Mei C., Zhou J., Peng X. Simulation and Optimization of Furnaces and Kilns for Nonferrous Metallurgical Engineering
Подождите немного. Документ загружается.

2 Modeling of the Thermophysical Processes in FKNME
4
2
cos
dd
π
k
k
kk l k
VA
k
T
HA A V
l
γ
γγ
γγ
γ
εσ
θ
τ
−
−
=
∫∫
•• • (2.43)
By defining the direct exchanging area
kr
sg , which represents the overall
influence resulting from geometry and physical properties:
2
cos
dd
k
rk l k
VA
k
k
gs A V
l
γ
γ
γ
γ
ε
θ
τ
−
π
∫∫
˙ •• • (2.44)
We simplify Eq.2.43 into:
4
γγ
σ
TSgAH
krkk
=
−
(2.45)
The above equation reveals that the heat flux from
γ
V to
k
A is the production
of the black body radiation and the direct exchanging area.
The total heat flux towards
k
A from all N volume elements should be:
∑
=
=
N
kr
k
k
TSg
A
N
1
4
1
γ
γγ
σ
(2.46)
On the other hand, all of the
M gray diffusive surface elements with area
j
k
A (j=
1, …,
M) radiate to
k
A :
2
cos cos d d
jj
k
j
k
jk k l
AA
kj
jk
J
AA
HA
l
θθ
τ
−
=
π
∫∫
•••
(2.47)
where
i
J is the effective radiation of
j
A . Eq.2.47 can be expressed in a way
similar to Eq. 2.45:
j
kk jki
HASSJ˙•
(2.48)
where
kj
SS is the direct exchanging area between the surfaces j and k with
definition out of Eq.2.47:
2
cos cos d d
π
kj
j
k
j
k
jk l
AA
jk
A
A
SS
l
θθ
τ
−
=
∫∫
•••
(2.49)
The total heat flux from the
M surface elements towards the surface element
k
A is then:
∑
=
=
M
j
ikj
k
j
sk
JSS
A
H
1
1
(2.50)
The big total of the heat flux towards
k
A is obtained by summarizing
contributions from both surfaces and bodies:
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
+=+=
∑∑
==
NM
j
ikjkr
k
jkkk
JSSTSg
A
HHH
11
4
1
γ
γγ
σ
(2.51)
As the effective radiation from
k
A is:
4
)1(
kkkkk
HTJ
εσε
−+= (2.52)
The difference between incoming and outgoing radiation flux is:
Ping Zhou, Feng Mei and Hui Cai
4
kkkkkkk
HTHJq
εσε
−=−= (2.53)
In case of unknown gases temperature
T
¤
, another N equations are needed to
close the equations set. The following energy balance equation can be used to
describe the relationship among volume elements.
+=
−
∗
∗
∗∗
∑
∫∫
γ
γγ
γ
γ
γ
γγ
γ
γγγ
ε
τ
σε
σε
V
L
VT
VT
l
N
VV
d
π4
d4
4
2
4
4
cos
d
π
M
kk
kl
VA
k
k
J
A
l
γ
γ
γ
ε
θ
τ
2
∗
−
∑
∫∫
+=
∫∫
∑
∗
−
∗
∗
=
∗
∗
γγ
γγ
γ
γ
γ
γ
τ
σε
VV
l
N
l
VV
T
2
1
42
π
dd
γ
γ
γ
γ
τ
θ
ε
VA
l
J
kl
M
V
k
A
k
k
k
dd
π
cos
∑
∫∫
2
−
(2.54)
Define
rk
gs as the direct exchanging area from a surface to a gaseous volume:
γ
γ
γ
γ
τ
θ
ε
VA
l
gs
kl
V
k
A
k
k
rk
dd
cos
π
∫∫
2
−
=
(2.55)
Knowing from Eq.2.44 that the direct exchanging area from a surface to a
gaseous volume should be identical to that from a gaseous volume to a surface,
namely:
krrk
sggs = (2.56)
Similar relationships exist for gases to gases and surface to surface:
ijji
ssss = ;
ijji
gggg =
The direct exchanging area between gaseous volumes
γγ
gg
∗
is defined as:
2
2
dd
l
VV
VV
gg
l
γγ
γγ
γγ
γγ
τ
ε
∗
∗
∗
∗
−
=
π
∫∫
(2.57)
Substituting all above definitions and relationships into Eq.2.54 leads to
kr
M
j
k
N
sgJggTVT
∑∑
=
∗
=
∗
∗
+=
1
1
44
4
γγ
γ
γ
γγγ
σσε
(2.58)
With the help of Eq.2.58, N energy balance equations can be established for
N gaseous volumes. Therefore the temperatures fields and the heat fluxes
among the elements in any enclosure can be determined by the following
procedure:
a) Determining the direct exchange areas of the involved elements.
