Mei C., Zhou J., Peng X. Simulation and Optimization of Furnaces and Kilns for Nonferrous Metallurgical Engineering
Подождите немного. Документ загружается.

5 Hologram Simulation of Aluminum Reduction Cells
eff eff eff
221/2
f
eff
1
21
( )
3
y
vuvvv p v v u
txy yx xy yx y
C
uk
uv v F
yyyH
ννν
ρ
ν
ρ
⎛⎞⎛⎞
∂∂∂ ∂∂∂∂∂∂∂
⎛⎞
++=− + + + +
⎜⎟⎜⎟
⎜⎟
∂∂ ∂ ∂∂ ∂ ∂ ∂ ∂ ∂
⎝⎠
⎝⎠⎝⎠
⎛⎞
∂∂ ∂
−− + +
⎜⎟
∂∂∂
⎝⎠
(5.40)
where
x
F and
y
F are the electromagnetic forces of the unit volume;
f
C is
friction resistance coefficiency between the melt and the wall;
H is the depth of
the melt layer. To describe the turbulent flow, k-
ε
equations are used and averaged
along the depth of the melt layer.
eff eff
() ()
νν
ε
σσ
⎛⎞⎛⎞
∂∂ ∂ ∂ ∂ ∂ ∂
++= + ++−
⎜⎟⎜⎟
∂∂ ∂ ∂ ∂ ∂ ∂
⎝⎠⎝⎠
kkv
kk
kkk
uk vk P P
tx y x x y y
(5.41)
2
eff eff
l2
() ()
ε
εε
νν
εεεεε
εε
σσ
⎛⎞⎛⎞
∂∂ ∂ ∂ ∂ ∂ ∂
++= + ++−
⎜⎟⎜⎟
∂∂ ∂ ∂ ∂ ∂ ∂
⎝⎠⎝⎠
kv
uv CPPC
tx
y
xx
yy
kk
(5.42)
2
eff
μ
νννν
ε
=+ =+
T
Ck
(5.43)
where
1
C =1.44,
2
C =1.92,
σ
k
=1.0,
ε
σ
=1.3,
μ
C =0.09. The initial conditions
are considered as static, and the boundary conditions are solid, non-slip walls.
Solutions of above equations should be jointed with wall functions on the side
walls.
With the Navier-Stocks equations, the height of two molten liquid layers (as
shown in Fig. 5.19) can be obtained by averaging the momentum equation of
z
direction:
me m m e e
mm ee
( ) [( ) ( ) ( )]
()()
zz
zz
P
P
g
Fh
g
FHh
h
gF gF
ρρ
ρρ
−− − + − −
′
=
−− −
••
(5.44)
where
m
P
and
e
P are the static
pressure respectively at the bottom
surface of the molten aluminum and the
top of the electrolyte;
m
ρ
and
e
ρ
are
the densities of the molten aluminum and
electrolyte;
z
F
m
and
z
F
e
are vertical
electromagnetic force in the molten
aluminum and electrolyte (the vectors
point to z direction, for solution of the
vectors, please refer to Section 5.2); and
g
is the gravity.
The above equations can be discretized with finite difference method. Using
SIMPLE algorithm and staggered-grid, the equations can be solved by assigning
velocity field and pressure field at different nodes. In references (Sun and Mei,
1989; Huang et al., 1994; Liang et al., 1998; Morris and Davidson, 2003; Gerbeau
Fig. 5.19 Interface of melt
Naijun Zhou
et al., 2003; Sun et al., 2004), the velocity field of various of prebaked anode cells
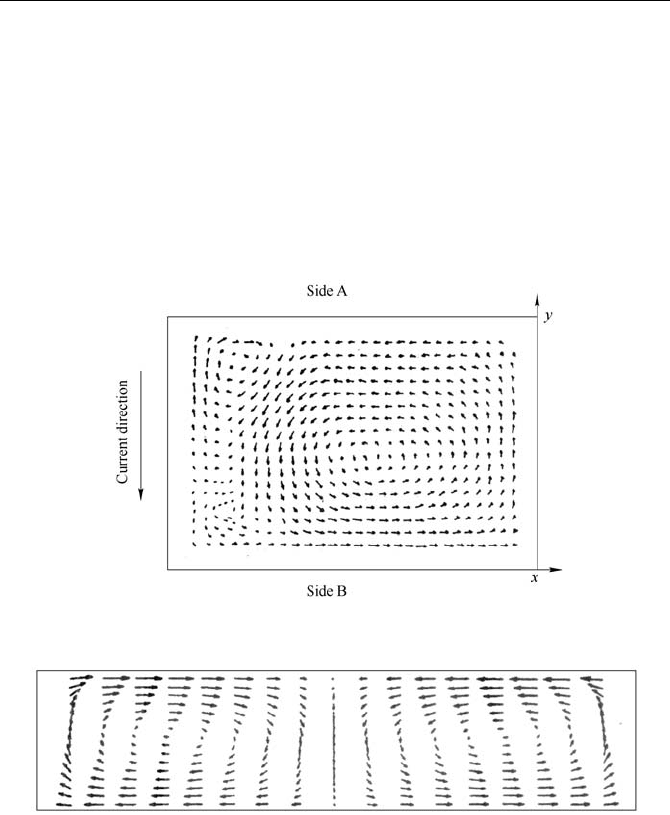
were computed and analyzed. Computational results of a 280kA cell in China
cited from reference (Huang et al., 1994) are shown in Fig. 5.20 and Fig 5.21. Cai
Qifeng et al. (Cai et al., 1993) had ever measured the velocity of the molten
aluminum in the industrial aluminum reduction cells using iron bar dissolution
method for many times.
