Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.


A special form of Eq. 14.61 for a system at equilibrium consisting of a liquid or solid
phase and a vapor phase can be obtained simply. If the specific volume of the liquid or solid,
is negligible compared with the specific volume of the vapor, and the vapor can be
treated as an ideal gas, Eq. 14.61 becomes
or
(14.62)
which is the Clausius–Clapeyron equation. The similarity in form of Eq. 14.62 and the van’t
Hoff equation, Eq. 14.43b, may be noted. The van’t Hoff equation for chemical equilibrium
is the counterpart of the Clausius–Clapeyron equation for phase equilibrium.
d ln p
sat
dT
h– h¿
RT
2
dp
sat
dT
h– h¿
RT
2
p
sat
v– R T
p
sat
,
v–,v¿,
706 Chapter 14 Chemical and Phase Equilibrium
14.6 Equilibrium of Multicomponent,
Multiphase Systems
The equilibrium of systems that may involve several phases, each having a number of compo-
nents present, is considered in this section. The principal result is the Gibbs phase rule, which
summarizes important limitations on multicomponent, multiphase systems at equilibrium.
14.6.1 Chemical Potential and Phase Equilibrium
Figure 14.1 shows a system consisting of two components A and B in two phases 1 and 2
that are at the same temperature and pressure. Applying Eq. 14.10 to each of the phases
(14.63)
where as before the primes identify the two phases.
When matter is transferred between the two phases in the absence of chemical reaction,
the total amounts of A and B must remain constant. Thus, the increase in the amount present
in one of the phases must be compensated by an equivalent decrease in the amount present
in the other phase. That is
(14.64)dn–
A
dn¿
A
,
dn–
B
dn¿
B
dG– 4
T,p
m–
A
dn–
A
m–
B
dn–
B
dG¿ 4
T,p
m¿
A
dn¿
A
m¿
B
dn¿
B
Phase 2
Component A, n´´
A
, ´´
A
Component B, n´´
B
, ´´
B
µ
µ
Phase 1
Component A, n´
A
, ´
A
Component B, n´
B
, ´
B
µ
µ
Figure 14.1 System consisting of two components in two
phases.
Clausius–Clapeyron
equation

14.6 Equilibrium of Multicomponent, Multiphase Systems 707
With Eqs. 14.63 and 14.64, the change in the Gibbs function for the system is
(14.65)
Since and can be varied independently, it follows that when dG]
T,p
0, the terms in
parentheses are zero, resulting in
(14.66)
At equilibrium, the chemical potential of each component is the same in each phase.
The significance of the chemical potential for phase equilibrium can be brought out sim-
ply by reconsidering the system of Fig. 14.1 in the special case when the chemical potential
of component B is the same in both phases: With this constraint, Eq. 14.65 reduces
to
Any spontaneous process of the system taking place at a fixed temperature and pressure must
be such that the Gibbs function decreases: dG]
T,p
0. Thus, with the above expression we
have
Accordingly,
when the chemical potential of A is greater in phase 1 than in phase 2 it
follows that That is, substance A passes from phase 1 to phase 2.
when the chemical potential of A is greater in phase 2 than in phase 1 it
follows that That is, substance A passes from phase 2 to phase 1.
At equilibrium, the chemical potentials are equal and there is no net transfer of
A between the phases.
With this reasoning, we see that the chemical potential can be regarded as a measure of
the escaping tendency of a component. If the chemical potential of a component is not the
same in each phase, there will be a tendency for that component to pass from the phase hav-
ing the higher chemical potential for that component to the phase having the lower chemi-
cal potential. When the chemical potential is the same in both phases, there is no tendency
for a net transfer to occur from one phase to the other.
In Example 14.10, we apply phase equilibrium principles to provide a rationale for the
model introduced in Sec. 12.5.3 for moist air in contact with liquid water.
(m¿
A
m–
A
),
dn¿
A
7 0.
(m–
A
7 m¿
A
),
dn¿
A
6 0.
(m¿
A
7 m–
A
),
1m¿
A
m–
A
2 dn¿
A
6 0
dG4
T,p
1m¿
A
m–
A
2 dn¿
A
m¿
B
m–
B
.
m¿
A
m–
A
and
m¿
B
m–
B
n¿
B
n¿
A
1m¿
A
m–
A
2
dn¿
A
1m¿
B
m–
B
2
dn¿
B
dG4
T,p
dG¿ 4
T,p
dG– 4
T,p
EXAMPLE 14.10 Equilibrium of Moist Air in Contact with Liquid Water
A closed system at a temperature of 20C and a pressure of 1 bar consists of a pure liquid water phase in equilibrium with a
vapor phase composed of water vapor and dry air. Determine the departure, in percent, of the partial pressure of the water
vapor from the saturation pressure of water at 20C.
SOLUTION
Known: A phase of liquid water only is in equilibrium with moist air at 20C and 1 bar.
Find: Determine the percentage departure of the partial pressure of the water vapor in the moist air from the saturation
pressure of water at 20C.

708 Chapter 14 Chemical and Phase Equilibrium
Schematic and Given Data:
Analysis: For phase equilibrium, the chemical potential of the water must have the same value in both phases:
1
v
,
where
1
and
v
denote, respectively, the chemical potentials of the pure liquid water in the liquid phase and the water vapor
in the vapor phase.
