Mei C., Zhou J., Peng X. Simulation and Optimization of Furnaces and Kilns for Nonferrous Metallurgical Engineering
Подождите немного. Документ загружается.

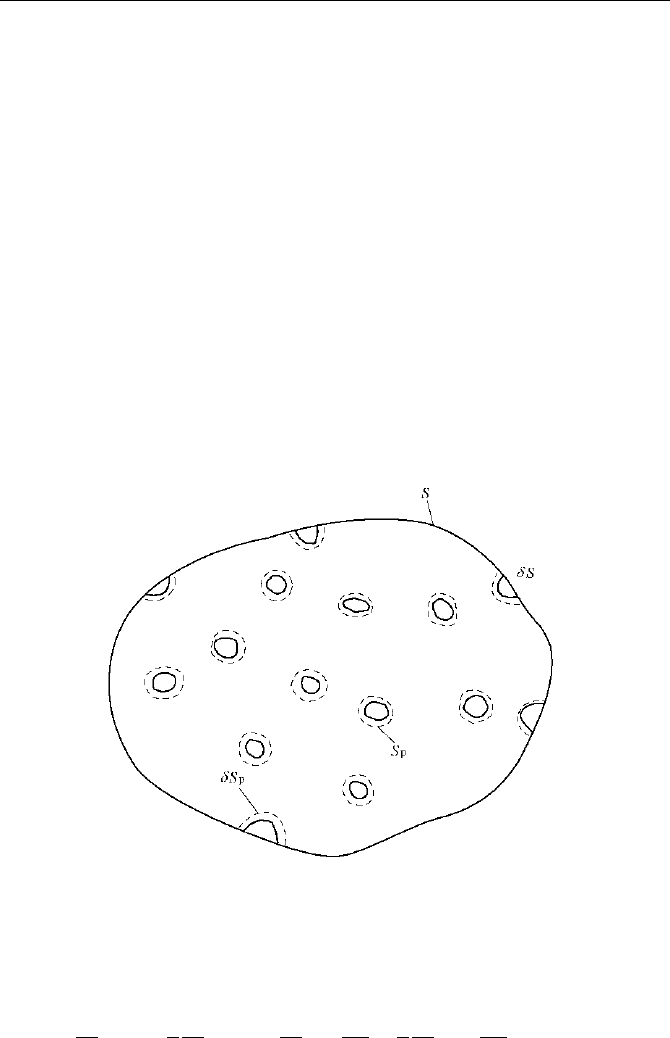
8 Modeling of Dilute and Dense Phase in Generalized Fluidization
8.3.4
Particle group trajectory model
Crowe and Smoot et al. brought up the conceptual model of particle groups in
1979 (Smoot and Partt, 1979). In this model a control volume with size
CV and
interface area
CS is defined as containing fluid and dispersed particle groups (also
called unit package), as shown in Fig. 8.6. Given that the surface area of a particle
p
S and the total interface area of the particle group ''SC , the model assumes
(Cen and Fan, 1990):
a) The gas phase is continuous whereas the particle phase is discrete.
The velocities and temperatures of the gas and particle phases are
different.
b)The particles are grouped according to their initial sizes and within the same
group all particles possess the same size, velocity and temperature.
c) Every particle group moves along the same trajectory. The model
follows up the changes on particle size, mass, velocity and temperature along
the trajectory.
Take the cylindrical reaction space in the presence of swirl-flow for example,
and the equation set governing the gas phase of the dispersed particle groups can
be written in a unified form as follows:
() ()
ϕϕϕϕ
ϕϕ
ϕρϕρ
p
11
SS
r
rΓ
rrx
Γ
x
vr
rr
u
x
++
⎟
⎠
⎞
⎜
⎝
⎛
∂
∂
∂
∂
+
⎟
⎠
⎞
⎜
⎝
⎛
∂
∂
∂
∂
=
∂
∂
+
∂
∂
(8.45)
where
ϕ
, Γ
ϕ
, S
ϕ
are listed in Table 8.5.
