Level
Figure 5.10 Illustration of the lifting condensation level (LCL).
each fall about 12% (using a scale height H of the atmosphere of about 8 km):
e(z) =
p(z)
w
0
0.622
, p(z) = p
0
e
−z/H
, w = w
0
= constant, e
−1/8
= 0.882. (5.43)
So the vapor pressure goes down by ≈12% while the saturation vapor pressure goes
down by 50%. This shows that the saturation vapor pressure is decreasing much
faster than the actual vapor pressure in the parcel. As this continues (the same
percentages for each kilometer of ascent) the curves will cross and condensation
will occur. The level at which an initially unsaturated parcel reaches its saturation
level while being lifted adiabatically is called the lifting condensation level (LCL).
If we know the temperature, pressure and mixing ratio of an air parcel, we can find
the LCL, where condensation starts to occur along the ascent.
If we know the mixing ratio, we can easily find the dew point and wet-bulb
temperature and vice versa. Figure 5.10 shows the LCL for a parcel at initial
temperature T , pressure p, and dew point T
D
. It is located at the intersection
of the line with constant potential temperature (the unsaturated parcel ascends
dry adiabatically) and the line of constant saturation mixing ratio starting at the
dew point temperature (this is because the mixing ratio is fixed at its initial value
during this noncondensing part of the ascent). The physical significance of these
parameters will be more clear when we start to work with thermodynamic diagrams
in Chapter 7.
Wet-bulb derivation In the preceding we indicated that the geometrical factors
cancel in the wet-bulb depression. To show this we can derive the relationship
between the relative humidity and the wet-bulb depression. For simplicity we take the
bulb to be a sphere of radius R
0
, but in the end it does not matter what the geometrical
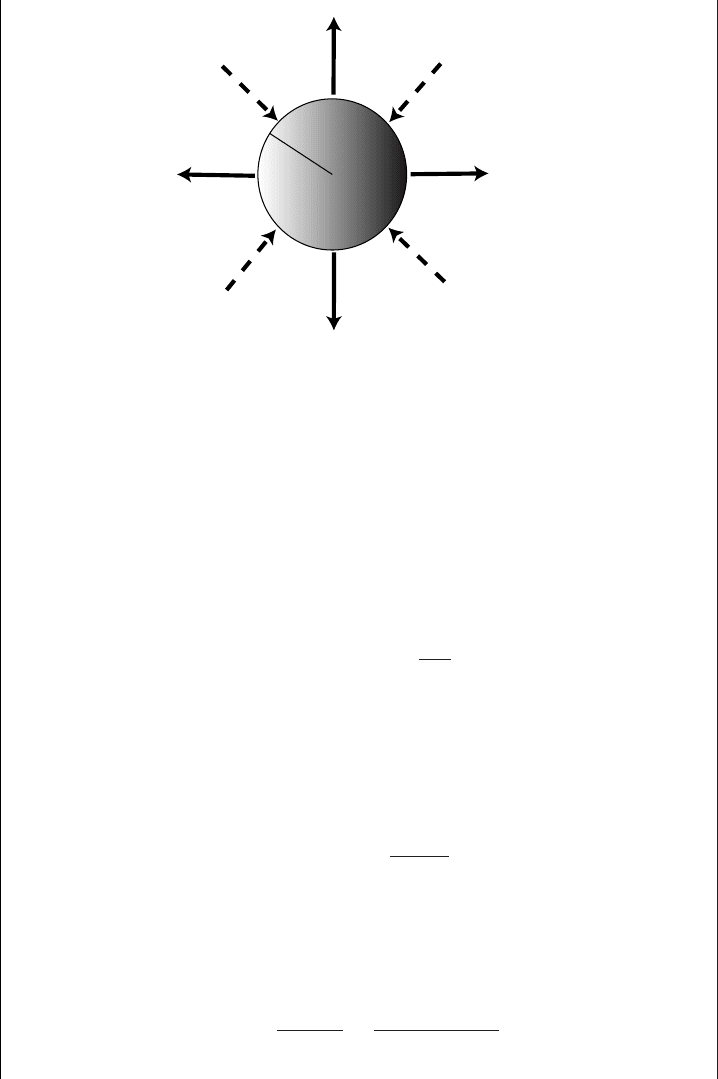
120 Air and water
R
o
water molecules
thermal energy flu
x
Figure 5.11 Water vapor molecules diffuse away from the wet surface as heat is
conducted towards the wet surface. When these fluxes match, the temperature at the
wet surface will be the wet-bulb temperature.
shape is. There are two fluxes from the sphere that have to be computed: (1) the flux
of water vapor molecules leaving the sphere due to evaporation toward less moist air
far away; (2) the heat conducted to the sphere because of the warmer air far away
(Figure 5.11).