The chemical potential
1
is the Gibbs function per mole of pure liquid water (Eq. 14.12)
Since the vapor phase is assumed to form an ideal gas mixture, the chemical potential
v
equals the Gibbs function per mole
evaluated at temperature T and the partial pressure p
v
of the water vapor (Eq. 14.16)
For phase equilibrium,
1
v
,or
With this can be expressed alternatively as
The water vapor is modeled as an ideal gas. Thus, the enthalpy is given closely by the saturated vapor value at tempera-
ture T
Furthermore, with Eq. 6.21b, the difference between the specific entropy of the water vapor and the specific entropy at the
corresponding saturated vapor state is
or
where p
sat
is the saturation pressure at temperature T.
With Eq. 3.13, the enthalpy of the liquid is closely
where and are the saturated liquid specific volume and enthalpy at temperature T. Furthermore, with Eq. 6.7
where is the saturated liquid specific entropy at temperature T.s
f
s 1T, p2 s
f
1T 2
h
f
v
f
h 1T, p2 h
f
v
f
1p p
sat
2
s
1T, p
v
2 s
g
1T 2 R ln
p
v
p
sat
s 1T, p
v
2 s
g
1T 2R ln
p
v
p
sat
h 1T, p
v
2 h
g
h 1T, p
v
2 T s 1T, p
v
2 h 1T, p2 T s 1T, p2
g
h T s,
g
1T, p
v
2 g 1T, p2
m
v
g 1T, p
v
2
m
1
g 1T, p2
Assumptions:
1. The gas phase can be modeled as an ideal gas
mixture.
2. The liquid phase is pure water only.
❶
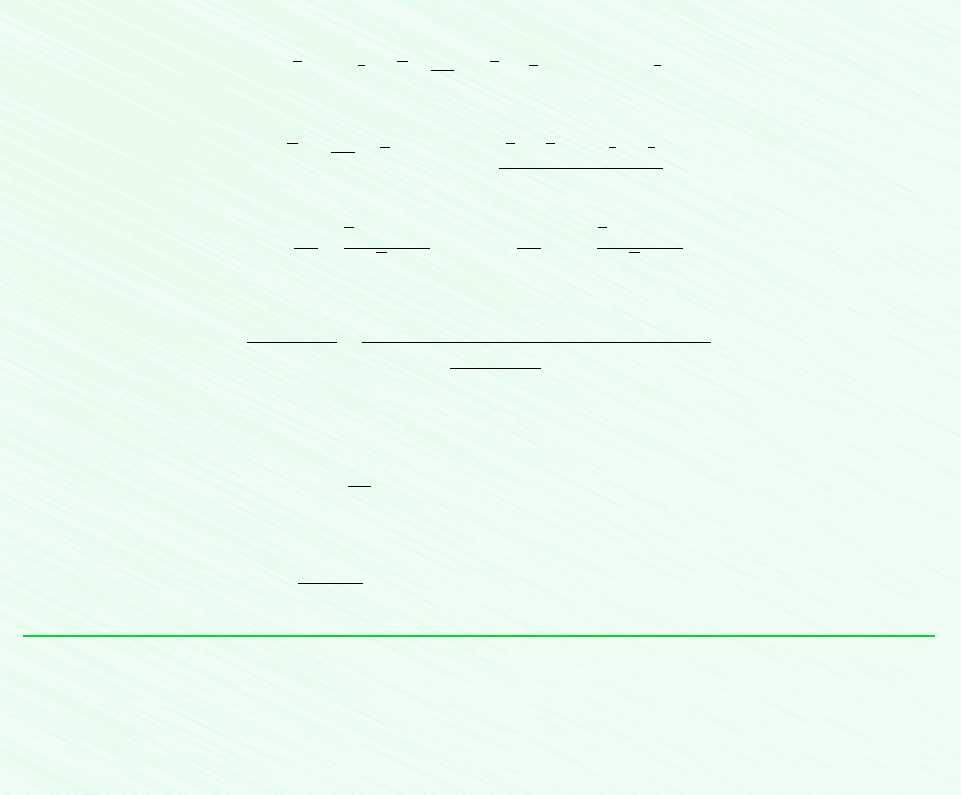
Figure E14.10
20C
Gas phase of
water vapor
and dry air
Liquid water
T
v
p = 1 bar
Sat. liquid
Sat. vapor
State of
the liquid
State of
the water
vapor
f
g
p
v
p
sat
14.6 Equilibrium of Multicomponent, Multiphase Systems 709
❷
Collecting the foregoing expressions, we have
or
The underlined term vanishes by Eq. 14.60, leaving
With data from Table A-2 at 20C, v
f
1.0018 10
3
m
3
/kg and p
sat
0.0239 bar, we have
Finally
When expressed as a percentage, the departure of p
v
from p
sat
is
For phase equilibrium, there would be a small, but finite, concentration of air in the liquid water phase. However, this
small amount of dissolved air is ignored in the present development.