Fig. 8.6 Control unit of fluid and dispersed particle group
Chi Mei and Shaoduan Ou
Table 8.5 Parameters in the gas-phase equations of the particle group trajectory model
Equation
ϕ
Γ
ϕ
S
ϕ
p
S
ϕ
Gas phase continuous 1 0 0
()
pm
Smn
k
k
k
=⋅
∑
&
Axial momentum
u
eff
μ
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
∂
∂
∂
∂
+
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
∂
∂
∂
∂
+
∂
∂
−
r
v
r
rrz
u
zz
p
effeff
1
μμ
pm
Suf
k
zk
∑
+
⋅
Radial momentum v
eff
μ
eff eff
eff
1
2
2
2
pu v
r
rz zrr r
v
r
r
μμ
ω
μρ
∂∂ ∂ ∂ ∂
−+ + −
∂∂ ∂ ∂ ∂
+
⎛⎞⎛ ⎞
⎜⎟⎜ ⎟
⎝⎠⎝ ⎠
pm
Svf
k
zk
∑
+
⋅
Tangential momentum
ω
eff
μ
rr
r
r
v
∂
∂
−−−
eff
2
eff
μ
ωω
μ
ωρ
pm
Sf
k
k
∑
+
⋅
ω
θ
Turbulent kinetic
energy
k
eff
k
μ
σ
ρε
−
k
G
0
Dissipation rate of
turbulent kinetic
energy
ε
eff
ε
μ
σ
()
ρε
ε
εε
21
CGC
k
k
−
0
Fraction of mixture
f
eff
f
μ
σ
0 0
Fluctuation value of f
f
g
eff
g
μ
σ
k
g
C
r
f
z
f
C
f
gg
ρεμ
2
22
eff1
−
⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
∂
∂
+
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
∂
∂
0
Fraction of the gas
mixture from particles
η
eff
η
μ
σ
0 S
pm
Fluctuation value of
η
η
g
eff
g
μ
σ
k
g
C
rz
C
gg
η
ρε
ηη
μ
2
22
eff1
−
⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
∂
∂
+
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
∂
∂
0
Gas component
j
Y
eff
Y
μ
σ
j
ω
−
a
j
S
pm
Gas energy h
eff
h
μ
σ
−q
r
ppm
k
kk
nQ cTS+
∑
8 Modeling of Dilute and Dense Phase in Generalized Fluidization
In Table 8.5,
⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
⎟
⎠
⎞
⎜
⎝
⎛
∂
∂
+
∂
∂
+
⎟
⎠
⎞
⎜
⎝
⎛
∂
∂
+
⎟
⎠
⎞
⎜
⎝
⎛
∂
∂
=
222
eff
22
z
v
r
u
r
v
z
u
G
k
μ
kz
f ,
kr
f and
θ
k
f in
ϕ
p
S are the three components of the external force
imposed on the particle groups in unit volume. If the pressure gradient force,
virtual mass force, Basset force, Magnus force, Safman force, thermophoresis
force and gravity of the particles imposed by the gas flow are neglected, and only
the gas flow resistance is considered, then:
()
gp
r
1
kz k
k
fuu
τ
=−
()
gp
r
1
kr k
k
fvv
τ
=−
()
gp
r
1
kk
k
f
θ
ωω
τ
=−
where
kr
τ
is the particle relaxation time determined by Stokes’ resistance law,
μρτ
18/
2
r kkk
d=
Table 8.6 lists the values of the constants obtained by numerical experiments.
Table 8.6 Values of the constants in Table 8.5
Constant
C
1
ε
C
2
ε
C
1g
C
2g
C
k
σ
f
σ
g
σ
η
σ
Y
σ
h
σ
ε
σ
Value 0.09 1.44 1.92 2.8 2.0 0.9 0.9 0.9 0.9 0.9 0.9 1.3
Eq. 8.45 actually presents the generalized Navier-Stokes equation set, and can
be solved in the way of solving any simple fluid flow.
8.3.4.1
Trajectory of particle groups
Considering the trajectory of the particles in Lagrange coordinates, the motion
equation of a single particle is as follows:
()
pp
p
pp p
d
d
dd
mv
m
Fmgv
tt
= ∑ ++
(8.46)
where
p
F
∑ is the sum of all external forces imposed on this particle; gm
p
is the
gravity of the particle. The last term on the right side represents the inertia force
caused by the mass change of the particle.
It is actually rather difficult to consider in the model all the external forces imposed
on a particle. For industrial bulks, such as pulverized coal and concentrate particle, a
more practical approach is to start from modeling the most important facts and
gradually add up the complexity to get closer to the reality step by step.