The water vapor molecules diffuse away with a flux F
w
(r) where r is the distance
from the center of the sphere, r ≥ R
0
. The flux at any value of r passing through a
surrounding sphere is
F
w
(r) =−D4π r
2
dn
w
dr
(5.44)
where D is the diffusion coefficient for this process (known from tables), n
w
(r) is the
number density of vapor molecules at radius r. This flux must be independent of r
since otherwise there would be a source of flux other than the wet bulb’s surface.
We can then set F
w
(r) = F
0
= constant. This allows us to integrate each side from
R
0
to ∞ after dividing through by 4πr
2
D. This produces:
n
∞
− n
R
0
=−
F
0
4πR
0
D
. (5.45)
We can recognize that
n
R
0
= n
sat
(T
w
) (5.46)
where n
sat
(T
w
) is the saturation number density for T = T
w
. If we divide through by
n
sat
(T
D
) we have
RH −
n
R
0
n
sat
(T
D
)
=
−F
0
4πR
0
Dn
sat
(T
D
)
(5.47)

5.10 Equilibrium vapor pressure over a curved surface 121
where RH = n
∞
/n
sat
(T
D
) is the relative humidity away from the wet bulb. So far we
do not know the value of F
0
in the above formula. Another measurement is required.
We must now turn to condition (2), the heat conduction. Heat flows from infinity
toward the wet bulb by heat conduction. The flux of heat crossing a spherical surface
of radius r is
H (r) = 4πr
2
κ
H
dT
dr
= H
0
= constant (5.48)
where κ
H
is the (available from tables) thermal conductivity coefficient and the same
argument is used as above for the constancy of the flux passing through spheres of
different radii.
As above we can integrate after dividing through by the factor 4π r
2
κ
H
. We get:
T
D
− T
w
=
H
0
4πR
0
κ
H
. (5.49)
There is one other condition, that the flux of heat has to be related to the flux of
vapor molecules. Each molecule of water vapor leaving to infinity cools the wet bulb
by =
L/N
A
where L is the enthalpy of vaporization per mole of water vapor and N
A
is Avogadro’s number. This says that
H
0
= F
0
. (5.50)
Now we can substitute for H
0
and divide the two equations above to obtain:
RH =
n
sat
(T
w
)
n
sat
(T
D
)
−
κ
H
D
(T
D
− T
w
)
n
sat
(T
D
)
. (5.51)
Using the psychrometer we measure both T
D
and T
w
. We can then calculate n
sat
(T
D
)
and n
sat
(T
w
) from the Clausius–Clapeyron relation. The only unknown above is RH,
which can now be calculated. The computation is tedious, hence, the tables. Note that
the geometric factors 4π R
0
cancelled out. It can be shown that no matter what the
geometrical shape of the web bulb, these geometrical quantities will cancel out and
the wet-bulb temperature is independent of the shape of the bulb. So why swing
the psychrometer around? The reason is to have fresh environmental air within a
millimeter or two of the wet bulb to insure that the moist air at infinity is
representative and not contaminated by the wet bulb’s evaporation.
5.10 Equilibrium vapor pressure over a curved surface
So far our discussion of the saturation vapor pressure has been restricted to that
over a plane surface of water. However, in atmospheric physics we also encounter
situations where the surface is curved. This is the case, for example, in cloud droplet
formation. Cloud droplets have approximately spherical shape, which means that
growth of a droplet implies an increase of the surface area of the drop. Increasing
122 Air and water
surface area of a liquid requires work (consider blowing a soap bubble). Growth of a
droplet then requires consideration of surface tension. Thus to find the equilibrium
vapor pressure over a droplet we have to include surface tension in the energy
balance. The energy required for an increase of surface area dA is σ dA, where σ
is the surface tension (surface energy per unit area), in J m
−2
(the value for water
is 0.0761 J m
−2
at 0
◦
C). The energy required to create a spherical drop
4
of radius
a is σ 4π a
2
.