The departure of p
v
from p
sat
is negligible at the specified conditions. This suggests that at normal temperatures and pressures
the equilibrium between the liquid water phase and the water vapor is not significantly disturbed by the presence of the
dry air. Accordingly, the partial pressure of the water vapor can be taken as equal to the saturation pressure of the water
at the system temperature. This model, introduced in Sec. 12.5.3, is used extensively in Chap. 12.
a
p
v
p
sat
p
sat
b 11002 11.00073 12 11002 0.072%
p
v
p
sat
exp 17.23 10
4
2 1.00072
7.23 10
4
v
f
1
p p
sat
2
RT
1.0018 10
3
m
3
/kg 11 0.02392 10
5
N/m
2
a
8314 N
#
m
18.02 kg
#
K
b 1293.15 K2
ln
p
v
p
sat
v
f
1p p
sat
2
RT
or
p
v
p
sat
exp
v
f
1p p
sat
2
RT
R
T ln
p
v
p
sat
v
f
1p p
sat
2 31h
g
h
f
2 T 1s
g
s
f
24
h
g
T as
g
R ln
p
v
p
sat
b h
f
v
f
1p p
sat
2 T s
f
❶
❷
14.6.2 Gibbs Phase Rule
The requirement for equilibrium of a system consisting of two components and two phases,
given by Eqs. 14.66, can be extended with similar reasoning to nonreacting multicomponent,
multiphase systems. At equilibrium, the chemical potential of each component must be the
same in all phases. For the case of N components that are present in P phases we have, there-
fore, the following set of N(P 1) equations:
(14.67)
where denotes the chemical potential of the ith component in the jth phase. This set of
equations provides the basis for the Gibbs phase rule, which allows the determination of the
m
j
i
N e
m
1
1
m
2
1
m
3
1
###
m
P
1
m
1
2
m
2
2
m
3
2
###
m
P
2
#
#
#
m
1
N
m
2
N
m
3
N
###
m
P
N
P 1
w

number of independent intensive properties that may be arbitrarily specified in order to fix
the intensive state of the system. The number of independent intensive properties is called
the degrees of freedom (or the variance).
Since the chemical potential is an intensive property, its value depends on the relative
proportions of the components present and not on the amounts of the components. In other
words, in a given phase involving N components at temperature T and pressure p, the chem-
ical potential is determined by the mole fractions of the components present and not the re-
spective n’s. However, as the mole fractions add to unity, at most N 1 of the mole fractions
can be independent. Thus, for a system involving N components, there are at most N 1
independently variable mole fractions for each phase. For P phases, therefore, there are at
most P(N 1) independently variable mole fractions. In addition, the temperature and pres-
sure, which are the same in each phase, are two further intensive properties, giving a max-
imum of P(N 1) 2 independently variable intensive properties for the system. But
because of the N(P 1) equilibrium conditions represented by Eqs. 14.67 among these
properties, the number of intensive properties that are freely variable, the degrees of free-
dom F,is
(14.68)
which is the Gibbs phase rule.
In Eq. 14.68, F is the number of intensive properties that may be arbitrarily specified and
that must be specified to fix the intensive state of a nonreacting system at equilibrium.
for example... let us apply the Gibbs phase rule to a liquid solution consisting of
water and ammonia such as considered in the discussion of absorption refrigeration (Sec. 10.5).
This solution involves two components and a single phase: N 2 and P 1. Equation 14.68
then gives F 3, so the intensive state is fixed by giving the values of three intensive properties,
such as temperature, pressure, and the ammonia (or water) mole fraction.
The phase rule summarizes important limitations on various types of systems. For ex-
ample, for a system involving a single component such as water, N 1 and Eq. 14.68
becomes
(14.69)
The minimum number of phases is one, corresponding to P 1. For this case,
Eq. 14.69 gives F 2. That is, two intensive properties must be specified to fix the
intensive state of the system. This requirement is familiar from our use of the steam
tables and similar property tables. To obtain properties of superheated vapor, say, from
such tables requires that we give values for any two of the tabulated properties, for
example, T and p.
When two phases are present in a system involving a single component, N 1 and
P 2. Equation 14.69 then gives F 1. That is, the intensive state is determined by a
single intensive property value. For example, the intensive states of the separate phases
of an equilibrium mixture of liquid water and water vapor are completely determined
by specifying the temperature.
The minimum allowable value for the degrees of freedom is zero: F 0. For a single-
component system, Eq. 14.69 shows that this corresponds to P 3, a three-phase
system. Thus, three is the maximum number of different phases of a pure component
that can coexist in equilibrium. Since there are no degrees of freedom, both temperature
and pressure are fixed at equilibrium. For example, there is only a single temperature
0.01C and a single pressure 0.6113 kPa for which ice, liquid water, and water vapor
are in equilibrium.
F 3 P
F 3P1N 12 24 N1P 12 2 N P
710 Chapter 14 Chemical and Phase Equilibrium
degrees of freedom
Gibbs phase rule

14.6 Equilibrium of Multicomponent, Multiphase Systems 711
The phase rule given here must be modified for application to systems in which chemical
reactions occur. Furthermore, the system of equations, Eqs. 14.67, giving the requirements for
phase equilibrium at a specified temperature and pressure can be expressed alternatively in
terms of partial molal Gibbs functions, fugacities, and activities, all of which are introduced in
Sec. 11.9. To use any such expression to determine the equilibrium composition of the differ-
ent phases present within a system at equilibrium requires a model for each phase that allows
the relevant quantities—the chemical potentials, fugacities, and so on—to be evaluated for the
components present in terms of system properties that can be determined. For example, a gas
phase might be modeled as an ideal gas mixture or, at higher pressures, as an ideal solution.