b) Resolving M
ˇN heat balance equations for temperatures in the M surface
elements and N gaseous volume elements.
c) Determining the difference in fluxes balance of the M surface elements as

2 Modeling of the Thermophysical Processes in FKNME
well as the total heat exchange in the system.
Obviously the accuracy of the zone method chiefly depends on how to define
the zones and how many zones to be defined. Larger number of zones helps to
improve accuracy but also exponentially raises the computational load. Automatic
zone partition becomes difficult if the geometry is complicated. Basically the zone
method requires large amount of human intervention for high computational
efficiency and accuracy. It has also been found that serious inconsistency between
the defined radiation zones and the mesh grids setup can also bring substantial
difficulties to numerical resolving process when applying finite element or finite
difference method.
2.2.3
Monte Carlo method
The Monte Carlo method is a computational algorithm, which relies on repeated
random sampling to simulate physical processes. In the case of simulating a
radiation process, a number of rays of radiation are sampled and simulated along
the entire process from emitting, traveling in and interacting with the medium to
finally arriving on a surface and being absorbed or reflected. So long as the
number of sampled radiation rays is sufficiently large (more than 100,000) the
Monte Carlo method may satisfyingly predict the overall heat transfer process.
The disadvantage of the Monte Carlo method is the demanding need to the
computational capacity. A relatively simple problem may also require large
amount of computation.
Assuming the elementary volume
γ
V emits N rays with ω Joule energy in
each ray. These rays are “emitted” following the directions randomly decided by
the random cumulative probability function R
θ
(Siegel and Howell, 1990; Zhu,
1989) .
θ
θ
R
1
sin
ˉ
= (2.59)
We assume the ray concerned goes in a randomly determined direction
R
θ
and is
absorbed after traveling over a distance of
l. The energy intensity of the ray should
be reduced to e
-αs
after traveling over a distance of s in a medium with constant
absorptivity. The probability function
()
sp represents the probability of this ray
traveling over a distance
s before it is entirely absorbed.
()
s
s
s
s
sp
α
α
α
α
−
∞
−
−
==
∫
e
de
e
0
Adopting the cumulative probability function
()
l
l
l
sspR
α
−
−==
∫
e1d
0
or

Ping Zhou, Feng Mei and Hui Cai
˅
l
Rl −−= 1ln(
1
α
The above expression can be equivalently written as
l
Rl ln
1
α
−= because R
l
is
a random value uniformly distributed between 0 and 1.
Under thermal equilibrium condition, the medium emits an identical number of
rays after having absorbed a certain quantity of radiation rays. The emission
direction (
γ
) over a sphere around the medium is determined by a cumulative
probability function
γ
R
()
γ
γ
R21cos
1
−=
ˉ
(2.60)
Together with
R
l
, the emission direction given by Eq.2.60 determines where this
ray will be absorbed.
This emitting
üabsorbingüemitting (diffusion) process is repeated until the ray
reaches its destination. We then may move on for the next ray until all of the
N
rays have been “emitted”. The number of the rays that have been absorbed by the
surfaces or the gaseous volumes will be then recorded.