It should be noted that the computation of the velocity field is so hard to be
highly accurate, because of the inaccurate analysis of the electromagnetic force
field computation as well as the complicated movement of fluid in the cells.
Fig. 5.20
Horizontal velocity distribution in melted aluminum for a 160kA cell (a half cell)
(Sun and Mei, 1989)
Fig. 5.21
Horizontal velocity distribution in electrolyte for a 280kA cell (Huang et al., 1994)
5.4
Analysis of Thermal Field in Aluminum Reduction Cells
The aim of analysis of the static thermal field is to solve the voltage distribution
and the temperature distribution inside the cell as well as the heat balance of the
cell under certain structure and working current conditions, thus to optimize the
design and structure of reduction cells. For such kind of analysis, steady-state
conduction and thermal conductivity models are used, with given melt

5 Hologram Simulation of Aluminum Reduction Cells
temperature, melt position and shape of the ledge. Hence it is a “static”
problem.
The original analysis of thermal field was made separately for three parts, i.e.,
the anode, the cathode and the bottom of slot, which may be limited by computer
capacity and speed at that time. Nowadays, there is no need for such a
separation.
Because of the strong coupling between the electric field and the temperature
field in reduction cells, the temperature field cannot be analyzed independently.
Instead, it has to be solved simultaneously with the electric field, as introduced in
Section 5.2. In fact, in Section 5.2.2, details of analysis of the thermal field of the
anode have been described, and here will not be repeated any more. In this section,
it is to treat the aluminum electrolysis cell as a whole to discuss models and
solutions of the analysis of thermal field.
5.4.1
Control equations and boundary conditions
Under the assumption of steady state, equations of electrical and thermal
conduction in anode and cathode are the same as Eq. 5.3 and Eq. 5.4. What is
different is that, at non-conductive part (i.e. the cell body outside ledge and below
the cathode), the equation of thermal conduction can be transformed into the
Laplace equation without solving the electrical conduction equation.