Chapter Summary and Study Guide
In this chapter, we have studied chemical equilibrium and
phase equilibrium. The chapter opens by developing criteria
for equilibrium and introducing the chemical potential. In the
second part of the chapter, we study the chemical equilibrium
of ideal gas mixtures using the equilibrium constant concept.
We also utilize the energy balance and determine the equi-
librium flame temperature as an application. The final part of
the chapter concerns phase equilibrium, including multicom-
ponent, multiphase systems and the Gibbs phase rule.
The following list provides a study guide for this chapter.
When your study of the text and end-of-chapter exercises has
been completed, you should be able to
write out the meaning of the terms listed in the margin
throughout the chapter and understand each of the related
concepts. The subset of key concepts listed below is
particularly important.
apply the equilibrium constant relationship, Eq. 14.35, to
determine the third quantity when any two of tempera-
ture, pressure, and equilibrium composition of an ideal
gas mixture are known. Special cases include applica-
tions with simultaneous reactions and systems involving
ionized gases.
use chemical equilibrium concepts with the energy bal-
ance, including determination of the equilibrium flame
temperature.
apply Eq. 14.43b, the van’t Hoff equation, to determine
the enthalpy of reaction when the equilibrium constant is
known, and conversely.
apply the Gibbs phase rule, Eq. 14.68.
Key Engineering Concepts
Gibbs function p. 719
equilibrium
criterion
p. 720
chemical
potential
p. 721
equation of reaction
equilibrium p. 725
equilibrium
constant p. 727
Gibbs phase rule p. 749
Exercises: Things Engineers Think About
1. Why is using the Gibbs function advantageous when study-
ing chemical and phase equilibrium?
2. For Eq. 14.6 to apply at equilibrium, must a system attain
equilibrium at fixed T and p?
3. Show that (dA)
T,V
0 is a valid equilibrium criterion, where
A U TS is the Helmholtz function.
4. A mixture of 1 kmol of CO and kmol of O
2
is held at am-
bient temperature and pressure. After 100 hours only an
insignificant amount of CO
2
has formed. Why?
5. Why might oxygen contained in an iron tank be treated as
inert in a thermodynamic analysis even though iron oxidizes in
the presence of oxygen?
1
2
6. For how does pressure affect the
equilibrium composition?
7. For each of the reactions listed in Table A-27, the value of log
10
K
increases with increasing temperature. What does this imply?
8. For each of the reactions listed in Table A-27, the value of the
equilibrium constant K at 298 K is relatively small. What does
this imply?
9. If a system initially containing CO
2
and H
2
O were held at fixed
T, p, list chemical species that might be present at equilibrium.
10. Using Eq. 14.12 together with phase equilibrium considera-
tions, suggest how the chemical potential of a mixture compo-
nent could be evaluated.
CO
2
H
2
S
d
CO H
2
O,

712 Chapter 14 Chemical and Phase Equilibrium
Problems: Developing Engineering Skills
Working with the Equilibrium Constant
14.1 Determine the change in the Gibbs function G at 25C,
in kJ/kmol, for the reaction
using
(a) Gibbs function of formation data.
(b) enthalpy of formation and absolute entropy data.
14.2 Calculate the equilibrium constant, expressed as log
10
K,
for at (a) 500 K, (b) 1800R. Compare with
values from Table A-27.
14.3 Calculate the equilibrium constant, expressed as log
10
K,
for the water-gas reaction CO H
2
O(g) CO
2
H
2
at
(a) 298 K, (b) 1000 K. Compare with values from Table A-27.
14.4 Calculate the equilibrium constant, expressed as log
10
K,
for at (a) 298 K, (b) 2000K. Compare with
values from Table A-27.
14.5 Using data from Table A-27, determine log
10
K at 2500 K
for
(a)
(b)
(c)
14.6 In Table A-27, log
10
K is nearly linear in 1T: log
10
K
C
1
C
2
T, where C
1
and C
2
are constants. For selected
reactions listed in the table
(a) verify this by plotting log
10
K versus 1T temperature rang-
ing from 2100 to 2500 K.
(b) evaluate C
1
and C
2
for any pair of adjacent table entries in
the temperature range of part (a).
14.7 Determine the relationship between the ideal gas equilib-
rium constants K
1
and K
2
for the following two alternative ways
of expressing the ammonia synthesis reaction:
1.
2.
14.8 Consider the reactions
1.
2.
3.
Show that K
1
(K
3
K
2
)
12
.
14.9 Consider the reactions
1.
2.
3. H
2
O
S
d
H
2
1
2
O
2
CO
2
S
d
CO
1
2
O
2
CO
2
H
2
S
d
CO H
2
O
2H
2
O
S
d
2H
2
O
2
2CO
2
S
d
2CO O
2
CO H
2
O
S
d
H
2
CO
2
N
2
3H
2
S
d
2NH
3
1
2
N
2
3
2
H
2
S
d
NH
3
2H
2
O
S
d
2H
2
O
2
.
H
2
1
2
O
2
S
d
H
2
O.
H
2
O
S
d
H
2
1
2
O
2
.
H
2
O
S
d
H
2
1
2
O
2
S
d
CO
2
S
d
CO
1
2
O
2
CH
4
1g2 2O
2
S
d
CO
2
2H
2
O 1g2
(a) Show that K
1
K
2
K
3
.