Under thermal equilibrium condition, the energy radiated by a medium volume
should equal to the energy it absorbs. Giving
S
dv
as the number of absorbed rays
by the medium finite volume d
v and
ω
as the energy contained in each ray, we
have
vTS
vpv
d4
4
dd
σαω
=⋅ (2.61)
where
p
α
is the Planck mean absorptivity. The average temperature in the finite
volume is then determined by
1/4
d
d
4d
v
v
p
S
T
v
ω
ασ
⎛⎞
=
⎜⎟
⎜⎟
⎝⎠
(2.62)
The heat flux through the surface
i is obtainable from the balance between the N
emitted rays and the number of absorbed rays. The dimensionless heat flux
4
/
ii
Tq
σ
can be written as
()
N
S
N
SN
T
Sq
T
q
wiwi
i
wi
i
i
−=
−
=
−
= 1
4
eff
4
ω
ω
σ
ω
σ
The readers are referred to references (Siegel and Howell, 1990; Zhu, 1989; Howell,
1964a, b; Perlmutter, 1964) for the expressions of the involved cumulative probability
functions.
Monte Carlo method may result in considerable error in cases of low optical
thickness (
lmLk =
λ
, namely the ratio between the geometrical scale and the
mean penetration distance of the photons). On the other hand, increased optical
thickness may lead to rapidly rising demands of computational capacity. Therefore
Monte Carlo method is considered efficient mostly in cases of complicated system
with sensitively changing radiation properties.

2 Modeling of the Thermophysical Processes in FKNME
2.2.4
Discrete transfer radiation model
DTRM (discrete transfer radiation model) is a method combining the zone
partition idea from zone method and the ray tracing principle from Monte Carlo
method. This makes DTRM generally applicable to wide range of cases with
relatively lower demand to computational efforts. DTRM works especially
effective with optically thin applications.
The radiation intensity of a randomly selected ray changes as it travels through
an absorptive-diffusible medium. The governing equation is:
() () () ()( )
4π
0
d
,,,d
d4π
(I) (II) (III) (IV)
ibiii
i
ai x i x a i x i x
x
λ
λλλλλ λ
σλ
σω
ϕ
λωω ω
′
′′′ ′
=− − + +
∫
(2.63)
Term
ĉ in Eq. 2.63 denotes the absorption of the medium. Term Ċ
represents the deterioration in x direction due to scattering. Term
ċ is the
emission in x direction from the medium itself. Term
Č include all radiations in
x direction scattered by all other rays. We usually ignore the scattering term when
there are only minor particles presented in the furnace gases, which simplifying
the equation into:
() () ()
4
d
π
ibib
T
iaixaixaixa
λλ λλ λ λ
σ
′′ ′ ′
=− + =− + (2.64)
By assuming the radiation intensity is independent of wavelength and direction,
we rewrite Eq.2.64 into:
πd
d
4
T
aai
s
i
σ
+−= (2.65)
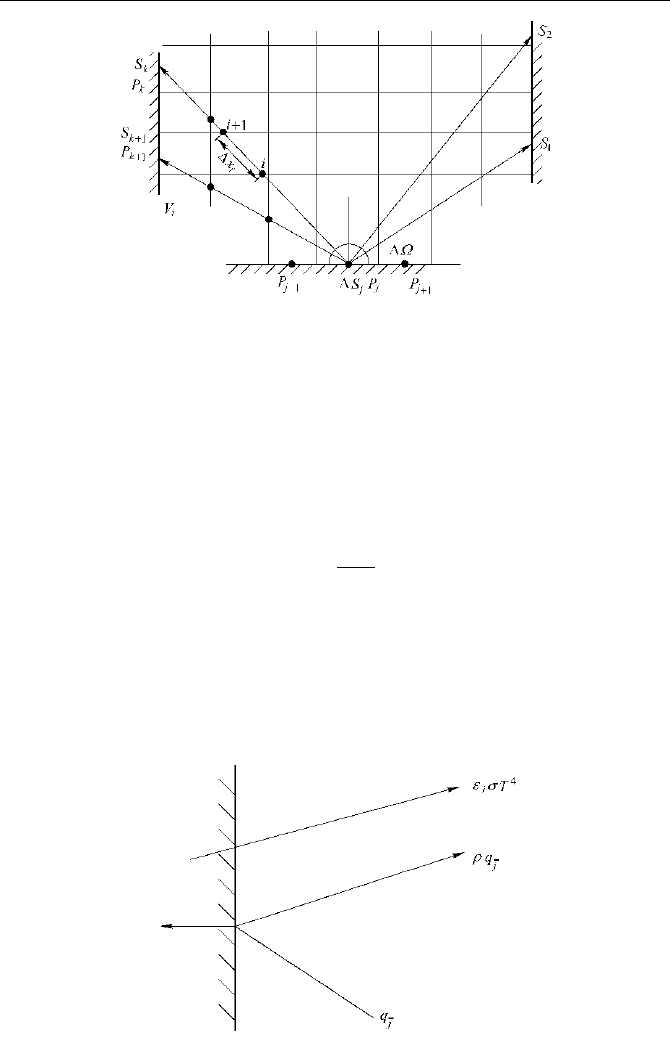
The procedure of applying DTRM is illustrated below by a 2D model.
