Massoud M. Engineering Thermofluids: Thermodynamics, Fluid Mechanics, and Heat Transfer
Подождите немного. Документ загружается.


642 Vb. Two-Phase Flow and Heat Transfer: Boiling
2.1. Dimensionless Groups
Practical aspects of boiling heat transfer are based on experimental data. To corre-
late such data we need to find the dominant factors in heat transfer associated with
phase change. Such factors include the involved forces, key fluid properties, and
the operational conditions. Viscous and buoyancy forces play a major role in heat
transfer with phase change. Pertinent fluid properties include latent and specific
heat (h
fg
, c
p
), density and thermal conductivity (
ρ
, k), and viscosity and surface
tension (
µ
,
σ
). Finally, operational conditions include pressure, fluid and surface
temperature, and the surface geometry (L). Since the effect of pressure appears in
fluid properties, we can reduce the number of variables to 10. These are
ρ
l
,
ρ
v
, h
fg
,
c
p
, k,
µ
,
σ
, ∆T, L and h. Incropera finds five dimensionless groups for these pa-
rameters; Nu = hL/k = f[
ρ
g(
ρ
l
–
ρ
v
)L
3
/
µ
2
, Ja, Pr, Bo] where the Bond number (Bo)
is similar to the Gr number (Table A.I.6).
2.2. Determination of Degree of Superheat
for Equilibrium Bubble
Homogeneous nucleation: Earlier we noticed that for a bubble to be in equi-
librium in the bulk of a liquid, three conditions must be met. To maintain equilib-
rium, we can use these conditions to find the minimum degree of superheat for a
liquid; i.e. T
l
– T
sat
. From the requirement for equal chemical potential, we obtain
the Clausius-Clapeyron equation dP/dT = h
fg
/(T
sat
v
fg
). We integrate this equation
assuming T
sat
v
fg
/h
fg
remains constant. We then substitute for P
v
– P
l
= 2
σ
/r
e
from
the second requirement to find:
egfg
sat
efg
fgsat
fg
fgsat
satl
rh
T
rh
T
P
h
T
TTT
ρ
σ
σ
2
2
vv
≅
¸
¸
¹
·
¨
¨
©
§
=∆=−=∆
Vb.2.1
where r
e
is the radius of the equilibrium bubble. Equation Vb.2.1 shows that the
degree of superheat is inversely proportional to the bubble radius. Thus, the
smaller the bubble, the higher the required degree of superheat. That is why the
homogenous nucleation requires very high degrees of superheat.
Heterogeneous nucleation: Regarding nucleation from a heated surface, we
noticed that the minimum radius of a growing bubble is when r
b
= r
c
, where r
c
is
the radius of the cavity. Substituting into Equation Vb.2.1, we conclude that bub-
bles that have made it to the mouth of the cavity will grow if the degree of super-
heat in the liquid is at least equal to ∆T as given by Equation Vb.2.1. This is in-
deed the case if the bulk liquid is superheated. Superheating is achieved by
heating the liquid in a pressurized vessel until liquid becomes saturated. When the
heating process is terminated and the vessel is perfectly insulated, we reduce the
liquid pressure. As the pressure drops, bubbles begin to form on the surface of the
vessel when the liquid superheat becomes at least equal to that given by Equa-
tion Vb.2.1.
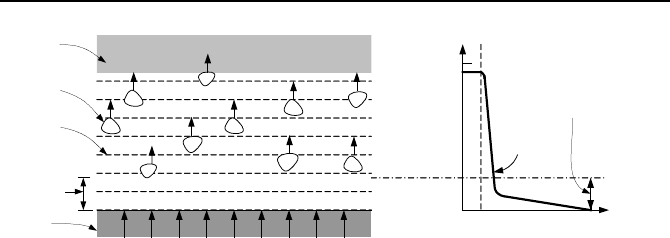
2. Convective Boiling, Analytical Solutions 643
T
T
s
T
sat
y
T(y)
δ
Thickness of
Thermal Sublayer
Solid
Vapor
Liquid
Bubble
Thermal
Sublayer
(a) (b)
Figure Vb.2.1. Heterogeneous boiling and temperature gradient in liquid
It is fascinating to note that if boiling is induced solely by continuing to heat the
vessel, the degree of superheat required for boiling is much higher than that pre-
dicted by Equation Vb.2.1. Indeed, some data have shown that the required de-
gree of superheat for bubble growth is three times as much as that predicted by
Equation Vb.2.1 (Hsu). The reason turns out to be the existence of a region (Fig-
ure Vb.2.1) referred to as the thermal sub-layer. Liquid temperature increases
markedly in this region from T
sat
to T
s
, Figure Vb.2.1(b). It is in this thermal sub-
layer near the heated surface that liquid becomes superheated to provide sufficient
heat for the bubble to grow and depart.
We may estimate the thickness of the thermal sub-layer by using the definition
of heat transfer coefficient (h). Thus, the thickness of the thermal sub-layer is re-
lated to the liquid thermal conductivity as
δ
= k
f
/h where h can be estimated from
a correlation such as that of Fishenden for turbulent natural convection from a
horizontal flat surface:
()
3/1
PrGr14.0Nu =
where the Gr and Pr numbers are calculated for the liquid phase. Next, we focus
on the heat transfer mechanism taking place in the thermal sub-layer.