0T
λ
∇∇=• (5.45)
Since the anode is symmetrical, it generally takes only half of the anode for a
prebaked cell(or even a quarter) as the computational geometry; but for a
self-baking cell, it is an area including two halves of conducting bar. It is assumed
that no transverse current and heat flow through the cross section. Obviously,
computation of thermal field for the anode must be treated as a three-dimensional
problem. Those of the cathode and other parts of the cell is actually a mix of two
and three dimensions, of which the cathode bar is to be treated as three-dimension,
while others can be computed with two-dimensional models. Temperature field of
the electrolyte and the molten aluminum are both homogeneous and their
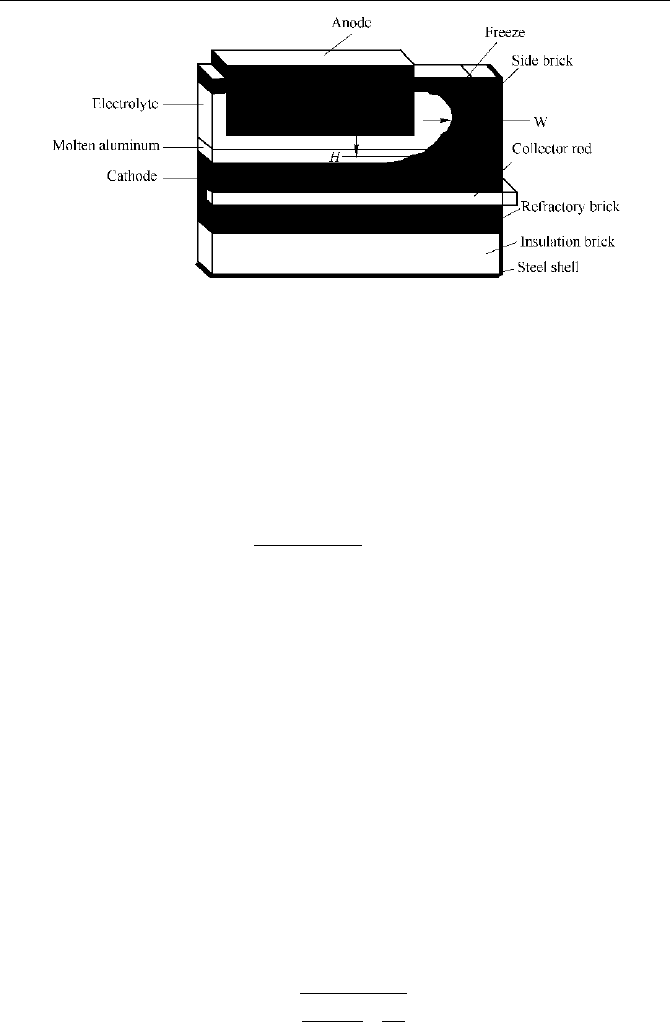
temperature are specified. It must be noted that the computational slices should be
taken from the big and end faces of the cell. A schematic of the computational
slice is given in Fig. 5.22.
The boundary conditions for the electrical conduction equation are:
a) Regarding the cathode block surface as basic potential surface, and the
potentials in the molten aluminum are considered as equal.
b) The ledge is not conductive and all currents pass through the cathode block.
c) The currents passing through each anode and cathode rod are known and the
value is an average.
Naijun Zhou
Fig. 5.22
Schematic of the computational slice of an aluminum reduction cell
The boundary conditions of the thermal conduction equation are:
a) The electrolyte and molten aluminum layers are an isothermal region, their
temperatures are to be decided with experience and by request.
b) The top of the cell is assumed to be adiabatic.
c) The environmental temperature around the cell is certain.
d) The coefficient of heat transfer between the external surface and the environment
α
is:
8
0.25 4 4 2
w
wa wa
wa
5.6 10
( ) ( ) (W/(m K))CT T T T
TT
ε
α
−
×
=− + −
−
•
•
(5.46)
where C is an experimental coefficient, which is 2.6 for the side walls, 2.0 for the
bottom, and 3.3 for the top plate;
w
ε
is the radiance of the external wall, of
which the value is usually taken as 0.82.
e) Factors that influence the heat transfer between the melt and the internal
faces are quite complicated, and no consist and reliable results can be referred (see
Table 5.2). Generally, the coefficient can be calculated with following empirical
formula (Mei and Tang, 1986; Mei et al., 1992; Zhou and Mei, 1992; Mei et al.,
1996).
Heat transfer from the melt to the ledge:
0.8 0.33
0.0365Nu Re Pr= • (5.47)
Heat transfer from the molten aluminum to the cathode block:
0.8
50.025( )Nu Re Pr=+ • (5.48)
Big error may be found in Eq. 5.48. So in practice, it is usually corrected with
additional thin film thermal-resistance method:
B
mB
1
L
Nu
α
δ
λλ
′
=
+
•
(5.49)
where
L is the inter width of the cell, m; Nu is the Nusselt number to be
determined with Eq. 5.47 and Eq. 5.48;
m
λ
and
B
λ
are respectively the

5 Hologram Simulation of Aluminum Reduction Cells
thermal conductivity of the melt and the boundary thinfilm, W/(m·K); and
B
δ
is the thickness of the boundary thinfilm, m. The melt velocity used above
equations are calculated with Eq. 5.36 and Eq. 5.37. Besides, the thermal
properties of main materials used in the computation are given in Table 5.3, or
refer to reference (Qiu, 1982).
Table 5.2 Coefficient of heat transfer between melts and ledge (W/(m
2
gK))
Author Electrolyte-ledge Aluminum-ledge Remark
[Japan]Haruhiko
Ikenouchi et al.