(b) Evaluate log
10
K
1
at 298 K, 1 atm using the expression from
part (a), together with log
10
K data from Table A-27.
(c) Check the value for log
10
K
1
obtained in part (b) by apply-
ing Eq. 14.31 to reaction 1.
14.10 Evaluate the equilibrium constant at 2000 K for
At 2000 K, log
10
K 7.469 for
and log
10
K 3.408 for
14.11 For each of the following dissociation reactions, deter-
mine the equilibrium compositions:
(a) One kmol of N
2
O
4
dissociates to form an equilibrium ideal
gas mixture of N
2
O
4
and NO
2
at 25C, 2 atm. For
G5400 kJ/kmol at 25C.
(b) One kmol of CH
4
dissociates to form an equilibrium ideal
gas mixture of CH
4
,H
2
, and C at 1000 K, 5 atm. For
log
10
K 1.011 at 1000 K.
14.12 The following exercises involve oxides of nitrogen:
(a) One kmol of N
2
O
4
dissociates at 25C, 1 atm to form an
equilibrium ideal gas mixture of N
2
O
4
and NO
2
in which
the amount of N
2
O
4
present is 0.8154 kmol. Determine the
amount of N
2
O
4
that would be present in an equilibrium
mixture at 25C, 0.5 atm.
(b) A gaseous mixture consisting of 1 kmol of NO, 10 kmol
of O
2
, and 40 kmol of N
2
reacts to form an equilibrium
ideal gas mixture of NO
2
, NO, and O
2
at 500 K, 0.1 atm.
Determine the composition of the equilibrium mixture. For
K 120 at 500 K.
(c) An equimolar mixture of O
2
and N
2
reacts to form an equi-
librium ideal gas mixture of O
2
,N
2
, and NO. Plot the mole
fraction of NO in the equilibrium mixture versus equilib-
rium temperature ranging from 1200 to 2000 K.
Why are oxides of nitrogen of concern?
14.13 One kmol of CO
2
dissociates to form an equilibrium
ideal gas mixture of CO
2
, CO, and O
2
at temperature T and
pressure p.
(a) For T 3000 K, plot the amount of CO present, in kmol,
versus pressure for 1 p 10 atm.
(b) For p 1 atm, plot the amount of CO present, in kmol,
versus temperature for 2000 T 3500 K.
14.14 One kgmol of H
2
O dissociates to form an equilibrium
ideal gas mixture of H
2
O, H
2
, and O
2
at temperature T and
pressure p.
(a) For T 3000K, plot the amount of H
2
present, in kgmol,
versus pressure ranging from 1 to 10 atm.
NO
1
2
O
2
S
d
NO
2
,
C 2H
2
S
d
CH
4
,
N
2
O
4
S
d
2NO
2
,
C 2H
2
S
d
CH
4
.C
1
2
O
2
S
d
CO,
CH
4
H
2
O
S
d
3H
2
CO.
11. Note 1 of Example 14.10 refers to the small amount of air
that would be dissolved in the liquid phase. For equilibrium, what
must be true of the chemical potentials of air in the liquid and
gas phases?
12. Water can exist in a number of different solid phases. Can
liquid water, water vapor, and two phases of ice exist in
equilibrium?
Problems: Developing Engineering Skills 713
(b) For p 1 atm, plot the amount of H
2
present, in kgmol,
versus temperature ranging from 2000 to 3500K.
14.15 An equimolar mixture of CO and O
2
reacts to form an
equilibrium mixture of CO
2
, CO, and O
2
at 3000 K. Determine
the effect of pressure on the composition of the equilibrium
mixture. Will lowering the pressure while keeping the tem-
perature fixed increase or decrease the amount of CO
2
pres-
ent? Explain.
14.16 An equimolar mixture of CO and H
2
O(g) reacts to form
an equilibrium mixture of CO
2
, CO, H
2
O, and H
2
at 1727C,
1 atm.
(a) Will lowering the temperature increase or decrease the
amount of H
2
present? Explain.
(b) Will decreasing the pressure while keeping the tempera-
ture constant increase or decrease the amount of H
2
pres-
ent? Explain.
14.17 Determine the temperature, in K, at which 9% of diatomic
hydrogen (H
2
) dissociates into monatomic hydrogen (H) at a
pressure of 10 atm. For a greater percentage of H
2
at the same
pressure, would the temperature be higher or lower? Explain.
14.18 Two kmol of CO
2
dissociate to form an equilibrium mix-
ture of CO
2
, CO, and O
2
in which 1.8 kmol of CO
2
is present.
Plot the temperature of the equilibrium mixture, in K, versus
the pressure p for 0.5 p 10 atm.
14.19 One kmol of H
2
O(g) dissociates to form an equilibrium
mixture of H
2
O(g), H
2
, and O
2
in which the amount of water
vapor present is 0.95 kmol. Plot the temperature of the equi-
librium mixture, in K, versus the pressure p for 1 p
10 atm.
14.20 A vessel initially containing 1 kmol of H
2
O(g) and x kmol
of N
2
forms an equilibrium mixture at 1 atm consisting of
H
2
O(g), H
2
,O
2
, and N
2
in which 0.5 kmol of H
2
O(g) is pres-
ent. Plot x versus the temperature T for 3000 T 3600 K.
