Mei C., Zhou J., Peng X. Simulation and Optimization of Furnaces and Kilns for Nonferrous Metallurgical Engineering
Подождите немного. Документ загружается.

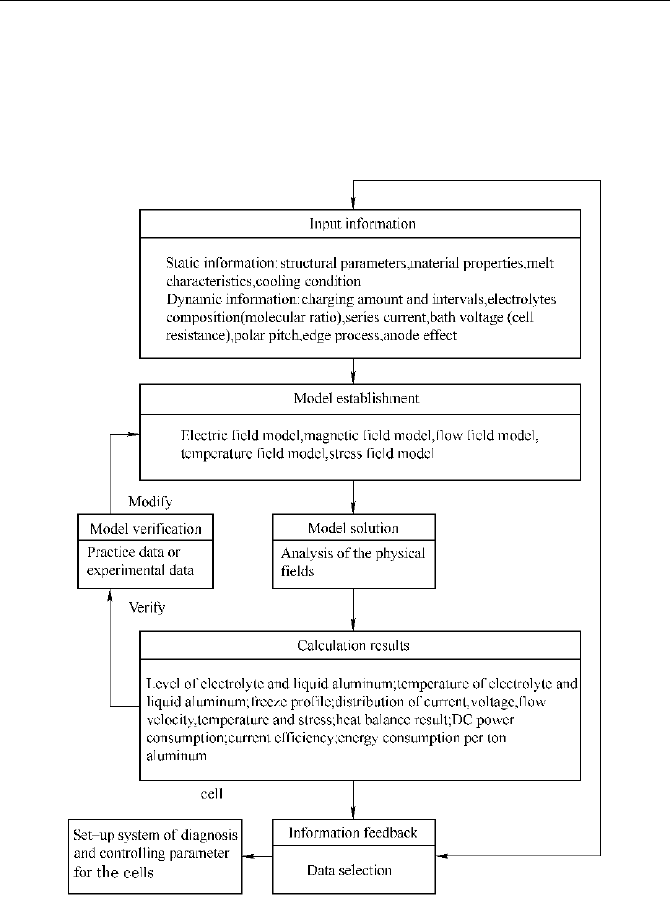
5 Hologram Simulation of Aluminum Reduction Cells
example on dynamic simulation for temperature field in an aluminum reduction
cell. The sixth section will introduce calculation model of the current efficiency.
Some typical simulation results will also be given in the chapter. It must be noted
that hologram simulation of aluminum cells are not perfect, hence unremitting
hard work is still required.
Fig. 5.3 Principle of hologram simulation technology for aluminum reduction cells
5.2
Computation and Analysis of the Electric Field and Magnetic
Field
The aluminum reduction cell is an electrochemistry reactor acted on high direct

Naijun Zhou
electric current. The current is the original cause of all the phenomena in
aluminum reduction cells. On one hand, high electric current results in strong
magnetic field and the electromagnetic force then causes melt motion and molten
aluminum flowing and influences heat
and mass transfer as well as the
operating conditions. On the other hand,
the electric current directly produces
heat, which keeps cells at an
appropriate temperature.