Consider the formation of a cloud droplet from pure water vapor (no aerosols
or other impurities present). Such a process is called homogeneous nucleation as
opposed to heterogeneous nucleation, when small aerosol particles take part in the
droplet formation. Suppose that initially, at t = t
1
, we have water vapor of mass M
at partial pressure e and temperature T . The Gibbs energy of the system, G
initial
,is
G
initial
= Mg
v
(5.52)
where g
v
is the specific (per unit mass) Gibbs energy of the water vapor. It depends
on the vapor pressure and the temperature. Suppose that at some later time, t = t
2
,
an embryonic droplet starts to form. It grows by occasional sticking collisions by
water vapor molecules and at some moment develops a radius a and mass
M
w
.
The spherical surface area of the droplet is A = 4πa
2
. The total mass is conserved,
so if
M
v
is the mass of water vapor remaining after condensation, then
M
v
+ M
w
= M. (5.53)
The total Gibbs energy of the system at time t
2
, G
final
,is
G
final
= g
v
M
v
+ g
w
M
w
+ σ A. (5.54)
The first term on the right-hand side of (5.54) is the Gibbs energy of the water
vapor, the second is the Gibbs energy of the liquid, and the last term is due to
surface tension.
The change of the Gibbs energy due to the droplet formation can be found by
subtracting (5.52) from (5.54) and taking into account (5.53):
G
final
− G
initial
= (g
w
− g
v
)M
w
+ σ A. (5.55)
The next step is to find the difference g
w
−g
v
. We know that at a constant temperature
(in our case the temperature is fixed) the change in the Gibbs energy is dg =
v dp,
4
The example of a soap bubble is helpful. Soapy water has a higher surface tension coefficient than pure water
so the surface tension is very important. Also one must remember that the bubble has twice the surface area
because there is an inside surface as well as an outside surface, in contrast to the water droplet.

5.10 Equilibrium vapor pressure over a curved surface 123
vapor pressure
e
vapor pressure
e
s
vapor
droplet
liquid with flat surface
Figure 5.13 Schematic diagram illustrating the notation for equilibrium vapor
pressure over a droplet, e, and that over a flat surface, e
s
.
where v is the specific volume (v = (density)
−1
). Then, for the vapor we have
dg
v
= v
v
de, for the liquid dg
w
= v
w
de, and the difference is equal to
d(g
v
− g
w
) = (v
v
− v
w
)de ≈ v
v
de (5.56)
since
v
v
v
w
. Substituting specific volume v
v
from the Ideal Gas Law, we obtain
d(g
v
− g
w
) = R
w
T
de
e
= R
w
T d(ln e) (5.57)
and, after the integration,
g
v
− g
w
= R
w
T ln e + constant. (5.58)
We can find the constant of integration by taking into account that in equilibrium
(see Figure 5.13), along the phase boundary where e = e
s
(T ), g
w
= g
v
(see (5.7)).
Then,
g
v
− g
w
= R
w
T ln
e
e
s
. (5.59)
Substituting (5.59) into (5.55) gives
G
final
− G
initial
=−R
w
T ln
e
e
s
M
w
+ σ A. (5.60)
The mass of a spherical water droplet with radius a and density ρ
w
is M
w
=
4
3
πρ
w
a
3
, and the surface area is A = 4πa
2
. Then the change in the Gibbs energy
due to the droplet formation, G = G
final
− G
initial
, is:
G =−
4
3
πa
3
ρ
w
R
w
T ln
e
e
s
+ 4πa
2
σ . (5.61)
From (5.61) we see that in the subsaturated air, when e < e
s
,ln(e/e
s
) is negative,
and G is always positive (see Figure 5.14). From Section 4.8.2 we know that
equilibrium occurs when the minimum of the Gibbs energy is achieved; or, in other
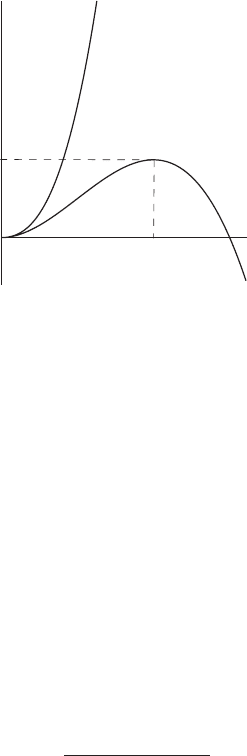
124 Air and water
∆G
a
e < e
s
e > e
s
a
*
Figure 5.14 The change of Gibbs energy due to the formation of a water droplet
as a function of the radius of the droplet.