14.21 A vessel initially containing 1 kmol of CO and 4.76 kmol
of dry air forms an equilibrium mixture of CO
2
, CO, O
2
, and
N
2
at 3000 K, 1 atm. Determine the equilibrium composition.
14.22 A vessel initially containing 1 kmol of O
2
, 2 kmol of
N
2
, and 1 kmol of Ar forms an equilibrium mixture of O
2
,
N
2
, NO, and Ar at 2727C, 1 atm. Determine the equilibrium
composition.
14.23 One kmol of CO and 0.5 kmol of O
2
react to form a
mixture at temperature T and pressure p consisting of CO
2
,
CO, and O
2
. If 0.35 kmol of CO is present in an equilibrium
mixture when the pressure is 1 atm, determine the amount of
CO present in an equilibrium mixture at the same temperature
if the pressure were 10 atm.
14.24 A vessel initially contains 1 kmol of H
2
and 4 kmol of
N
2
. An equilibrium mixture of H
2
, H, and N
2
forms at 3000 K,
1 atm. Determine the equilibrium composition. If the pressure
were increased while keeping the temperature fixed, would
the amount of monatomic hydrogen in the equilibrium mixture
increase or decrease? Explain.
14.25 A gaseous mixture with a molar analysis of 20% CO
2
,
40% CO, and 40% O
2
enters a heat exchanger and is heated
at constant pressure. An equilibrium mixture of CO
2
, CO, and
O
2
exits at 3000 K, 1.5 bar. Determine the molar analysis of
the exiting mixture.
14.26 An ideal gas mixture with the molar analysis 30% CO,
10% CO
2
, 40% H
2
O, 20% inert gas enters a reactor operating
at steady state. An equilibrium mixture of CO, CO
2
,H
2
O, H
2
,
and the inert gas exits at 1 atm.
(a) If the equilibrium mixture exits at 1200 K, determine on
a molar basis the ratio of the H
2
in the equilibrium mix-
ture to the H
2
O in the entering mixture.
(b) If the mole fraction of CO present in the equilibrium mix-
ture is 7.5%, determine the temperature of the equilibrium
mixture, in K.
14.27 A mixture of 1 kmol CO and 0.5 kmol O
2
in a closed
vessel, initially at 1 atm and 300 K, reacts to form an equilib-
rium mixture of CO
2
, CO, and O
2
at 2500 K. Determine the
final pressure, in atm.
14.28 Methane burns with 90% of theoretical air to form an
equilibrium mixture of CO
2
, CO, H
2
O(g), H
2
, and N
2
at 1000 K,
1 atm. Determine the composition of the equilibrium mixture,
per kmol of mixture.
14.29 Octane (C
8
H
18
) burns with air to form an equilibrium
mixture of CO
2
,H
2
, CO, H
2
O(g), and N
2
at 1700 K, 1 atm.
Determine the composition of the products, in kmol per kmol
of fuel, for an equivalence ratio of 1.2.
14.30 Acetylene gas (C
2
H
2
) at 25C, 1 atm enters a reactor op-
erating at steady state and burns with 40% excess air entering
at 25C, 1 atm, 80% relative humidity. An equilibrium mixture
of CO
2
,H
2
O, O
2
, NO, and N
2
exits at 2200 K, 0.9 atm.
Determine, per kmol of C
2
H
2
entering, the composition of
exiting mixture.
Chemical Equilibrium and the Energy Balance
14.31 Carbon dioxide gas at 25C, 5.1 atm enters a heat ex-
changer operating at steady state. An equilibrium mixture of
CO
2
, CO, and O
2
exits at 2527C, 5 atm. Determine, per kmol
of CO
2
entering,
(a) the composition of the exiting mixture.
(b) the heat transfer to the gas stream, in kJ.
Neglect kinetic and potential energy effects.
14.32 Carbon at 25C, 1 atm enters a reactor operating at steady
state and burns with oxygen entering at 127C, 1 atm. The
entering streams have equal molar flow rates. An equilibrium
mixture of CO
2
, CO, and O
2
exits at 2727C, 1 atm. Deter-
mine, per kmol of carbon,
(a) the composition of the exiting mixture.
(b) the heat transfer between the reactor and its surroundings,
in kJ.
Neglect kinetic and potential energy effects.

714 Chapter 14 Chemical and Phase Equilibrium
14.33 Methane gas at 25C, 1 atm enters a reactor operating at
steady state and burns with 80% of theoretical air entering at
227C, 1 atm. An equilibrium mixture of CO
2
, CO, H
2
O(g),
H
2
, and N
2
exits at 1427C, 1 atm. Determine, per kmol of
methane entering,
(a) the composition of the exiting mixture.
(b) the heat transfer between the reactor and its surroundings,
in kJ.
Neglect kinetic and potential energy effects.
14.34 Gaseous propane (C
3
H
8
) at 25C, 1 atm enters a reactor
operating at steady state and burns with 80% of theoretical air
entering separately at 25C, 1 atm. An equilibrium mixture of
CO
2
, CO, H
2
O(g), H
2
, and N
2
exits at 1227C, 1 atm. Determine
the heat transfer between the reactor and its surroundings, in
kJ per kmol of propane entering. Neglect kinetic and potential
energy effects.