If only the resistances of the particle are considered (including the inertial force
due to the mass change), particle motion equations in cylindrical coordinates will
Chi Mei and Shaoduan Ou
be stated as:
()
()
()
⎪
⎪
⎪
⎪
⎭
⎪
⎪
⎪
⎪
⎬
⎫
−−=
+−=
+−=
p
pp
pg
r
p
p
2
p
pg
r
p
pg
r
p
1
d
d
1
d
d
1
d
d
r
v
t
r
vv
t
v
guu
t
u
k
k
k
ω
ωω
τ
ω
ω
τ
τ
(8.47)
where
p
r is the radius of the particle position,
p
u ,
p
v and
p
ω
are the axial,
radial and tangential velocity components respectively
Eq. 8.47 can be solved numerically. The particle trajectory can also be obtained
by Runge-Kutta integration method:
p
p
p
p
p
p
d
d
d
d
d
d
z
u
t
r
v
t
r
t
θ
ω
⎫
=
⎪
⎪
⎪
=
⎬
⎪
⎪
=
⎪
⎭
(8.48)
8.3.4.2
Computation of the particle source term
The particle groups may affect the gas-phase flow and in the mean time the gas
phase flow may also influence the trajectory and motion of the particle groups.
The coupling effect between the gas flow expressed by Euler’s equation and the
particles trajectory expressed in Lagrange equation is realized by the source term
(
m
S
p
,
u
S
p
,
v
S
p
,
w
S
p
,
h
S
p
).
When solving the particle motion by Lagrange method, every particle trajectory
represents the motion of a group of particles with the same size. The flow rate
expressed as the particl number of a group moving along the jth trajectory with
size of
i
d
p
is
ij
n
ijijij
mmn
pp
/
&
= (number/s) (8.49)
where
ij
m
p
&
is the total flow rate of the particles;
ij
m
p
is the mass of a single
particle.
When a particle passes through a computational cell k following trajectory
j
,
a particle mass source term is generated:
() ()()
ppp
out in
Δ
ij ij ij ij
k
k
mnm m
⎡⎤
=−
⎣⎦
&
(8.50)
The “out” and “in” in the subscripts represent the particle mass leaving and
entering cell k. n
ij
keeps constant along the trajectory. The total particle mass
source term in cell k is:

8 Modeling of Dilute and Dense Phase in Generalized Fluidization
k
ij
ijkm
VmS
⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
Δ
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
=
∑∑
/
p,p
&
(kg/m
3
) (8.51)
where
∑
i
is the sum of the whole size group passing through cell k ;
∑
j
is
the sum of all particles with the same size but different initial position or trajectory
j .
Similarly, the source term in the momentum equation can be written as:
()()
p, pp pp
out in
1
u k ij ij ij ij ij
k
ij
Snumum
V
⎧⎫
⎡⎤
=−
⎨⎬
⎣⎦
Δ
⎩⎭
∑∑
(kg/(m
2
gs)) (8.52)
()()
p, p p p p
out in
1
v k ij ij ij ij ij
ij
k
Snvmvm
V
⎧⎫
⎡⎤
=−
⎨⎬
⎣⎦
Δ
⎩⎭
∑∑
(kg/(m
2
gs)) (8.53)
()()
p, p p p p
out in
1
w k ij ij ij ij ij
ij
k
Snwmwm
V
⎧⎫
⎡⎤
=−
⎨⎬
⎣⎦
Δ
⎩⎭
∑∑
(kg/(m
2
gs)) (8.54)
The source terms in the energy equation of the particles include the heat effect
of reactions of the particles, the convection between particles and gas and the
radiation.
() ()
[]
k
ijij
ij
ijkh
mmTCQn
V
S
⎪
⎭
⎪
⎬
⎫
⎪
⎩
⎪
⎨
⎧
−+−
Δ
=
∑∑
in
p
out
pp,p
1
(8.55)
where
k
Q is the convection and radiation heat transfer between particles and gas
in cell k.
The source terms for the turbulent energy and dissipation equations are usually
taken as zero.
Once the trajectories and the velocities on the trajectories of the particle groups
are determined by the Lagrange method, the particle concentration and velocity
fields can be solved by the volume average method.