words, the system tends spontaneously toward its equilibrium state by having the
Gibbs energy diminish more and more until it reaches its minimum. We conclude
that there are no favorable conditions for cloud droplet (a > 0) formation in
unsaturated air.
The situation is different when the air is supersaturated, which means that e > e
s
and ln (e/e
s
) is positive (see Figure 5.14). In this case G increases as the radius
a increases, then it reaches a maximum at some radius, and then decreases with the
further increase of a. If the cloud droplet has a radius less than the critical radius a
∗
,
it will disappear by evaporation. If, however, the cloud droplet reaches the critical
radius a
∗
, then it will continue to grow. We can find an expression for the critical
radius by equating the derivative ∂G/∂a to zero. The result is
a
∗
=
2σ
ρ
w
R
w
T ln (e/e
s
)
(5.62)
which is called Kelvin’s formula. It allows one to find the radius a
∗
of a droplet
which is in equilibrium with air with the vapor pressure e. This equilibrium state
is unstable. This becomes evident if we consider a slight growth of the droplet.
This growth might be due to the condensation of water vapor in the vicinity of the
droplet, which means that the relative humidity decreases just above the surface.
Since the air in the vicinity of the droplet becomes drier, there is a diffusive flux of
moist air toward the droplet, and the condensation process continues, leading to the
further growth of the droplet. On the other hand, if the droplet evaporates slightly,
the relative humidity just above the droplet surface increases, the water vapor starts
to diffuse from the droplet, and the droplet will continue to evaporate to maintain
the relative humidity corresponding to radius a
∗
.

5.10 Equilibrium vapor pressure over a curved surface 125
We can rewrite (5.62) in order to determine the equilibrium vapor pressure e over
a droplet with radius a,
e = e
s
exp
2σ
ρ
w
R
w
Ta
= e
s
e
b/a
(5.63)
where the parameter b is defined by
b =
2σ
ρ
w
R
w
T
. (5.64)
If we substitute σ = 0.076 J m
−2
, ρ
w
= 1000 kg m
−3
, and R
w
= 461.5 J kg
−1
K
−1
,
we obtain
b =
3.30 × 10
−7
T
(5.65)
(in meters). At 273 K, b = 1.21 nm (typically b a). Formula (5.63) shows that
the equilibrium vapor pressure over a spherical droplet is not equal to the saturation
vapor pressure as determined over a plane surface of water. This happens because
of the surface tension. If the radius of the droplet goes to infinity, a →∞, which
corresponds to a plane surface, we obtain the result for a flat surface e = e
s
(see
Figure 5.13).
Example 5.8 Water droplets are in equilibrium with surrounding vapor at a
temperature of 2
◦
C. Calculate the vapor pressure and relative humidity over the
droplet with radius 0.008 µm.
Answer: The saturation vapor pressure at 2
◦
C is: e
s
= 2.497 ×10
9
exp(−5417/
275.2) (hPa) = 7.06 hPa. From (5.63) we obtain e = 7.06 × exp(3.3 ×10
−7
/
(275.2 ×0.008 ×10
−6
)) = 8.2 hPa. RH = 116%. A supersaturation of 16% is
required for the creation of a cloud droplet with radius 0.008 µm by homogeneous
nucleation at 2
◦
C.
