Mei C., Zhou J., Peng X. Simulation and Optimization of Furnaces and Kilns for Nonferrous Metallurgical Engineering
Подождите немного. Документ загружается.


2 Modeling of the Thermophysical Processes in FKNME
particle surface. The value of k
d
is given by.
0.75
7
pg
A
d
p
2.53 10
2
TT
p
k
Rp
−
+
⎛⎞
×
=
⎜⎟
⎝⎠
(2.128)
where R
p
is the particle radius; T
p
is the particle temperature; T
g
is the far-field
temperature
˗p is the local pressure and p
A
is atmospheric pressure. The char
oxidation rate per unit area of particle surface is given by k
c
p
s
. The chemical
reaction rate coefficient k
c
is given by
c
ccp
p
exp
T
kAT
T
⎛⎞
=−
⎜⎟
⎜⎟
⎝⎠
(2.129)
where A
c
and T
c
depend on the type of coal and their values can refer to the
relative references (Smoot and Pratt,1983;Zhou,1986;Wall,1986). For this model,
k
d
and k
c
are in units of kg/(m
2
• atm • s). The overall reaction rate of a particle is
given by:
()
1
11
dc g
A
4π
−
−−
+
p
kk pR
p
Q
(2.130)
and is controlled by the smaller rates of the k
d
and k
c
.
Gibb model (Gibb, 1985) takes into account the void fraction
ε
of the char
particle, the particle volume /internal surface ratio a, the effective internal
diffusion coefficient D
p
of oxygen within the pores, and the molar ratio
φ
of
carbon atoms/oxygen molecules.
The oxidation mechanism of carbon can be characterized by the parameter
φ
,
then the oxides are produced according to the equation:
() ( )
22
CO 2 1CO 2 CO
φφφ
+⎯⎯→− +−
(2.131)
The value of
φ
is assumed to depend on the particle temperature T
p
()
s
s
p
21
exp
2
T
A
T
φ
φ
⎛⎞
−
=−
⎜⎟
⎜⎟
−
⎝⎠
(2.132)
where A
s
and T
s
are constants. Gibb suggests A
s
=2500K and T
s
=6240K.
By solving the oxygen diffusion equation analytically, the following equation is
obtained for the rate of decrease in the char mass m
c
()
()
1
1
1
cc
123
c
d
3
d1
mM
C
kkk
tM
φ
ερ
−
−
−
∞
=− + +
−
0
(2.133)
where the far field oxygen concentration C
∞
is taken to be the time-averaged value
obtained from the gas phase calculation;
ρ
c
is the density of the char; k
1
is the rate
of external diffusion; k
2
is the surface reaction rate; k
3
is the rate of internal
diffusion and surface reaction. These are defined as follows:
1
2
p
D
k
R
=
(2.134)

Ping Zhou, Feng Mei and Hui Cai
where D represents the external diffusion coefficient of oxygen in the surrounding
gas.
()
c
2
p
1
k
k
R
ε
=−
(2.135)
where k
c
is the carbon oxidation rate, defined by the modified Arrhenius equation:
c
ccp
p
exp
T
kAT
T
⎛⎞
=−
⎜⎟
⎜⎟
⎝⎠
(2.136)
where A
c
and T
c
are constants. Gibb recommends A
c
=14m/s and T
c
=21580K.
Further
()
2
3c
coth 1 /kk a
ββ β
=− (2.137)
0.5
c
p
p
k
R
Da
β
ε
⎛⎞
=
⎜⎟
⎜⎟
⎝⎠
(2.138)
Field model may predict sufficiently accurate when the temperature is not too
high but correction on the reduction and combustion of CO
2
must be considered if
the temperature is high (Zhou, 1982).
2.3.3.4
Oil combustion
The simulation on oil combustion is similar to that on coal combustion. The
mass fractions of fuel, oxidant and product in gaseous phase are predicted
through computing mixture fraction, which is identical to the aforementioned
gaseous combustion model (Guo, 1997). The difference is that both coal
devolatilization and carbon oxidation need to be considered in coal
combustion, which are the chemical reactions in solid and gaseous phase.