A 2D space as shown in Fig. 2.2 is divided into i volume elements and j
surface elements. In most practices the zones are divided following the mesh
grids for computing the convection and diffusion processes of fluid flow. We
name the center point of a surface element as P
j
. The hemisphere around P
j
is
divided into k solid angles sections. For each of these angular sections an
emitting direction can be defined, following which a radiation ray will be
“emitted” and traced. The prediction accuracy rises if a larger number of sections
(k) is selected.
To carry out the computation, we assume as well:
a) Thermophysical properties within an element stay constant.
b) Surface elements are diffusive and gray.
c) Radiation intensity of each ray is identical.
d) All consequent effects from rays incident to surface element j are contributed
to the center point P
j
.
Ping Zhou, Feng Mei and Hui Cai
Fig. 2.2 A schematic diagram for 2D DTRM
The implementing of DTRM becomes pretty easy once the above assumptions
having been established.
a) Going through all angular sections and tracing back each incident rays until
their emitting point. Along the tracing process, the variation of the radiation
energy is updated for each volume element the ray has traveled through. The
variation is considered as the contribution of this ray to this particular volume
element, which is described by the restructured Eq.2.65:
4
1
e(1e)
π
ax ax
ii
T
ii
σ
−Δ −Δ
=+ −
ˇ
(2.66)
where xΔ denotes the gain in traveling distance in this volume element; a denotes
the absorptivity of the volume concerned.
b) Once having traced back to the original emitting surface of the ray, the
radiation intensity on this surface is calculated by Eq.2.67 (Fig.2.3):
Fig. 2.3 Radiation intensity of surface element
(² is reflection coefficient; ¦ is radiation coefficient)

2 Modeling of the Thermophysical Processes in FKNME
()
π
1
ππ
_
jj
P
qTq
i
j
ε
σ
ε
ϕ
−+==
4+
+
(2.67)
where
+
j
q denotes total radiation energy;
_
j
q denotes the obtained incident
energy from elsewhere;
¦ denotes the emissivity of the surface, which equals to
the absorptivity.
In case the boundary is not defined by surface temperature but by net radiation
heat flux
net
q , Eq.2.68 is used:
π
1(
ππ
_
net
jj
p
q
q
q
i
j
)−+==
+
+
ε
(2.68)
The above 2D case can be easily expanded into 3D (Fig.2.4) simply by
rewriting Eq.2.67 into:
()
ωθεσε
ϕθ
dcos1
π2
0
π/2
0
_4
∫∫
==
+
−+=
jjij
iTq
()
ωθεσε
ω
Δ−+=
∑
Δ
cos1
_4
jji
iT (2.69)
Eq.2.66 is still applicable for determining the radiation intensity within a
volume element.
Fig. 2.4 Solid angle θ and φ in a hemisphere
Before closing the discussion about the radiation models, we go back to the
starting point of Section 2.2.1 where the source term in the energy conservation
equation is discussed. To determine the radiation source term for each volume i,
we add up contributions from all involved parts:
()
1
dd
J
jj
vs
ii
j
v
ΔΔ
=
∇= =
∑
∫∫
••qqsJ (2.70)
where
()
jjjnnj
sii ΔΔ−=
+
ω
θ
cos
1
J is the contribution of surface element j towards
the concerned volume element i. The radiation intensities i
n+1
and i
n
are

Ping Zhou, Feng Mei and Hui Cai
computable from Eq.2.66 with the starting point at i
+
.