200
20~150
400
Effective heat transfer
coefficient during side
feeding (Ikenouchi et al.,
1978);
measured
value(Ikenouchi et al.,
1978)
[USA]W.E.Haupin 370
1500
1100
Calculated with criterion
equation (Haupin,1971);
measured value
(Haupin, 1971)
[Japan]Arai et al. 232
793 (
δ
B
=0.3mm)
334 (
δ
B
=1.0mm)
183 (
δ
B
=2.0mm)
Arai and Ramazaki, 1975
[USA]J.G.Peacey 175~210 300~384 Peacey and Medlin, 1979)
[Norway]Perutne 200 Perutne, 1982
[USSR]
U.D.Dekopov et al.
14000
mf
=Q
W/m
2
10500
mf
=Q
W/m
2
Dekopov, 1978
[USSR]
B.P.Romanov et al.
141~184 150~268 Romanov, 1980
[Norway]A.Solhiem
For prebaked cells:
250~500
For self-baking cells:
300~650
Solhiem et al., 1983

Naijun Zhou
Table 5.3 Thermal properties of materials
Material
Conductance
/W
g(mgK)
−1
Density
/kggm
−3
Specific heat
capacity
/J
gkg
−1
gK
−1
Semi-graphited cathode
carbon block (Aparotsev,
1998)
8.81
−0.072T
−3
1573/(1+7.9
h10
−6
T)
307/(1+4.3
h
10
−4
T)
Cathode iron
rod (Aparotsev, 1998)
58.85
−0.0414 T
−3
7830/(1+11.1
h10
−6
T)
465/(1+12.1
h
10
−4
T)
Cast iron (filled in iron rod
and carbon block)
(Aparotsev, 1998)
51.25
−0.064 T
−3
7270/(1+12.1
h10
−6
T)
419/(1+5.7
h
10
−4
T)
Side ledge
(CSIOFMAM, 1977)
2.16
−0.365h10
−2
T
+0.0558
h10
−4
T
2
Bottom ledge
(CSIOFMAM, 1977)
4.97
0.869h10
−2
T
+0.098
h10
−4
T
2
Insulation impervious
material
0.319+1.37
h10
−4
T
5.4.2
Calculation methods
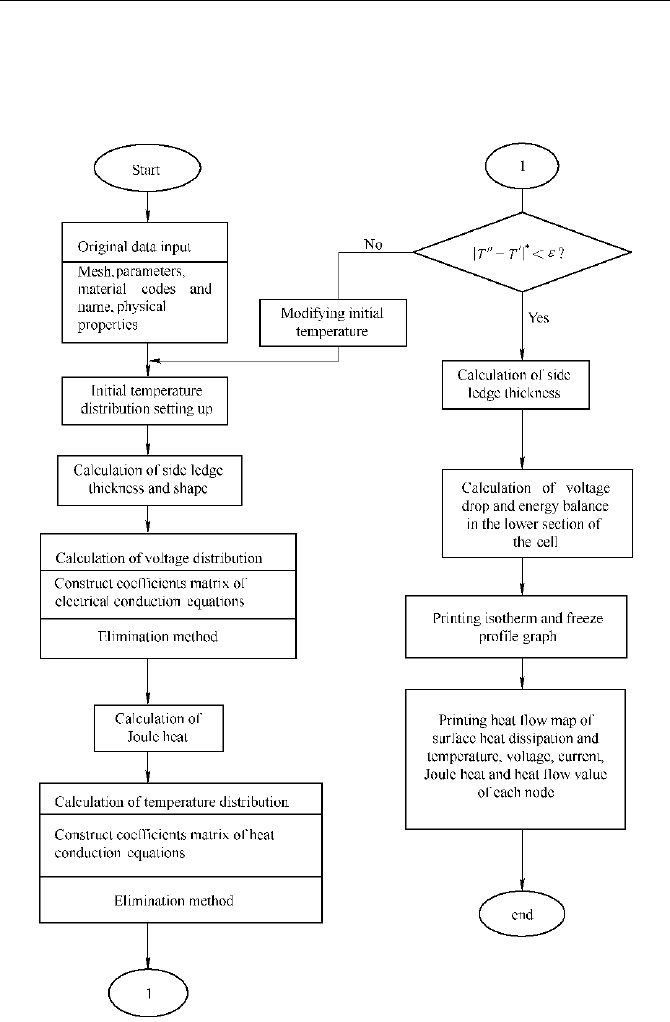
In computation of the thermal field, finite difference method and Gaussian
elimination method (or other methods) are usually used to solve the linear
equations. The program chart of the computation is shown in Fig. 5.23. This
algorithm was used in references (Mei and Tang,1986;Mei et al., 1992; Zhou and
Mei, 1992; Mei et al., 1996; Mei et al., 1997; Mei et al; 1998; Zuo, 1996; You,
1997; You et al., 1998; Zhou et al., 1998) to analyze the thermal fields of various
types of reduction cells, which provided examples of systematic research on the
design of thermal fields and optimization of cell structures. A typical analytical
result is shown in Fig. 5.24.