For each computational cell, the total density of particle number can be
computed by the following equation:
()
k
ij
ijijk
Vnn
⎪
⎭
⎪
⎬
⎫
⎪
⎩
⎪
⎨
⎧
ΔΔ=
∑∑
/
r
τ
(number/m
3
) (8.56)
where
ijr
τ
Δ is the residence time of the particle in the cell. Then the particle
volumetric concentration (or volumetric fraction) is:
()
k
ij
ijijijkv
VvnC
⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
ΔΔ=
∑∑
/
pr,
τ
(8.57)
The particle velocity in this cell can be obtained by the momentum average
method:
Chi Mei and Shaoduan Ou
()()
k
ij
ijij
ij
ijijijk
nvnv
⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
ΔΔ=
∑∑∑∑
rpr,p
/
ττ
(m/s) (8.58)
8.3.5
Solution of the particle group trajectory model
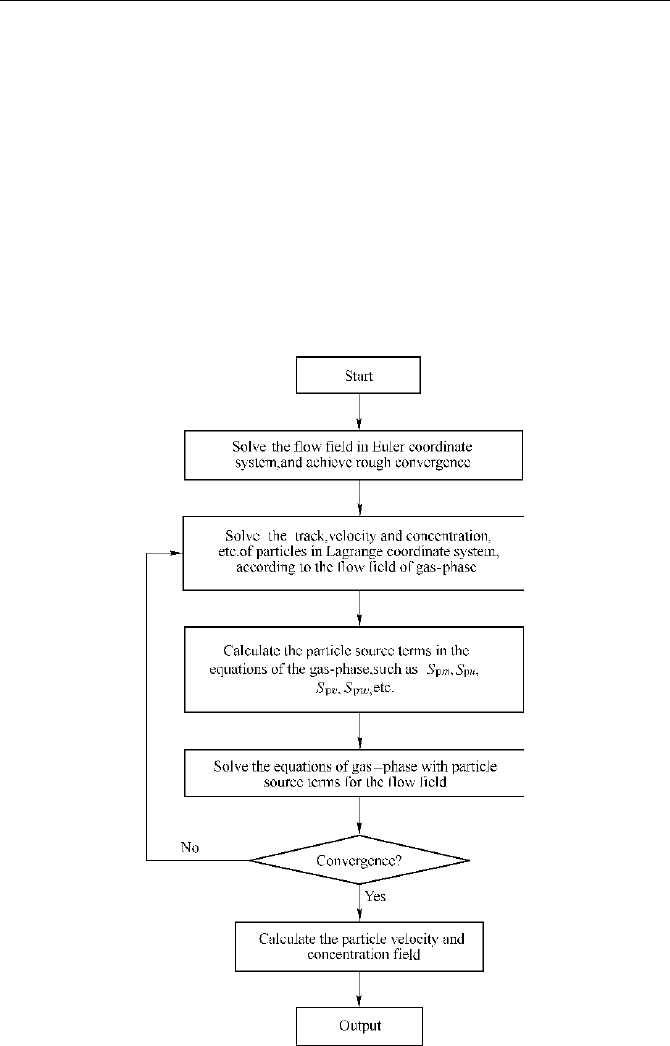
A coupling method, called PSIC (particle-source-in-cell) for computing gas-particle
two-phase flow, was introduced by Crowe in 1977. The method can be used to predict
the relatively large slip between particle groups and the gas phase, as well as the changes
in size, velocity and temperature of the particle groups along the trajectories. PSIC is
simpler than IPSA. The computation procedure is shown in Fig. 8.7.
Fig. 8.7 PSIC computation scheme
8 Modeling of Dilute and Dense Phase in Generalized Fluidization
8.4
Mathematical Models for Dense Phase
Generally, when the velocities of the gas flow passing through the bulk bed are
between the critical fluidization velocity (
mf
u ) of the particle and the terminal
velocity (or carry out velocity,
t
u ), the bulk layer is considered to be a dense
fluidized bed. The Reynolds number
mf
Re with respect to
mf
u is determined
by the following experimental equation:
4.75
mf
0.5
4.75
18 0.61
Ar
Re
Ar
ε
ε
•
=
⎡⎤
+
⎣⎦
(8.59)
where
p
mf
mf
g
du
Re
v
•
≡ (8.60)
()
gp
2
g
3
p
ρρ
−≡
v
dg
Ar (8.61)
where
ε
is the void fraction of the fluidized system. For critical fluidized bed,
45.0~40.0=
ε
in general. V
g
is the dynamic viscosity of gas phase Given
40.0=
ε
, then
5.0
mf
38.51400 Ar
Ar
Re
+
= (8.62)
The gas flow velocity reaches the terminal sedimentation velocity when 1=
ε
,
5.0
t
61.018 Ar
Ar
Re
+
= (8.63)
where
p
t
t
g
du
Re
v
•
≡ (8.64)
In the fluidized reactor, the overflow outlet divides the dense and dilute phase;
the fluid below this position is regarded as dense phase whereas that above is
regarded as dilute phase. Even though from the point of view of reactor designing,
the dense phase part and the dilute phases part should be considered as a unity
because they are both within the reaction zone, there are still large differences
between the dense and dilute phases in terms of fluidization condition, structural
features, transport properties and the major interests of researching.