The distribution of magnetic field
in a cell depends on the electric
current distribution in the cell and
the adjacent cells. The electric
current is usually divided in several
sections such as bus bar, anode, melt
and cathode etc. and can be treated
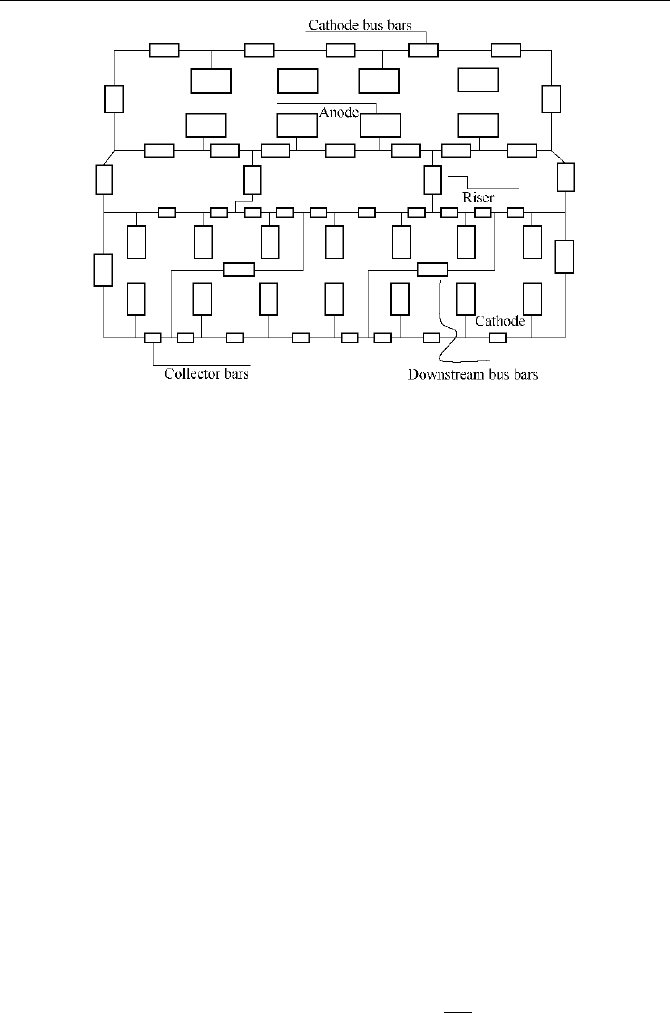
respectively. Fig.5.4 is a schematic
of the conduction structure in a
typical aluminum reduction cell.
5.2.1
Computation model of electric current in the bus bar
According to Kirchhoff’s law, current that flows through each part of the bus bar
system can be calculated as:
EIR=
∑∑
• (5.1)
0
j
j
I =
∑
(5.2)
where
j is the node number.
In calculation, all sections of the bus bar are replaced with equivalent resistance.
Then on the basis of series-parallel connection relation among conductors of the
bus bar, a network chart of electric circuit is obtained, and electric potential at
each node and current along each section of the bus bar are computed using a
program.
In calculation, because of the complicated relation of the bus bar sections,
computation is quite difficult and fussy. Fig.5.5 shows a typical bus bar calculation
network chart. For details of the computational methods, program and the
examples, please refer to references (Imery, 1989; Vao, 1990).
Fig. 5.4
Conduction structure in an
aluminum reduction cell
5 Hologram Simulation of Aluminum Reduction Cells
Fig. 5.5
Computation current meshwork of bus bars
5.2.2
Computational model of electric current in the anode
Based on the calculated current that flows through anode rod into anode block and
appropriate assumption of boundary conditions, electric potential and current of
each control volume (node) can be calculated with Kirchhaff’s law and resistivity
of anode block by using three-dimensional finite difference method or finite
element method. The procedure is the same as that of calculation of the bus bar.
However, it must be noted that, since the resistivity is usually a function of
temperature, the heat of the control element should be computed before working
out the current distribution (electric field). Then the temperature distribution in
anode block can be obtained by solving heat and conduction differential equations
simultaneously.
The control equations are:
a) Electric conduction differential equation:
0
V
σ
∇∇=• (5.3)
b) Heat conduction equation:
vol
0Tq
λ
∇∇+ =• (5.4)
where σ is the electric conduction rate of the anode material; λ is the heat
conduction coefficient;
q
vol
is Joule heat in control volume that is given by:
1,,
,,
1,,
,,
,1,
,,
,1,
,,
,,1
,,
,,1
,,
,,vol
+−+−+−
+++++=⇒
kji
kji
kji
kji
kji
kji
kji
kji
kji
kji
kji
kji
kji
qqqqqqqq (5.5)
where
ΔΔ
1, , 2
,, 1,, ,,
Δ
()
yz
ijk
ijk x i jk ijk
x
qVV
σ
−
−
=−•• (5.6)
And the rest may be inferred by analogy.