2.3
The Simulation of Combustion and Concentration Field
Combustion is a flow phenomenon with chemical reactions producing a lot of
heat. So it also involves the characteristics of mass transfer and chemical
reactions. Apart from the characteristics of turbulent flow and heat transfer
discussed in the former sections. The interaction and strong coupling between
turbulence and combustion make the differential equations of combustion very
complicated. Generally, these equations can hardly be solved by analytical
method but numerical simulation method.
Knowing that the actual combustion processes in the FKNME applications are
mostly in turbulence, we focus in this section the simulation of turbulent
combustion, including gas phase and gas-particle two-phase combustions. The
objective of combustion simulation is to gain better understanding about the
temperatures, velocities, concentrations (species mass fraction) and heat release
(chemical reaction rate) based on the conservation of mass, momentum and energy,
and the rules governing the reaction rates as a function of temperature, pressure
and reactant concentration.
2.3.1
Basic equations of fluid dynamics including chemical reactions
For a chemical reaction system consisting of N
S
chemical species and probably
involving N
R
basic reversible reactions, the jth reaction can be written in the form
...
2211
++ XaXa
jrjr
= ...
2211
++ XaXa
jpjp
(2.71)
X
i
(i=1, …, N
S
) refer to chemical species. Reactants are not distinguished from
products in this equation because reactants and products are reversible in the
reactions. a
rij
and a
pij
are the stoichiometric coefficients of species i at both
sides of j th basic reaction equation. Their values are positive or zero. The
difference:
n
ij
= a
pij
a
rij
(2.72)
is the overall stoichiometric coefficient for species i in reaction j, and is positive
for products and negative for reactants.
The impacts of chemical reaction on turbulent combustion are mainly
repnesented by the source terms in transport equations. Since the momentum
equations are identical to what have been discussed in the previous sections, in
this section we mainly discuss the conservation equations of chemical species and
enthalpy and the reaction rates in connection with the source terms of these
equations.
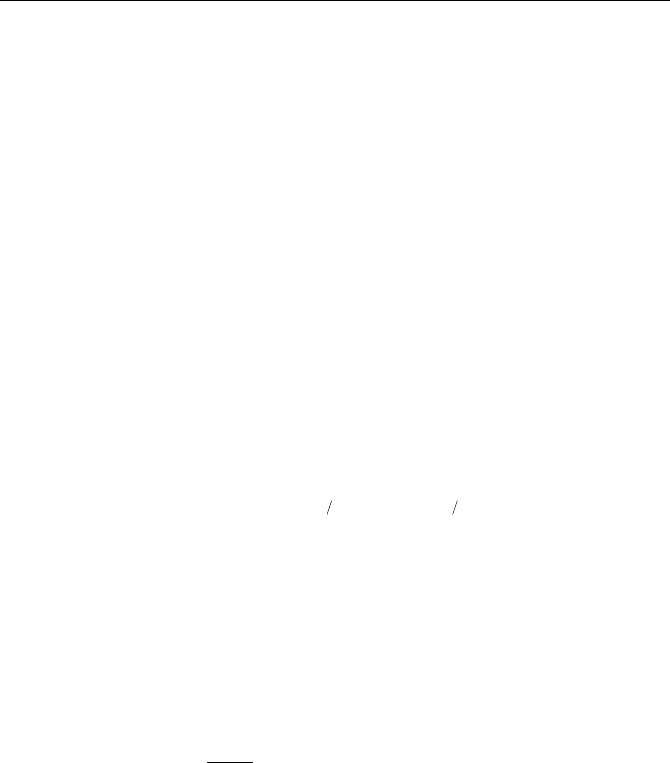
2 Modeling of the Thermophysical Processes in FKNME
2.3.1.1
Reaction rate
Chemical reaction rate means the species quantity produced or consumed at unit
time, unit volume (mol/(m
3
• s)) and is modeled as a function of reactant and
product
concentration (Fan and Wang, 1992; Fan et al., 1987), by
[] []
11
ij ij
SS
NN
jfj i bj i
ii
Rk x k x
αω
==
=−
∏∏
(2.73)
or in case of reaction rates influenced by concentrations of third non-reacting third
bodies (inert species), by
[] [] []
1
11
ij ij
SS
S
NN
N
jijifjibji
i
ii
Rxkxkx
αω
γ
=
==
⎛⎞
⎛⎞
⎜⎟
=−
⎜⎟
⎜⎟
⎝⎠
⎝⎠
∑
∏∏
(2.74)
where [x
i
] is molar concentration of species i;
ij
α
is forward rate exponent;
ij
ω
is
backward rate exponent;
ij
γ
is efficiency of species i as a third body (0İ
ij
γ
İ
1);
k
fj
is forward rate constant; k
bj
is backward rate constant.