The transport phenomena, either between the gas and particles in the dense
phase or between different bed layers, are very complicated, featuring high
fluctuation, randomicity and nonlinearity. The investigations in this area are still
far from enough and consensus still has to be reached regarding the understanding
and conclusions of the involved phenomena. Most of the published experiments

Chi Mei and Shaoduan Ou
stay within the range of
dp
dρ İ2.0kg/m
2
. Attempts to apply these data in practice
are subject to some limitations.
In this book only the frameworks of a few widely used physical models and
numerical solutions regarding dense phase reaction zone will be discussed.
Most of the currently available dense phase models focus on modeling the
bubbles’ structure, motion and change of sizes as well as correlating these
phenomena to variables about the fluidized beds. The major difference of
these models is basically the degree of complexity of the models and
correlations.
8.4.1
Two-phase simple bubble model
The following are the basic assumptions: (Chemical Engineering Handbook,
1989):
a) Gases flow through the bed layers either in bubble phase or in emulsion
phase. The gas velocity in emulsion phase equals the critical fluidized velocity
(
mf
u ); the parts exceeding
mf
u are considered in bubble phase.
b) The difference between the actual height of the bed layer (
f
L ) and the height
of critical bed layer is considered as filled with bubbles.
c) The change of the bubbles sizes and the influence of this change on other
variables in bed layer are negligible.
d) Chemical reactions only take place in the emulsion phase.
e) The exchange rate between the bubble phase and the emulsion phase is the
sum of penetration flow rate and the diffusion rate.
Based on the above assumptions and under the first-order chemical reaction
condition, the composition of the gas phase leaving the bed layer is as follows.
When emulsion phase is assumed to be fully mixed flow:
()
()
2
o
i
1e
e
'1 e
x
x
x
Z
C
Z
C
kZ
−
−
−
−
=+
+−
(8.65)
where
o
C
and
i
C
are respectively the concentrations of gas phase at the
outlet and inlet of the bed layer,
mf
cmf r
0.5 0.25
mf
fbb mf g b
0.5
bb
1
'/ /
6.34
/1.3(/)
()
u
Z
u
kkLukpWF
L
XQLuv u D gd
dgd
⎫
=−
⎪
⎪
⎪
==
⎬
⎪
⎪
⎡⎤
== +
⎣⎦
⎪
⎭
(8.66)
where
c
k
and
r
k
are the first-order reaction rate constants respectively based
on volume-concentration and weight-partial pressure of solid particles;
p
is the

8 Modeling of Dilute and Dense Phase in Generalized Fluidization
total pressure;
W
is the catalyst weight;
F
is the total gas mole flow rate;
g
D
is the molecular diffusion coefficient of reactant gas;
b
d
is the equivalent bubble
size (diameter). The relative velocity
br
u
can be determined by the empirical
equation reported by Grace and Clift:
()
0.5
br b
0.711ugd= (8.67)
On the other hand, the assumptions of the present model indicate that the relative
velocity can also be expressed as:
mf
br f mf mf f mf
fmf
()/()
L
uuu uu
L
L
ε
=− =−
−
(8.68)
Joining the above two equations, the equivalent bubble size (
b
d ) can be estimated
as follows:
2
fmf mf
b
fmf
1
0.711
uu L
d
gLL
⎛⎞
−
=
⎜⎟
−
⎝⎠
• (8.69)
This is a model proposed in the early time for rough estimation of the bubbles in a
steady state bubble. Subsequent studies (Grace and Clift, 1974) reported that the
part of the gases flowing through the bed layer in the emulsion form (
mf
uA• ) is
much greater than that predicted by this model (Cen et al.,1997).