The difference equations of Eq. 5.3 and Eq. 5.4 can be expressed as:
Naijun Zhou
, , 1 1, , 1, , 2 , 1, , 1,
3,,1 ,,1 123
[( ) ( )
()]/2()
ijk i jk i jk ij k ij k
ijk ijk
VaV V aV V
aV V a a a
−+ −+
−+
=++++
+++
••
•
(5.7)
,, 1 1,, 1,, 2 , 1, , 1,
3,,1 ,,1 ,, 123
[( ) ( )
()]/2()
ijk i jk i jk ij k ij k
ijk ijk ijk
TbT T bT T
bT T q bb b
−+ −+
−+
=++++
++ ++
••
•
(5.8)
where,
123
123
,,
,,
xyz
xyz
y
zxzx
y
aaa
x
y
z
y
zxzx
y
bbb
x
y
z
σσσ
λλλ
ΔΔ ΔΔ ΔΔ
ΔΔΔ
ΔΔ ΔΔ ΔΔ
ΔΔΔ
===
===
•••
•••
Eq.5.7 and Eq.5.8 can be solved by Gaussian elimination or iteration. The
boundary conditions are listed as following:
a) The electric potential at molten aluminum surface is evenly distributed.
b) The electric on the cathode carbon surface is evenly distributed.
c) The temperature in the electrolyte is uniform everywhere, and the value can
be obtained by measuring.
d) The temperature at the joint of anode bus bar and rod should be given.
e) The thermal boundary conditions on the carbon surface are defined as the
type of heat convection boundary.
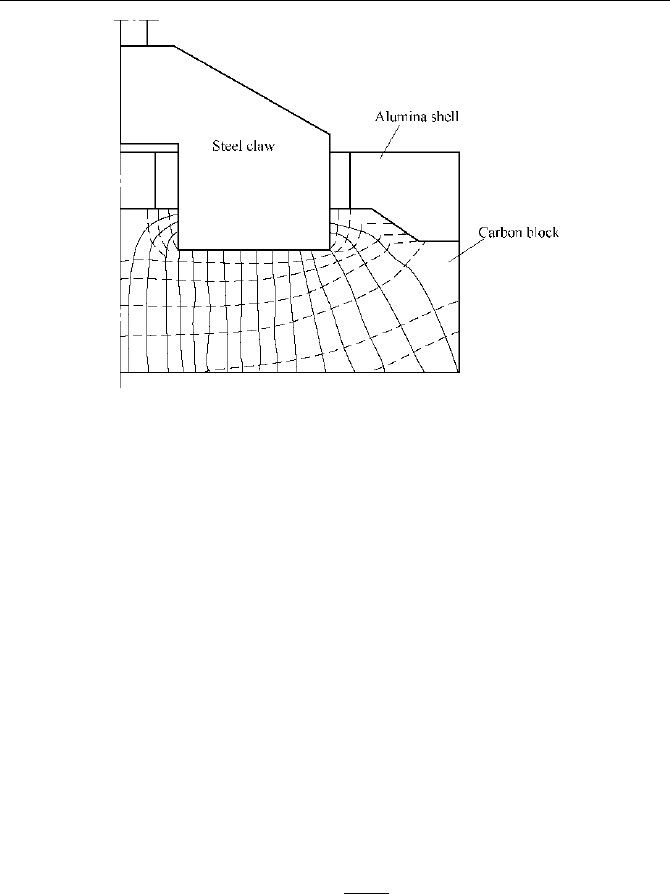
The program chart of the computation is showed in Fig.5.6. This procedure was
used to compute and analysis the electric field of anode, as introduced in
references (Gao, 1991; JLMRI, 1980; Zen, 1996; Zen, 2004). Fig.5.7 gives a
computation result.
5.2.3
Computation and analysis of electric field in the melt
The melt in aluminum reduction cells is composed of electrolyte and molten
aluminum. Because of the great difference of their electrical resistivity, the current
distribution in the melt is evident. For instance, since the electrical resistivity in
electrolyte is larger than in the molten aluminum, the current density in the
electrolyte below the anode bottom is almost uniform, and pointing vertically
downwards; however in the electrolyte at the side face of anode, the current
density is much smaller. Haupin had proposed a sector coefficient method to
approximate the current in these parts(Haupin,1990). Of course it can also be
accurately computed by finite difference method or finite element method with
nonlinear boundary conditions.