Rate constant can be calculated as a function of temperature from the modified
Arrhenius law. For the j th forward reaction, this is
ee
fj fj fj fj
ERT TT
fj fj fj
kAT AT
ββ
−−
==
(2.75)
where A
fj
is pre-exponential factor or frequency factor;
fj
β
is temperature
exponent; E
fj
is the activation energy; T
fj
is the activation temperature (or E
fj
/R).
The backward rate constant can be defined similarly.
2.3.1.2
Species conservation and mass continuity equation
General mass fraction Y
i
(i=1, …, N
S
) represents chemical species concentration,
then mass conservation equation for species can be written as (Fan et al, 1987)
()
i
ii i
Y
YS
t
ρ
ρ
∂
+∇ + =
∂
• UJ (2.76)
where
U=˄u, v, w˅ is average velocity of the mixture (u, v, w stand for velocity
components in three orthogonal coordinate directions, respectively );
ρ
is the
density of the mixture; t is time; J
i
is the mass flux of species i relative to the
mean (bulk) flow, kg/(m
2
• s); S
i
is the product and consume rate of species i due to
chemical reaction. Here
()
iii
Y
ρ
≡−JUU (2.77)
where
U
i
is the velocity of species i. The first, second, third term on the left hard side
and the term on the right hard side in Eq. 2.76 are called respectively the time
variation rate term, convection term, diffusion term and reaction source term of
species i.
According to Fick’s law

μ
μ
μ
(2.78)
40 Ping Zhou, Feng Mei and Hui Cai
J
j
==-[rj+~:)v~
where IlT is the turbulent viscosity,
oy
is turbulent Prandtl numbers. The mass
conservation equation
of
species i is defined by
a~;
+ V
.{PU~
-[
r,+
~
)v~
}
==
S, (2.79)
the species source term due to chemical reaction (kg/nrr' • s)) is summed from the
contributions
of
the reaction rates by
N
R
s,
=
MiLnijR
j
j=l
where M is the molecule weight for species i, R
j
is the j th reaction rate.
2.3.1.3 Enthalpy equation
(2.80)
(2.81)
Enthalpy equation is the application
of
the first law
of
thermodynamics to fluid
dynamics and can be expressed as that the increase
of
total energy per volume is
equal to the sum
of
the net incoming stagnation enthalpy, the heat transfer and the
work from the surroundings. How to choose the variables in the energy equation has
a great influence on the equation solving process. For the combustion system with
multi-species and chemical reactions, the four kinds
of
mixture enthalpy are usually
used as dependent variable in energy equation: thermal enthalpy, thermal enthalpy
+
chemical enthalpy, total enthalpy (thermal enthalpy + chemical enthalpy + kinetic
energy) and sensible enthalpy (thermal enthalpy + kinetic energy).
If
the work from external bulk force and other external heat sources apart from
thermal radiation are neglected, the equation for total enthalpy is (Zhou, 1994;
Carol, 1987)
~t
[p(
H,
-~)]+
V•
(pUH,
)
==
cIJ+Qrad
-V
-s,
where (/J is viscous dissipation, or shear deformation work;
Qrad
is source term
(radiative heat); qt is total energy flux (including the energy carried into by
thermal conduction and species diffusion):
N
s
qt
=-AVT+
LJiH
ti
i
A is thermal conductivity; H; is total enthalpy
of
species i and can be written by
tt,
==
hfi
+
~ (~U2
+k) (2.82)
hfi is specific enthalpy
of
formation
of
species i, U is the averaged velocity
of
the
mixture, and
k is the turbulent kinetic energy.
The energy equation with sensible enthalpy (H) is more generally applied in the