However, for oil combustion, the evaporation of the drops needs only to be
considered. Generally, there are no chemical reactions at the surface of oil
droplets and the combustions only carry out in volatiles given off by the
evaporation of the drops.
Spherical liquid droplets are assumed to be heated up to their boiling point
temperature and then to evaporate at a rate determined by the heat transfer to the
droplet and the latent heat of the liquid. The decrease in droplet diameter is
computed from the evaporative mass loss based on the assumption that the liquid
density remains constant.
In 1993, Barreiros suggested a mathematic model for oil combustion
(Barreiros et al.,1993). At any instant t, the droplet is assumed to have
diameter d
p
, a uniform temperature T
p
, while liquid density
ρ
p
and specific
heat capacity c
p
are assumed to be constant. According to the film theory for
the evaporation of liquid droplets, once the gas temperature T
g
exceeds the

2 Modeling of the Thermophysical Processes in FKNME
boiling point temperature T
b
, the evaporation equation for the droplets is
given by:
()
2*
pg
pp
d( ) 4
ln 1
d
dNu
B
tc
λ
ρ
=− + (2.139)
that is
()
*
pg
pp p
d2
ln 1
d
dNu
B
tcd
λ
ρ
=− + (2.140)
where
λ
g
is thermal conductivity; B is the mass transfer number defined by:
()
g
gb
c
BTT
L
=− (2.141)
where L is the latent heat of vaporization of the liquid and c
g
is specific heat of gas,
Nu
*
is the modified Nusselt number and is given by:
*
0
Re
Nu C Nu
=
= •
where
0.5 0.3333
20.55Nu Re Pr=+
(2.142)
0.5
0.5 0.3333
1.3333
1.237
1 0.278 1CRePr
RePr
−
⎛⎞
=+ +
⎜⎟
⎝⎠
(2.143)
where Re is the Reynolds number of the liquid droplet, that is:
gp
gp
g
d
Re v v
ρ
μ
=−
(2.144)
where
ρ
g
is gas density, v
g
and v
p
are gas velocity and droplet velocity respectively;
Pr is the Prandtl number:
gg
g
C
Pr
μ
λ
=
(2.145)
Hence, the decrease rate of droplet diameter is given by:
()
pg
pp p
d4
ln 1
d
d
CB
tcd
λ
ρ
=− +
(2.146)
Heat transfer to the droplet is modeled by the equation:
()
()
pg p
4
gp p 0p
2
Pp pPp
pp p
d6 d
36
dd
TNu d
L
TT I T
tcdtcd
cd
λ
ε
σ
ρ
ρ
=−++− (2.147)
where I
p
is the radiative heat flux through environment to droplets˗
ε
is emissivity
of droplet˗
σ
0
is the Stefan-Boltzmann constant.
Ping Zhou, Feng Mei and Hui Cai
2.3.4
NO
x
models
NO
x
given off from combustion consist of mostly nitric oxide NO and nitrogen
dioxide NO
2
. Besides, there is also minor nitrous oxide N
2
O. During the
combustion, the concentration of NO is higher than that of NO
2
, and NO
2
is
produced from NO. Thus, the key to the reaction dynamic model of NO
x
is to
research into the formation of NO. NO
x
is classified as thermal NO
x
, prompt
NO
x
and fuel NO
x
, based on three distinct chemical kinetic processes that form
NO
x
.
Thermal NO
x
is formed by oxidation of atmospheric molecular nitrogen N
2
at
high temperature. According to Zeldovich mechanism, it is generally described by
˖
N
2
ˇO NOˇN (2.148)
N
ˇO
2
NOˇO (2.149)
Prompt NO
x
is formed by a series of reactions and many possible intermediate
species between hydrocarbon and N
2
. Based on Fenimore mechanism, it is
generally simplified as:
CH
ˇN
2
HCNˇN (2.150)
CH
2
ˇN
2
HCNˇNH (2.151)
1
2
N
2
ˇO
2
NOˇO (2.152)
N
ˇOH NOˇH (2.153)
CN
ˇO
2
NOˇCO (2.154)
Fuel NO
x
is produced by oxidation of nitrogenous compound in devolatilized
fuel. Although the reaction mechanism for the formation and reduction of fuel
NO
x
is unclear, the following observations can be made based on the researches
reported in the past a few years (Mao et al., 1998):
a) In the normal combustion conditions, nitrogenous organic compound in fuel
are heated and decomposed into HCN, NH
3
and intermediate product CN, etc,
which are emitted from fuel with volatiles. They are called volatile-N.