The current distribution in the molten aluminum is complicated. The molten
aluminum is a good conductor. Moreover, because of the existence of frozen ledge
and deposits, there is usually large horizontal current found in the molten aluminum,

5 Hologram Simulation of Aluminum Reduction Cells
Fig. 5.6
Computation procedure of current field for the anode system
which results in the fluctuation and flow of the melt, and causes some unwanted
influences on the operation. Therefore, the current distribution in the melt must be
carefully studied and analyzed.
To compute the current distribution in the melt, there are many kinds of
methods, such as finite difference method, finite element method, network graph
theory, and boundary element method etc. (Arita, 1983; Tarapore, 1982; Richand,
1976; Chen, 1986). The basic control equations are Eq. 5.3 and Eq.5.4. However,
Naijun Zhou
Fig. 5.7
Computational result of electric distribution in anode (Gao, 1991)
different from that in last section, because of the symmetrical layout of the current
carriers, the current distribution in the melt is symmetrical along the longitudinal
axis in aluminum reduction cells, which therefore can be treated as a
two-dimensional problem. The computation is to be illuminated with method of
network graph theory as below.
The electrical potential drop occurs mainly in the electrolyte. In computation of
the electric field in the electrolyte, the anode and molten aluminum can be
regarded as equipotential volumes. In this case, the electric field in the electrolyte
can be studied as a potential boundary value problem with given electric current.
The potential drop is very small, so the potential difference at the electrolyte-metal
interface and the metal-cathode blocks interface must be taken into account. With
the current density at the interface of metal being determined, the electric field in
it can be studied as a potential field problem with the second boundary condition.
For an electric current field as shown in Fig. 5.8, the medium is homogeneously
isotropic and the thickness is
zΔ . The potential of node A is
ji
V
,
, and the
conductance of branch
AB is:
yz
G
x
σ
ΔΔ
Δ
=
• (5.9)
Current of branch AB is:
,,1,
()
ij ij i j
IVVG
−
=− • (5.10)
According to Kirchhoff’s junction current law:
0I =
∑
(5.11)
For the inner node
()
,
A
ij:
,1, ,,1 1,, ,1,
[( )( )( )( )]0
ij i j ij ij i j ij ij ij
GV V V V V V V V
−−++
−+−−−−−=•
(5.12)
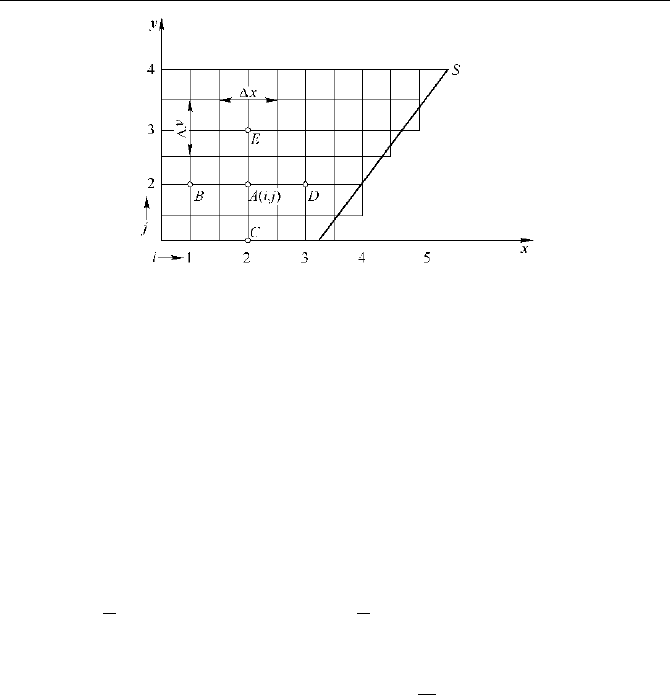
5 Hologram Simulation of Aluminum Reduction Cells
Fig. 5.8
Mesh nodes of current field
Namely:
04
,1,,11,,1
=−+++
+−−+ jijijijiji
VVVVV
(5.13)
Similarly, for the edge node
()
jiB ,:
010343
,1,,11,
=−++
−++ jijijiji
VVVV (5.14)
Potential equations of other edge nodes can be obtained in the same way. For
the boundary problem, the boundary condition of the electrolyte can be expressed
as a matrix after discretization as:
=
A
VB (5.15)
For the node that is of the second boundary condition problems such as
()
jiC , ,
if the effluent current is
()
jiI ,
0
, then:
,1, ,,1 1,, 0
11
()()()(,)0
22
[]
ij i j ij ij i j ij
GVV VV V V Iij
++−
−+−− −+ =• (5.16)
That is:
1, , 1 1, , 0
2
24(,)
i j ij i j ij
VVVV Iij
G
++−
++−=• (5.17)
A matrix can then be obtained with the potential equations at every boundary
nodes as:
0
*,7 = (5.18)
After the relative potential has been determined (for example, set the potential
at the electrolyte-metal interface to be zero, i.e.