14.35 One kmol of CO
2
initially at temperature T and 1 atm is
heated at constant pressure until a final state is attained con-
sisting of an equilibrium mixture of CO
2
, CO, and O
2
in which
the amount of CO
2
present is 0.422 kmol. Determine the heat
transfer and the work, each in kJ, if T is (a) 298 K, (b) 400 K.
14.36 Hydrogen gas (H
2
) at 25C, 1 atm enters an insulated re-
actor operating at steady state and reacts with 250% excess oxy-
gen entering at 227C, 1 atm. The products of combustion exit
at 1 atm. Determine the temperature of the products, in K, if
(a) combustion is complete.
(b) an equilibrium mixture of H
2
O, H
2
, and O
2
exits.
Kinetic and potential energy effects are negligible.
14.37 For each case of Problem 14.45, determine the rate of
entropy production, in kJ/K per kmol of H
2
entering. What can
be concluded about the possibility of achieving complete
combustion?
14.38 Hydrogen (H
2
) at 25C, 1 atm enters an insulated reac-
tor operating at steady state and reacts with 100% of theoret-
ical air entering at 25C, 1 atm. The products of combustion
exit at temperature T and 1 atm. Determine T, in K, if
(a) combustion is complete.
(b) an equilibrium mixture of H
2
O, H
2
,O
2
, and N
2
exits.
14.39 Carbon monoxide at 25C, 1 atm enters an insulated re-
actor operating at steady state and burns with excess oxygen
(O
2
) entering at 25C, 1 atm. The products exit at 2950 K,
1 atm as an equilibrium mixture of CO
2
, CO, and O
2
.
Determine the percent excess oxygen. Kinetic and potential en-
ergy effects are negligible.
14.40 Methane at 25C, 1 atm enters an insulated reactor op-
erating at steady state and burns with oxygen entering at 127C,
1 atm. An equilibrium mixture of CO
2
, CO, O
2
, and H
2
O(g)
exits at 3250 K, 1 atm. Determine the rate at which oxygen
enters the reactor, in kmol per kmol of methane. Kinetic and
potential energy effects are negligible.
14.41 Methane gas at 25C, 1 atm enters an insulated reactor
operating at steady state, where it burns with x times the the-
oretical amount of air entering at 25C, 1 atm. An equilibrium
mixture of CO
2
, CO, O
2
, and N
2
exits at 1 atm. For selected
values of x ranging from 1 to 4, determine the temperature of
the exiting equilibrium mixture, in K. Kinetic and potential en-
ergy effects are negligible.
14.42 A mixture consisting of 1 kmol of carbon monoxide
(CO), 0.5 kmol of oxygen (O
2
), and 1.88 kmol of nitrogen (N
2
),
initially at 227C, 1 atm, reacts in a closed, rigid, insulated ves-
sel, forming an equilibrium mixture of CO
2
, CO, O
2
, and N
2
.
Determine the final equilibrium pressure, in atm.
14.43 A mixture consisting of 1 kmol of CO and the theoreti-
cal amount of air, initially at 60C, 1 atm, reacts in a closed,
rigid, insulated vessel to form an equilibrium mixture. An
analysis of the products shows that there are 0.808 kmol of
CO
2
, 0.192 kmol of CO, and 0.096 kmol of O
2
present. The
temperature of the final mixture is measured as 2465C. Check
the consistency of these data.
Using the van’t Hoff Equation, Ionization
14.44 Estimate the enthalpy of reaction at 2000 K, in kJ/kmol,
for using the van’t Hoff equation and equi-
librium constant data. Compare with the value obtained for the
enthalpy of reaction using enthalpy data.
14.45 Estimate the enthalpy of reaction at 2000 K, in kJ/kmol,
for using the van’t Hoff equation and equi-
librium constant data. Compare with the value obtained for the
enthalpy of reaction using enthalpy data.
14.46 Estimate the equilibrium constant at 2800 K for
using the equilibrium constant at 2000 K
from Table A-27, together with the van’t Hoff equation and en-
thalpy data. Compare with the value for the equilibrium con-
stant obtained from Table A-27.
14.47 Estimate the equilibrium constant at 2800 K for the
reaction using the equilibrium constant at
2500 K from Table A-27, together with the van’t Hoff equa-
tion and enthalpy data. Compare with the value for the equi-
librium constant obtained from Table A-27.
14.48 At 25C, log
10
K 8.9 for Assuming
that the enthalpy of reaction does not vary much with temper-
ature, estimate the value of log
10
K at 500C.
14.49 If the ionization-equilibrium constants for
at 1600 and 2000 K are K 0.78 and K 15.63, respectively,
estimate the enthalpy of ionization, in kJ/kmol, at 1800 K using
the van’t Hoff equation.
14.50 An equilibrium mixture at 2000 K, 1 atm consists of Cs,
Cs
, and e
. Based on 1 kmol of Cs present initially, determine
the percent ionization of cesium. At 2000 K, the ionization-
equilibrium constant for is K 15.63.
14.51 At 2000 K and pressure p, 1 kmol of Na ionizes to form
an equilibrium mixture of Na, Na
, and e
in which the amount
of Na present is x kmol. Plot the pressure, in atm, versus x for
0.2 x 0.3 kmol. At 2000 K, the ionization-equilibrium
constant for is K 0.668.Na
S
d
Na
e
Cs
S
d
Cs
e
Cs
e
Cs
S
d
C 2H
2
S
d
CH
4
.