Nitrogenous compound remained in char are called char-N.
b) The percentages of HCN and NH
3
among volatile-N depend not only on the
types of fuel and the character of volatiles, but also on the chemical character such
as combination state between N and hydrocarbon. Besides, they are related to the
combustion conditions such as temperature.
c) In oxidizing atmosphere, HCN is oxidized into NO; In reducing atmosphere,
NO and HCN are reduced into N
2
.
The simplified model representing the forming and reducing mechanism of fuel
NO
x
is showed in Fig. 2.5.
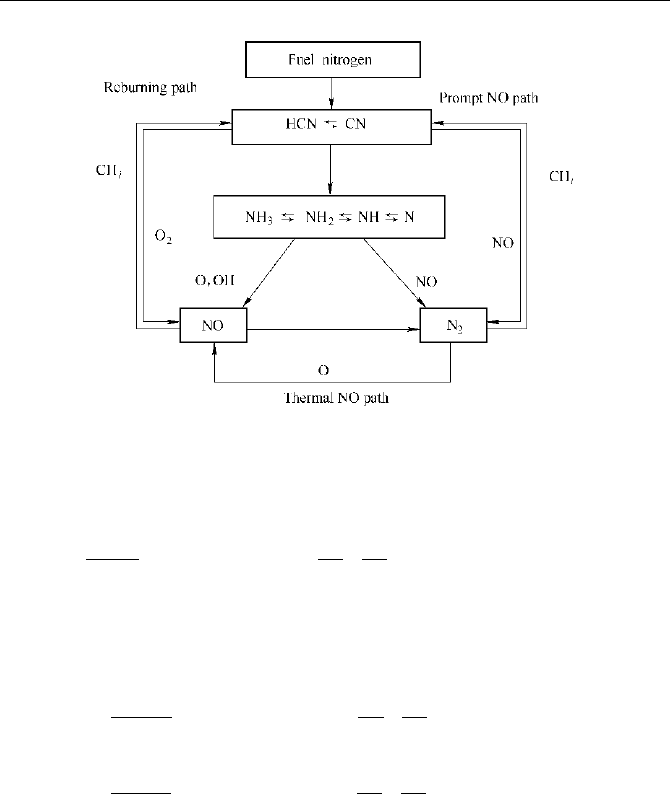
2 Modeling of the Thermophysical Processes in FKNME
Fig. 2.5 Main path for fuel NO
x
formation and reduction
For thermal and prompt NO
x
, only the transport equation for NO species is
needed:
()
NO
N
ONONO
T
TL
Y
YYS
t
ρ
μ
μ
ρ
σσ
⎛⎞
⎛⎞
∂
+∇ −∇ + ∇ =
⎜⎟
⎜⎟
⎜⎟
∂
⎝⎠
⎝⎠
••U (2.155)
For coal and oil combustion, in addition to the above equation, the transport
equations for HCN and NH
3
species are needed:
()
HCN
HCN HCN HCN
T
TL
Y
YYS
t
ρ
μ
μ
ρ
σσ
⎛⎞
⎛⎞
∂
+∇ −∇ + ∇ =
⎜⎟
⎜⎟
⎜⎟
∂
⎝⎠
⎝⎠
••U (2.156)
()
NH
3
N
HNHNH
333
T
TL
Y
YYS
t
ρ
μ
μ
ρ
σσ
∂
⎛⎞
⎛⎞
+∇ −∇ + ∇ =
⎜⎟
⎜⎟
⎜⎟
∂
⎝⎠
⎝⎠
••U (2.157)
where Y
NO
, Y
HCN
and
3
N
H
Y are respectively mass fractions of NO, HCN and NH
3
;
S
NO
, S
HCN
and
3
N
H
S are respectively source or sink term in the mass fraction
transport equations for NO, HCN and NH
3
.