()
0,
0
=jiV ), the potential of
each node can be solved with matrix 5.15 by using elimination method or
over-relaxation method, and the current density at the upper interface of the
molten aluminum
()
jiI ,
0
can be obtained as well. The current density at lower
interface
()
jiI ,
0
can be obtained in the same way. Substituting the current
densities into matrix 5.18, the potential at each node of the molten aluminum can
be solved using over-relaxation method, and the horizontal and vertical currents
can thus be decided. A computed typical current distribution in the melt is shown
in Fig. 5.9.
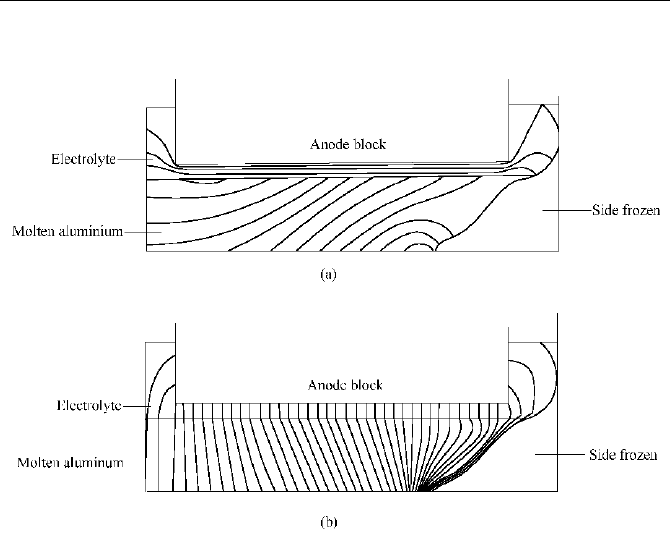
Naijun Zhou
Fig. 5.9 Computational result of current distribution in the melt
(a) Potential distribution; (b) Current distribution
5.2.4
Computation and analysis of electric field in the cathode
The computation and analysis methods stated in the above two sections are also
applicable for that of the cathode. Because of the existence of collector bar, the
model of cathode is usually three-dimensional (or a mix of two dimensions and
three dimensions). When taking the change of side ledge shape and deformation of
the molten aluminum height into accounts, computation will be more convenient
by using the boundary element method.
The Boundary element method is to solve a group of integral equations with
boundary conditions(Zhou,1983). The procedure is:
a) To discretize the boundary into units with certain shapes.
b) To construct interpolating function in each unit.
c) To convert the boundary integral equations into a group of algebraic equations.
d) To solve the equations.