8.4.2
Bubbling bed model
Based on the simple two-phase model, further assumptions are made that the
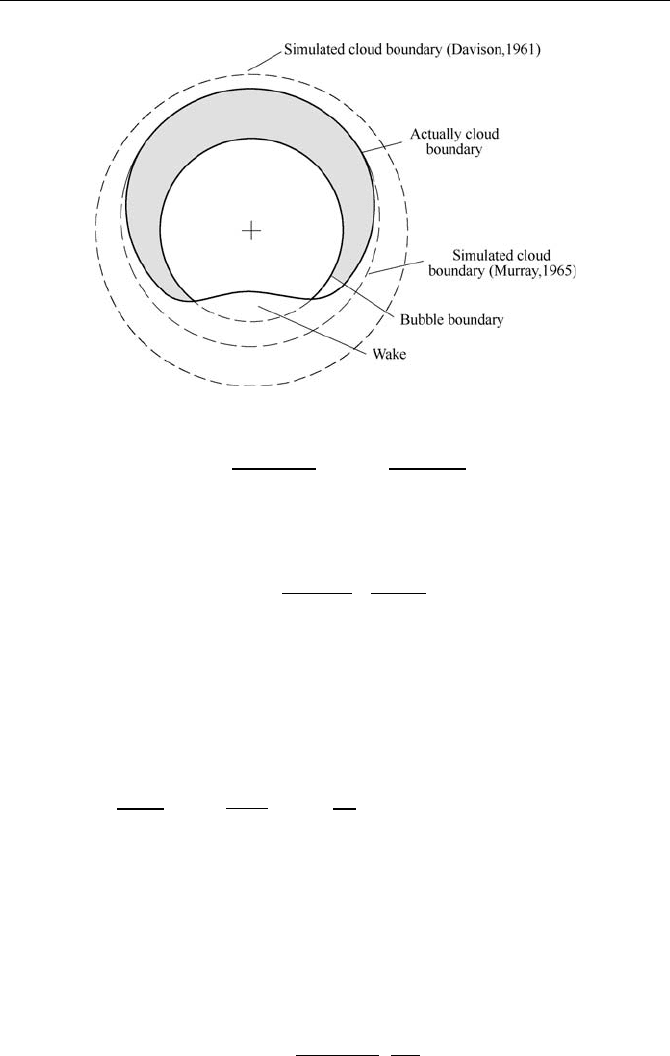
bubble phase contains bubble holes and bubble clouds (Fig. 8.8) surrounding
the bubbles, indicating. that the bed layer is composed of bubbles, bubble
clouds and emulsion phase. Similar to the simple two-phase model, it is still
assumed that the sizes of the bubbles do not change with the height of the bed
layer. The mass transfer between phases takes place only between bubble
clouds and emulsion phase. The transfers within gas phase take place between
the bubble holes and clouds as well as between the bubbles and the emulsion
phase (Kim and Han, 2007).
Under the first order reaction condition, the ratio between the compositions of
the bubble phase leaving and entering the bed layer is:
f
o
i
e
k
C
C
−
=
(8.70)
where
f
k is the mean overall mass transfer coefficient. Based on the above
assumptions,
f
k is expressed as(Kunii and Levenspiel,1969):
Chi Mei and Shaoduan Ou
Fig. 8.8 Structure schematic of the bubble and bubble cloud
()
2
ss bc
fbt
s
16
ydF
D
kjSh
yd D
ϕ
ε
ε
⎛⎞
=+
⎜⎟
−
⎝⎠
(8.71)
where D is the mass diffusivity;
b
c
F
is the gas exchange coefficient between the
bubbles and the clouds (Davison and Harrison,1963):
0.5 0.25
mf
bc
1.25
b
b
4.5
5.85
u
Dg
F
d
d
=+
(s
−1
) (8.72)
where
b
j
is the ratio of the particle volume in the bubble holes to the volume of
the bubble holes;
ε
is the ratio of the total volume of the holes to the total
volume of the bed layer;
t
Sh
is the Sherwood number, which is calculated based
on the terminal velocity of particles:
0.5 0.33
tt
2.0 0.6Sh Re Sc=+
(8.73)
where,
f
s
t
kd
y
Sh
D
=
,
f
sf
t
v
du
Re =
,
D
v
Sc
f
=
.
where
y
is the logarithmic mean fraction of the non-diffusion component.
The bubbling bed model further assumes that the particles in the bubble clouds
are drawn into the wakes of the bubbles when the particles are moving downward.
Within the wakes, the particles are homogenously mixed with equal flow rates
while entering and leaving the wakes. This assumption allows us to estimate the
particle exchange coefficient
()
ec
b
s
F
among the bubbles, the wakes of the
bubbles and the emulsion phase:
()
()
()
mf
mf
ec
bs
mf b
31
1
u
F
d
ε
δε
•
−
≈
−
(s
-1
) (8.74)