Since the mass fractions of those pollutants are generally small (<10
−3
), they are
calculated as passive combustion scalars, that is their influence on flow velocity
and other scalars, such as temperature, pressure and the concentration of other
species, can be neglected.
2.3.4.1 Thermal NO
x
formation
According to Zeldovich mechanism, the net rate of formation of thermal NO is

Ping Zhou, Feng Mei and Hui Cai
given by
[
]
[][] [][] [][] [][]
12221 2
dNO
ON NO NON NOO
d
kkk k
t
−−
=+− −
(2.158)
In order to calculate the formation rate of NO, the steady state assumption for
[N] is used (Mao, 1998), thus we obtain:
[
]
[
]
[
]
[
]
[
]
[] [ ]
2
12 2 2 1 2
22 1
dNO 2O( O N NO )
dONO
kk k k
tkk
−−
−
−
=
+
(2.159)
where [NO], [O
2
], [N
2
], [O] and [N] refer to the molar concentration of
corresponding species respectively, and their units are mol/m
3
. Their reaction rate
coefficients are respectively given by
(Dong, 1998):
k
1
=1.8×10
8
exp(−38370/T) (m
3
molgV
k
-1
=3.8×10
7
exp(−425/T) (m
3
molgV
k
2
=1.8×10
4
Texp(−4680/T) (m
3
molgV
k
-2
=3.8×10
3
Texp(−20820/T) (m
3
molgV
Based on the assumption that the dissociation reactions of [O] reach a partial
equilibrium, we have:
[] [ ]
0.5
0.5
2
O 36.64 O exp( 27123/ )TT=− (mol/m
3
) (2.160)
In the transport equation for mass fraction of NO, the NO source term due to
thermal NO
x
mechanism is:
[
]
thermal,NO NO
dNO
d
SM
t
= (2.161)
where M
NO
represents the molecule weight of NO.
2.3.4.2 Prompt NO
x
formation
Prompt NO
x
mainly result from the combustion circumstance with poor
oxygen and rich fuel in which there are more CH
i
atomic groups. In
hydrocarbon flame, the atomic groups of CH and CH
2
, via the reactions
Eq.2.150 and Eq.2.151, form the intermediate products, and they are further
oxidized into prompt NO
x
. Prompt NO
x
formation is proportional to the
number of carbon atoms present per unit volume. Soete’s research results show
that, for most hydrocarbon fuel, the control equation of prompt NO
x
formation
rate can be described as (Soete,1975):
[
]
[][][ ]
spr 2 2
dNO
ONFuelexp
d
n
n
E
fk
tRT
α
α
⎛⎞
−
=
⎜⎟
⎝⎠
(2.162)
where both
p
r
n
k and
n
E
α
are experimental constants and their values can refer to
reference (Dupont and Porkashnian,1993).
α
is the order of oxidation reaction
which depends on combustion conditions, as
2 Modeling of the Thermophysical Processes in FKNME
2
2
2
2
2
2
3
O
32
OO
2
OO
O
1.0, <4.1 10
3.95 0.9ln , 4.1 10 <1.1 10
0.35 0.1ln , 1.1 10 <0.03
0 , 0.03
x
xx
xx
x
α
−
−−
−
⎧
×
⎪
−− ×< ×
⎪
⎪
=
⎨
−− ×<
⎪
⎪
>
⎪
⎩
(2.163)
where
2
O
x is oxygen molar fraction; f
s
is Soete model’s correction factor which
incorporates the fuel type, as
23
s
4.75 0.0819 23.2 32 12.2fn
φφ φ
=+ − + − (2.164)
where n is the number of carbon atoms per molar hydrocarbon fuel;
φ
is the
equivalence ratio.