To obtain the relative humidity, divide both sides of (5.63)bye
s
:
e
e
s
= e
b/a
. (5.66)
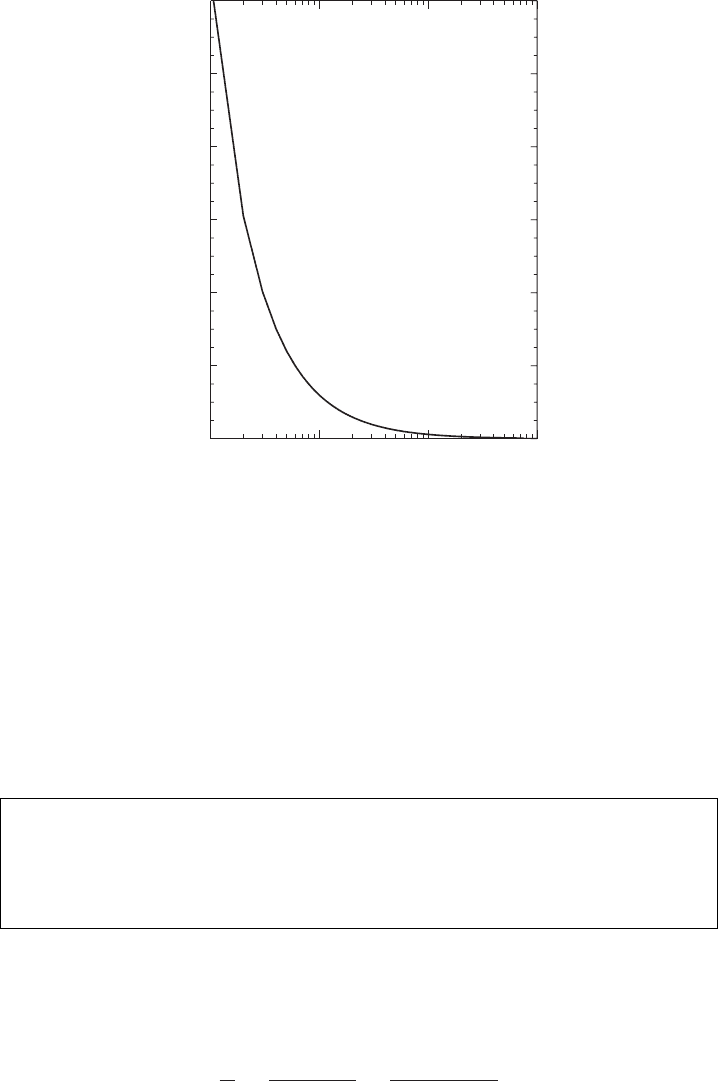
This relationship is illustrated in Figure 5.15 for a temperature of 5
◦
C. As shown
in the figure, the formation of a droplet with radius 0.01 µm requires supersaturation
of 112%.At the same time, in real clouds the relative humidity rarely exceeds 101%.
As we see from Figure 5.15, 1.0% supersaturation is required to form a droplet with
radius larger than 0.1 µm. Large droplets in a cloud simply cannot form by random
collisions of molecules on their surfaces. From the above discussion it follows
that the process of homogeneous nucleation is unlikely. In nature cloud droplets
form by heterogeneous nucleation on atmospheric aerosols. Consider then how the
126 Air and water
0.10 1.00 10.000.01
100
102
104
106
108
110
112
Droplet radius, µm
Relative humidity,%
Figure 5.15 The relative humidity as a function of droplet radius. The curve is
plotted for a temperature of 5
◦
C. This graph shows the relative humidity necessary
to form a droplet of the size indicated on the abscissa.
equilibrium vapor pressure over the droplet changes if the droplet contains dissolved
electrolytes (usually from aerosols, e.g. sea salt). We will consider hygroscopic
particles (those that are soluble in water). The most common of these are sodium
chloride (NaCl) and ammonium sulfate ((NH
4
)
2
SO
4
) which dissolve when water
condenses onto them. In this case a water droplet can be treated as a solution with
the water as a solvent and the salt as a solute.
Chemistry refresher: Raoult’s Law The ratio of the equilibrium vapor pressure
over a solution, e
, to the equilibrium vapor pressure over pure solvent, e, is equal to
the mole fraction of the solvent in the solution, f , e
/e = f . The presence of salt in
solution always lowers the vapor pressure.
Consider a cloud droplet that contains n
salt
molecules of salt and n
w
molecules
of water per unit volume. If e
is the equilibrium vapor pressure over the solution
(see Figure 5.16) then, according to Raoult’s Law,
e
e
=
n
w
n
w
+ n
salt
=
1
1 + n
salt
/n
w
(5.67)