In Section 2.4.2, the integral equation and fundamental solution of liner
constant current field boundary problem have been introduced. Here the solving
process is to be discussed. Assuming
V is the potential function and
*
V is the
fundamental solution, Eq. 2.190 and Eq. 2.191 can then be expressed as:

5 Hologram Simulation of Aluminum Reduction Cells
*
*
d
1
1
2
0
()
ii
S
i
VV
CV V V S
nn
i
CiS
iS
⎧
∂∂
=−
⎪
∂∂
⎪
⎪
∈Ω⎪
⎧
⎨
⎪
⎪
⎪
=∈
⎨
⎪
⎪
⎪
∉Ω
⎪
⎪
⎩
⎩
∫
U
(5.19)
For three-dimensional isotropic medium:
r
V
π4
1
*
= (5.20)
where
r
is the distance from a field point to the source point.
For two-dimensional field:
r
V
1
ln
π2
1
*
= (5.21)
For a two-dimensional field,
S can be divided into N units using liner
interpolation function:
⎪
⎩
⎪
⎨
⎧
+=
+=
+
+
121
121
jj
jj
qqq
VVV
φφ
φφ
(5.22)
where
)1(
2
1
1
ζφ
−= , )1(
2
1
2
ζφ
+= , and
ζ
is the local coordinate. Eq. 5.19 can
then be discretized as:
∑∑
==
−=
N
j
N
j
jijjijii
VHqGVC
11
(5.23)
where:
1
**
21
dd
jj
ij
SS
VV
HSS
nn
φφ
−
∂∂
=+
∂∂
∫∫
1
**
21
dd
jj
ij
SS
GVSVS
φφ
−
=+
∫∫
For
i =1, 2, …, N ( Si ∈ ), Eq. 5.23 can be written into a matrix as:
(2)7 = (5.24)
For the Dirichlet problem,
V is a known variable, then:
7)(2
1−
= (5.25)
Note that for the Neumann problem,
V cannot be uniquely determined. Hence,
a reference point must be decided before to solve
V . For the hybrid problem, the
unknown variables in Eq. 5.24 can be moved to the left of equation:
x
A
F= (5.26)
where
x is the column vector of V and q , the unknown variables at the
boundary. After solving the unknown variables in
S , the potential at each points
in
Ω can be obtained by solving Eq. 5.23. Then, with the following equation
(Eq.5.27),current density J
x
and J
y
of each points in Ω can be solved.
Naijun Zhou
∫
∫
∂
∂
−
∂
∂
=
∂
∂
−
∂
∂
=
S
y
S
x
S
y
V
q
y
q
VJ
S
x
V
q
x
q
VJ
d
d
)(
)(
**
**
σ
σ
(5.27)
where
n
V
q
∂
∂
=
*
*
;
σ
is conductivity.
In the model, there are following a ssumptions:
a) The electric current filed is approximated to be two-dimension.
b) The side ledge is an insulator.
c) The outlet of the cathode block is isopotential.
d) The cathode-electrolyte interface is isopotential.
e) Temperature inside the cathode blocks and collector bar are uniform, and every
subregions have the same resistivity.
f) The cross sections of the cathode rod are the same, an equivalent height of the
rod is used instead of the real value.
It must be pointed out that, because the cathode carbon block is orthogonal
anisotropic, the computation of the electric field in the cathode is a problem to
solving a multi-media electric field in orthogonal anisotropic material. Lu Jiayu
had proposed a method for this kind of medium(Chen et al., 1986).
The
computation procedure is given in Fig. 5.10, and a typical result is shown in Fig.
5.11.
5.2.5
Computation and analysis of the magnetic field
The operation of aluminum reduction cells is ensured by high DC current.
According to the electromagnetics, when current runs through a conductor, a
magnetic field will be produced in the space around, and an electromagnetic force
field will be produced under the interaction of the magnetic field and the current. It
is the electromagnetic force that results in the melt movement, which has a negative
effect on the process of aluminum production. Once an aluminum reduction cell is
designed and installed, its magnetic field is basically fixed. Therefore optimization
of bus bar collocation in design process is very important, so as to predict the
magnetic field in the operation and to minimize the negative effect.
With the results of the electrical fields as introduced in Section 2.4, computation
and analysis of magnetic field can be carried out. In computation and analysis of
the magnetic field in the cells. An equivalent mathematical model is usually
employed. That is, to replace the rectangular bus bars (including the melt) with
columnar bus bars that is of the same length and cross-sectional area, then for one