Therefore, in the transport equation for mass fraction of NO, the NO source
term due to prompt NO
x
mechanism is:
[
]
prompt,NO NO
dNO
d
SM
t
= (2.165)
In the common combustion, prompt NO
x
accounts for a very small part, namely,
for coal combustion, prompt NO
x
formation is less than 5% of the total.
2.3.4.3
Fuel NO
x
formation
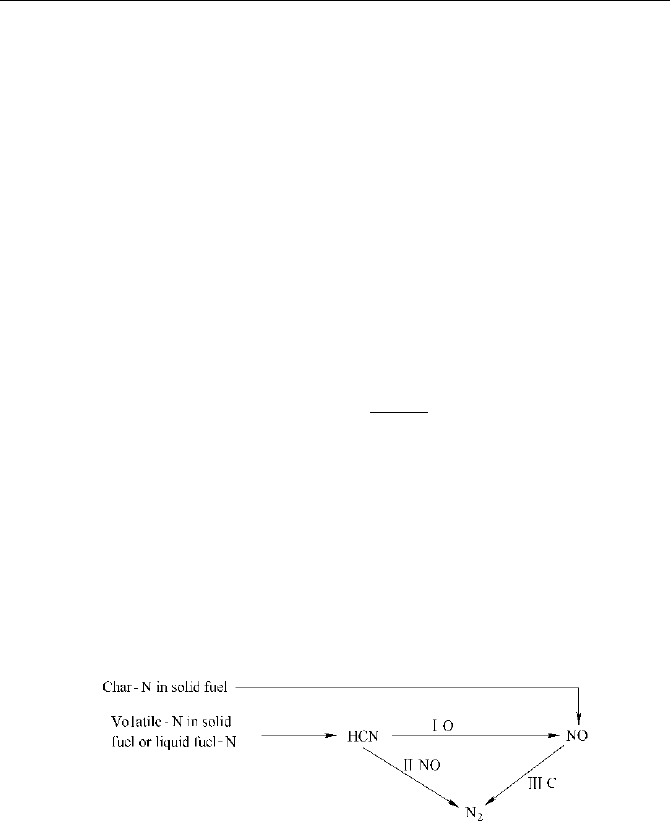
HCN path
The main reaction paths of fuel NO
x
by the intermediate products HCN are shown
as Fig. 2.6.
Fig. 2.6 The main reaction paths of fuel NO
x
by the intermediate products HCN
In terms of the NO reaction mechanism suggested by Lockwood and the
assumption that all char-N can be directly converted into NO (Lockwood,1992),
the source terms in the equations for mass fraction can be described as:
HCN pf,HCN HCN 1 HCN 2
SS S S
−−
=++ (kg/(m
3
• s)) (2.166)
N
OC,NONO1NO2NO3
SS S S S
−− −
=++ + (kg/(m
3
• s)) (2.167)
The rate of HCN production is equivalent to the rate of liquid fuel released into

Ping Zhou, Feng Mei and Hui Cai
the gas phase through evaporation or the rate of solid fuel released into the volatile
through volatilization. That is:
pf,HCN pf NF HCN N
//SSmMMV= (2.168)
where S
pf
is the rate of fuel release into the gas phase through evaporation or
volatilization; m
NF
is the mass fraction of nitrogen in fuel; M
HCN
and M
N
are
respectively the molecule weight of HCN and N; V is the cell volume.
The mass consumption rates of HCN which appear in Eq.2.168 are computed as:
HCN 1 1 HCN
/SRMpRT
−
=− (2.169)
HCN 2 2 HCN
/SRMpRT
−
=− (2.170)
where R
1
and R
2
are conversion rates of HCN in reactions (ĉ) and (Ċ)
respectively, s
-1
; p is pressure, Pa, T is the mean temperature, K; R is the general
gas constant.