5 Hologram Simulation of Aluminum Reduction Cells
Fig. 5.23
Program chart of static analysis of the thermal field (Mei and Tang., 1986)
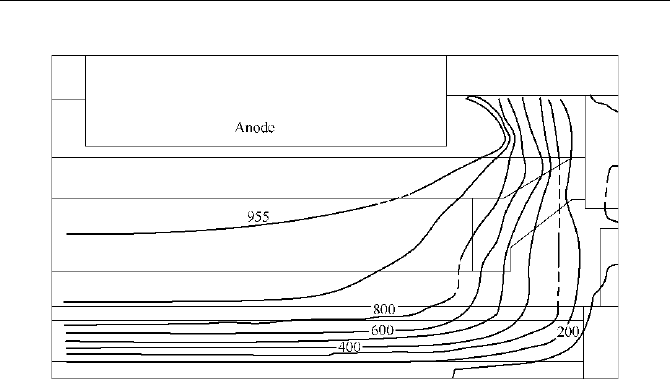
Naijun Zhou
Fig. 5.24
Analytical result of the temperature field in cathode (Mei et al., 1996)
5.5
Dynamic Simulation for Aluminum Reduction Cells
An aluminum electrolysis cell is a complex high temperature electrochemical
reactor, whose operation conditions are effected by many factors. Any changes of
the factors will cause unstable state of the cells. A worse operation condition will
even result in an increasing of power consumption, decreasing of production, and
shorter cell life. Obviously, it’s difficult to accurately predict the effects of various
technical parameters on the operation conditions with the static models discussed
in previous sections. Hence, the dynamic simulation is needed and will be
introduced in this section.
The aim of the dynamic simulation is to simulate the process in the cell with
realtime information obtained by computer and output (forecast) the results, in
order for online monitoring the operation conditions and to instruct the operations.
Equipped with automatic control system, it can then realize functions of online
optimization and monitoring.
Practices indicate that there are three critical factors for operations of
aluminum reduction cells, that is, the electrolyte temperature, the cell freeze
profile and the current efficiency. Since effective online measurements of these
factors haven’t been found so far, it is hence of particular practical significance
to simulate the parameters. At present, the primary task of the dynamic
simulation of aluminum reduction cells is the dynamic forecasting of the
electrolyte temperature and the current efficiency, as well as the online
simulation of the cell freeze profile. The Central South University and some

5 Hologram Simulation of Aluminum Reduction Cells
other institutes had carried out research in this fields for many years, and made
great progresses (Mei et al., 1997; Mei et al., 1998; Zuo, 1996; You, 1997; You
et al., 1998; Zhou et al., 1998). In this section, it is to be discussed the methods
for online simulation of the electrolyte temperature and the dynamic forecasting
of the cell freeze profile.
5.5.1
Factors influencing operation conditions and principle of
the dynamic simulation
You Wang (You, 1997; You et al., 1998)
had studied factors influencing the
operation conditions of the aluminum reduction cells. In his opinion, the factors
can be put into two categories. One is the static factors, including bus bar
configuration, cell structure, electro-thermal properties of materials, melt
properties, and heat transfer conditions of the cell body etc. The other is the
dynamic (transient) factors, including processing parameters such as series current,
cell voltage, anode-cathode distance (ACD), molecular ratio, temperature of the
electrolyte and molten aluminum, height of the as well as the electrolyte and
molten aluminum, anode effect coefficient etc., as well as the routine operations
such as alumina feeding, aluminum discharging, ACD adjusting, anode changing,
edge treating, effect treating and so on. The static factors only influence medium
and long term behavior of the cell, thus they can be used to establish the basic
energy balance of the cell. The dynamic factors influence dynamic behaviors and
they are the basis of dynamic simulation. All these factors must be quantitatively
transformed into energy budget of the cell or corresponding disturbance
parameters.