H
2
O
S
d
H
2
1
2
O
2
CO
2
S
d
CO
1
2
O
2
H
2
O
S
d
H
2
1
2
O
2
,
CO
2
S
d
CO
1
2
O
2

Problems: Developing Engineering Skills 715
14.52 At 12,000 K and 6 atm, 1 kmol of N ionizes to form an
equilibrium mixture of N, N
, and e
in which the amount of
N present is 0.95 kmol. Determine the ionization-equilibrium
constant at this temperature for
Considering Simultaneous Reactions
14.53 Carbon dioxide (CO
2
), oxygen (O
2
), and nitrogen (N
2
)
enter a reactor operating at steady state with equal molar flow
rates. An equilibrium mixture of CO
2
,O
2
,N
2
, CO, and NO
exits at 3000 K, 5 atm. Determine the molar analysis of the
equilibrium mixture.
14.54 An equimolar mixture of carbon monoxide and water va-
por enters a heat exchanger operating at steady state. An equi-
librium mixture of CO, CO
2
,O
2
,H
2
O(g), and H
2
exits at
2227C, 1 atm. Determine the molar analysis of the exiting
equilibrium mixture.
14.55 Butane (C
4
H
10
) burns with 100% excess air to form an
equilibrium mixture at 1400 K, 20 atm consisting of CO
2
,O
2
,
H
2
O(g), N
2
, NO, and NO
2
. Determine the balanced reaction
equation. For at 1400 K, K 8.4 10
10
.
14.56 Steam enters a heat exchanger operating at steady state.
An equilibrium mixture of H
2
O, H
2
,O
2
, H, and OH exits at
temperature T, 1 atm. Determine the molar analysis of the
exiting equilibrium mixture for
(a) T 2800 K.
(b) T 3000 K.
Considering Phase Equilibrium
14.57 For a two-phase liquid–vapor mixture of water at 100C,
use tabulated property data to show that the specific Gibbs
functions of the saturated liquid and saturated vapor are equal.
Repeat for a two-phase liquid–vapor mixture of Refrigerant
134a at 20C.
14.58 Using the Clapeyron equation, solve the following prob-
lems from Chap. 11: (a) 11.32, (b) 11.33, (c) 11.34, (d) 11.35,
(e) 11.40.
14.59 A closed system at 20C, 1 bar consists of a pure liquid
water phase in equilibrium with a vapor phase composed of
water vapor and dry air. Determine the departure, in percent,
of the partial pressure of the water vapor from the saturation
pressure of pure water at 20C.
14.60 Derive an expression for estimating the pressure at which
graphite and diamond exist in equilibrium at 25C in terms of
the specific volume, specific Gibbs function, and isothermal
compressibility of each phase at 25C, 1 atm. Discuss.
14.61 An isolated system has two phases, denoted by A and
B, each of which consists of the same two substances, de-
noted by 1 and 2. Show that necessary conditions for equi-
librium are
1. the temperature of each phase is the same, T
A
T
B
.
2. the pressure of each phase is the same, p
A
p
B
.
3. the chemical potential of each component has the same
value in each phase, m
A
1
m
B
1
, m
A
2
m
B
2
.
N
2
2O
2
S
d
2NO
2
N
S
d
N
e
.
14.62 An isolated system has two phases, denoted by A and B,
each of which consists of the same two substances, denoted by
1 and 2. The phases are separated by a freely moving, thin wall
permeable only by substance 2. Determine the necessary con-
ditions for equilibrium.
14.63 Referring to Problem 14.63, let each phase be a binary
mixture of argon and helium and the wall be permeable only
to argon. If the phases initially are at the conditions tabulated
below, determine the final equilibrium temperature, pressure,
and composition in the two phases.
T (K) p (MPa) n(kmol) y
Ar
y
He
Phase A 300 0.2 6 0.5 0.5
Phase B 400 0.1 5 0.8 0.2
14.64 Figure P14.65 shows an ideal gas mixture at temperature
T and pressure p containing substance k, separated from a gas
phase of pure k at temperature T and pressure p by a semi-
permeable membrane that allows only k to pass through. As-
suming the ideal gas model also applies to the pure gas phase,
determine the relationship between p and p for there to be no
net transfer of k through the membrane.
Ideal gas mixture
at T, p. Mole
fraction of substance
k is y
k
, partial
pressure p
k
= y
k
p
Membrane permeable
only to k
Ideal gas k
at, T, p´
Figure P14.64
14.65 What is the maximum number of homogeneous phases
that can exist at equilibrium for a system involving
(a) one component?
(b) two components?
(c) three components?
14.66 Determine the number of degrees of freedom for systems
composed of
(a) ice and liquid water.
(b) ice, liquid water, and water vapor.
(c) liquid water and water vapor.
(d) water vapor only.
(e) water vapor and dry air.
(f) liquid water, water vapor, and dry air.
(g) ice, water vapor, and dry air.
(h) N
2
and O
2
at 20C, 1 atm.
(i) a liquid phase and a vapor phase, each of which contains
ammonia and water.
(j) liquid mercury, liquid water, and a vapor phase of mercury
and water.
(k) liquid acetone and a vapor phase of acetone and N
2
.
14.67 Develop the phase rule for chemically reacting systems.