According to Soete’s research result, we have:
211HCNO 1
exp( /( ))RAxx ERT
α
=− (2.171)
22HCNNO 2
exp( /( ))RAxx ERT=− (2.172)
where
α
is the order of oxidation reaction and is the same as Eq.2.163, T is the
instantaneous temperature; x is the molar fraction and
A
1
=3.5×10
10
˄s
1
˅
A
2
=3.0×10
12
˄s
1
˅
E
1
=2.805×10
5
˄J/mol˅
E
2
=2.512×10
5
˄J/mol˅
NO
x
is produced in reaction (ĉ) but destroyed in reaction (Ċ), thus the source
terms for Eq.2.167, i.e. S
NO−1
and S
NO−2
, are evaluated as follows:
NO 1 HCN 1 NO HCN 1 NO
//()SSMMRMpRT
−−
=− = (2.173)
NO 2 HCN 2 NO HCN 2 NO
//()SSMM RMpRT
−−
==− (2.174)
The mass consumption rates of NO in reaction (
ċ), S
NO-3
(kg/(m
3
• s)), can be
expressed as:
NO 3 BET s NO 3
/1000SACMR
−
= (2.175)
where A
BET
is BET surface area
Ზ
(m
2
/kg); C
s
is the concentration of particles, kg/m
3
;
ᲖBET surface area is a method for measuring specific surface area, which is suggested by Brunauer,
Emmet and Teller and based on multi-layer absorption theory for the gas absorbed by the surface of solid
particles (refer to “Chemical Engineering Handbook”(in Chinese),1989). It is called as BET method and the
specific surface area tested by means of this method is called as BET surface.
2 Modeling of the Thermophysical Processes in FKNME
and the heterogeneous reaction rate of NO reduction on the char surface R
3
can be
modeled
by (Dong,1998):
33NO 3
exp( /( ))RAx ERTp=− (2.176)
where A
3
=230mol/ (atm • m
2
BET
• s); E
3
=1.428×10
5
J/mol; T is the mean
temperature, K; p is pressure, atm,latm=101325Pa; x
NO
is the molar fraction of
NO.
The formation rate of NO from char-N is given by:
C,NO C NC NO N
//SSmMMV= (2.177)
where S
C,NO
is the char burnout rate, kg/s; and m
NC
is the mass fraction of nitrogen
in the char.
For the liquid fuel, both S
C,NO
and S
NO−3
are equal to zero.
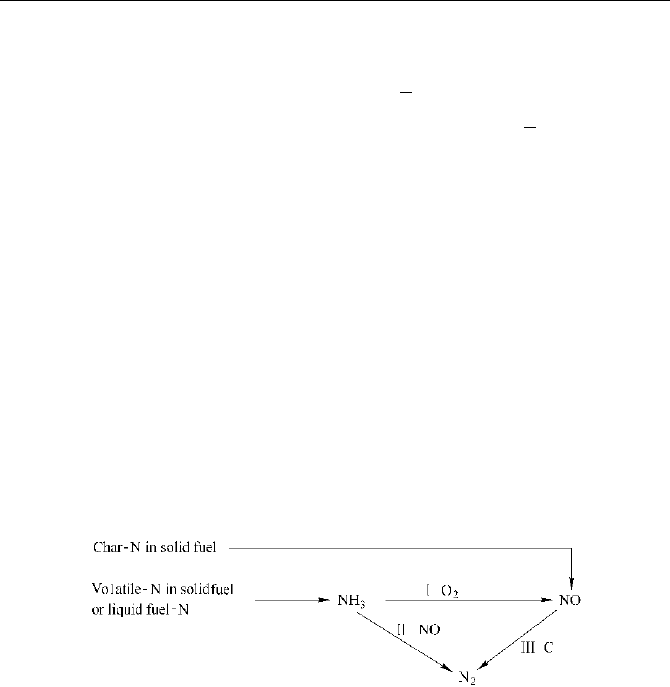
NH
3
path
The main reaction paths of fuel NO
x
by the intermediate products NH
3
are
shown as Fig.2.7. The overall reaction are:
Fig. 2.7 The main reaction paths of fuel NO
x
by the intermediate products NH
3
NH
3
ˇO
2
1
k
⎯
⎯→ NOˇH
2
Oˇ0.5H
2
(2.178)
NH
3
ˇNO
2
k
⎯
⎯→ N
2
ˇH
2
Oˇ0.5H
2
(2.179)
According to Soete’s research result, the reaction rates are respectively given
by:
32
6
1NHO
4.0 10 exp( 32000 /( ))kxx RT
α
=× − (2.180)
3
8
2NHNO
1.8 10 exp( 27000 /( ))kxx RT=× − (2.181)
where
α
is the oxidizing reaction order just as Eq.2.163.