The principle of the dynamic simulation is based on following facts. When a
disturbance factor causes the change of energy inputting into the cell, it will firstly
cause changes of the melt temperatures (especially, the change of the electrolyte
temperature). As the liquidus temperature T
f
is fixed corresponding to certain
composition of the electrolyte, the change of the melt temperatures will result in
melting or freezing of the electrolyte crust, or in other word, changing in the
thickness of the side ledge. As a result, heat transfer through the ledge will also
change until a new balance of energy is reached. Therefore, based on the
contribution of every factor to the energy budget of the system, the energy balance
of a cell can be continuously obtained to calculate the bath temperature with
measured intensity and duration of the disturbance, thus to simulate the freeze
profile by solving the unsteady heat conduction equation. The program chart of
the process is given in Fig. 5.25.
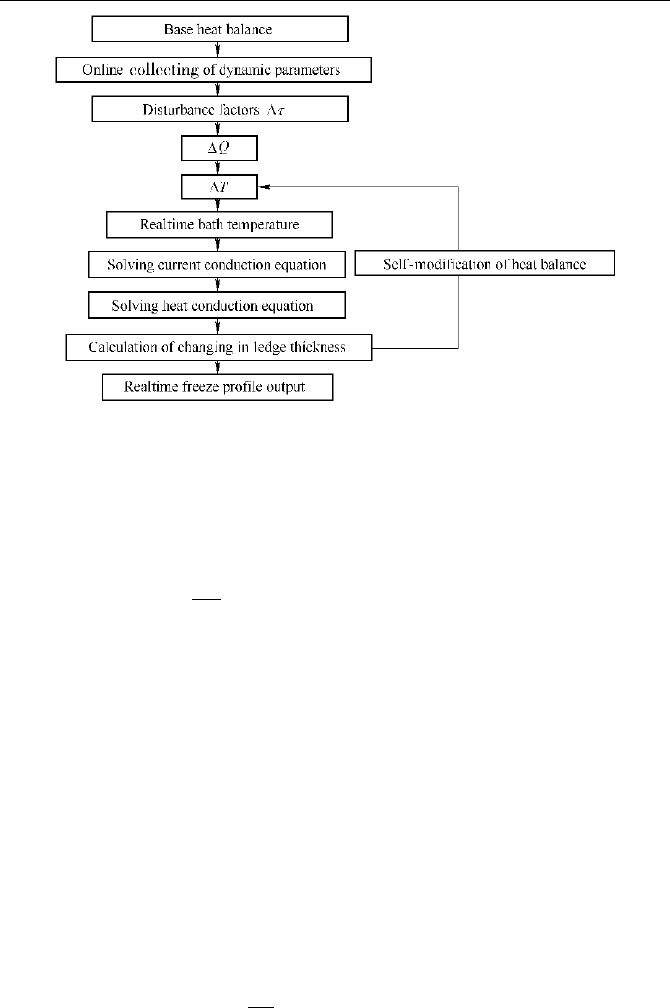
Naijun Zhou
Fig. 5.25
Program chart of the dynamic simulation for aluminum reduction cells
5.5.2
Models and algorithm
When viscous dissipation of the melt flow is neglected, energy equation of the
system is:
vol
div( ) div( grad )
H
HkTq
ρρ
τ
∂
+= +
∂
r
•v (5.50)
The relation between the enthalpy and temperature is:
For solid phase
f
TT < :
p
HcT= •
For liquid phase
f
TT > :
p
HcT
λ
=+•
At the interface
f
TT = :
p
HcTn
λ
=+••
where
f
T
is the melting point of the electrolyte;
p
c is the specific heat capacity;
λ
is the latent heat of melting, n is the molten fraction (0İnİ1).
As the melt motion in reduction cells is complicated. Eq. 5.50 is actually hard to
be solved. A simple method is: to replace the thermal conductivity
k with the
effective thermal conductivity
eff
k , so that the convection term can be eliminated:
teff
kkk += (5.51)
where k is the molecular thermal conductivity;
t
k is the turbulent thermal
conductivity.
Furthermore, assuming c
p
is a constant, Eq. 5.50 can then be simplified as:
eff vol
div( grad )
p
T
ckTq
ρ
τ
∂
=+
∂
•• (5.52)
This is an unsteady conduction equation with internal heat sources, in which the
source term
vol
q
is the heat produced when current passing through the melt, that