The source terms in the transport equations can be described as follows:
NH pf,NH NH NH
333132
SS S S
−−
=++ (kg/(m
3
• s)) (2.182)
123
N
OC,NONO NO NO
SS S S S
−−−
=+++ (kg/(m
3
• s)) (2.183)

Ping Zhou, Feng Mei and Hui Cai
The calculating method of S
pf, NH3
is similar to that in HCN paths, and there is:
33
pf,NH pf NF NH N
//SSmMMV= (2.184)
where
3
N
H
M is the molecule weight of NH
3
.
31 3
NH 1 NH
/SkMpRT
−
=− (2.185)
32 3
NH 2 NH
/SkMpRT
−
=− (2.186)
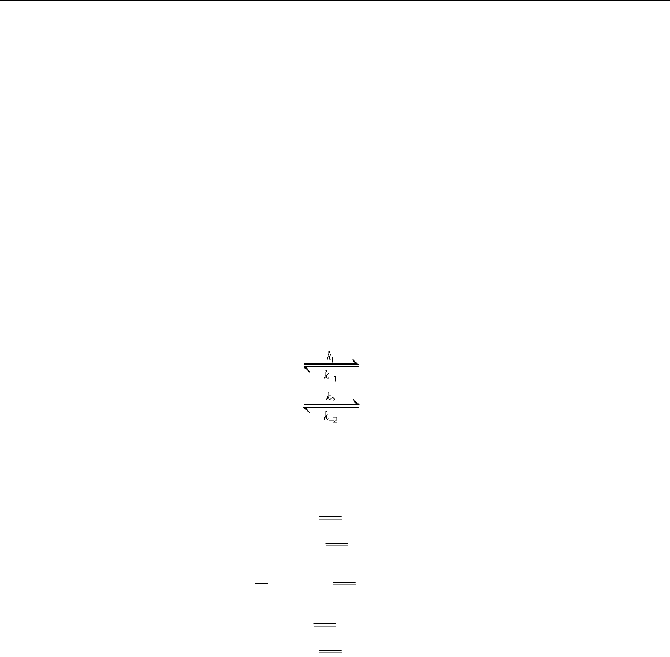
NO is produced in reaction (
ĉ) but destroyed in reaction (Ċ) and (ċ), we
have
131
3
NO NH NO 1 NO
NH
//()SSMMkMpRT
−−
=− = (2.187)
232 3
NO NH NO NH 2 NO
//()SSMM kMpRT
−−
==− (2.188)
The calculating method of S
C,NO
and S
NO-3
are similar to that in HCN paths.
2.4
Simulation of Magnetic Field
Electric field and magnetic field widely exist in the power equipments in
FKNME. The interaction between the magnetic field and the current flow in the
melt generates electromagnetic forces causes the melt flow and interface wave
thus effecting the production directly. So, to simulate the melt flow and its flow
field, the current field must be first computed before the magnetic field can be
determined.
2.4.1
Physical models
The magnetic field in FKNME is a joint effect of the currents in the
electrode (anode, cathode, bus bar), the electric heating elements and the
melt. Meanwhile, because of the ferromagnetic materials such as the jack
and steel structure of the furnace, influences of ferromagnetic shields
should also be considered in order to simulate precisely the magnetic field
in the furnace.
Simplification has to be made if the electric field and the magnetic field are to
be simulated in a complex FKNME. Besides simplifying the furnace structure
and the shape of electrical conductor, the following simplification still has to be
done.
The time-averaged value is used to represent the current flow in the melt to
eliminate the current fluctuation with the operation process. The fluctuation is
actually the outcome of many factors and it is difficult to be described in a
mathematical way.
The current flow in the electric conduction elements is simplified into the