Let’s investigate the relation between the bubble equilibrium temperature
(Equation Vb.2.1) and water temperature in the thermal sub-layer. Shown in Fig-
ure Vb.2.2 is a cavity of radius r
c
on the heated surface.
Originally, both liquid and surface have the same temperature as the bulk liquid
T
f
(line ZO). We add heat to the surface and bring its temperature to T
A
. The tem-
perature in the thermal sub-layer is shown by line ZA where we have assumed a
linear temperature profile in the thermal sub-layer. Since we do not observe any
bubble in the liquid we increase the surface temperature to T
B
with the liquid tem-
perature shown by line ZB. As we heat up the surface, the bubble in the cavity
begins to grow. We keep increasing the surface temperature until eventually the
line representing temperature in the thermal sub-layer (line ZC) becomes tangen-
tial to the curve representing Equation Vb.2.1 for an equilibrium bubble. At this
point, the bubble in the cavity has reached the mouth of the cavity and has the
smallest radius of curvature. Hsu’s condition for the bubble to grow is that the
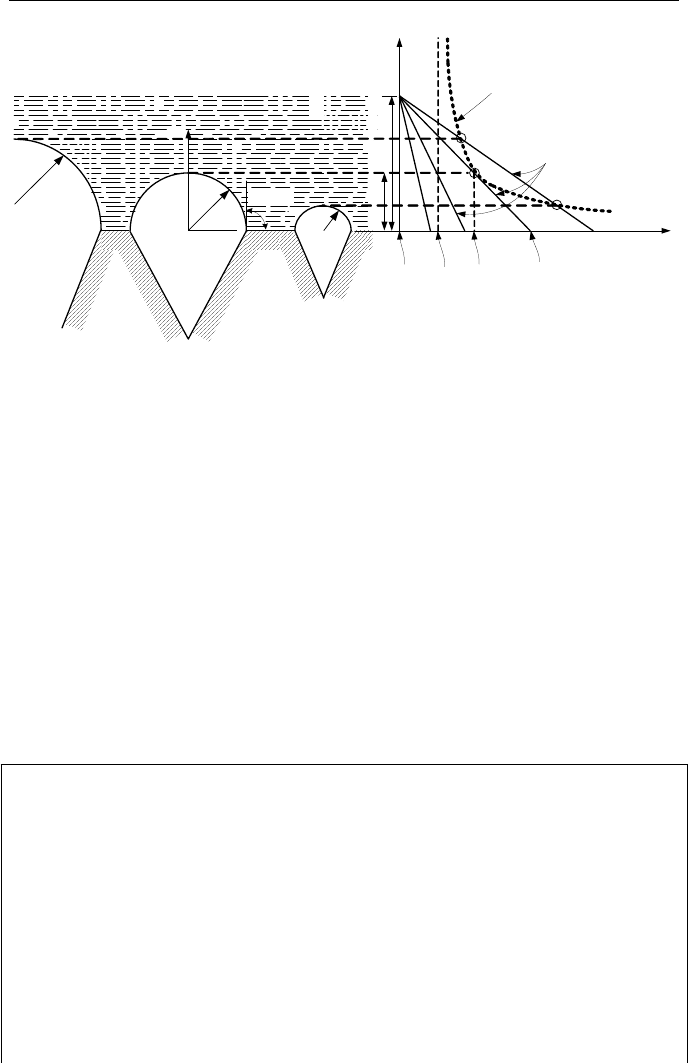
644 Vb. Two-Phase Flow and Heat Transfer: Boiling
r
c
δ
y
T
sat
Equation Vb.2.1
Liquid Temperature
r
max
r
min
r
e
T
T
ONB
A
B
CD
T
f
O
Z
φ
=90
o
r
min
r
max
y
T
g
r
c
T
f
Figure Vb.2.2. Depiction of the onset of nucleate boiling (Hsu)
liquid temperature at y = r
c
must be at least equal to the bubble interior tempera-
ture for the bubble to grow. Thus, when the line for liquid temperature is tangent
to the curve representing the equilibrium bubble (Equation Vb.2.1), cavities of ra-
dius r
c
are active sites for nucleation. The corresponding temperature, is known as
onset of nucleate boiling temperature (T
ONB
).
If the surface temperature is further increased (say to T
D
corresponding to line
ZD for the thermal sub-layer), then at lower superheats the larger radii cavities and
at higher superheats smaller radii cavities become active. But if a cavity of size r
c
does not exist on the surface, the increasing surface temperature results in higher
degrees of liquid superheat. This will continue until the temperature profile in liq-
uid becomes tangent to the vapor temperature calculated from Equation Vb.2.1 for
the cavity size that is present in the surface. When various cavity sizes exist and
the bulk liquid is at saturation, we may approximate r
c
as r
c
=
δ
/2. Using similar
triangles, we find that (
δ
– r
c
)/r
c
= ∆T
sat
/∆T
ONB
resulting in ∆T
ONB
= 2∆T
sat
.
Example Vb.2.1. Find the degree of superheat (T
s
– T
sat
) for a horizontal flat
plate in water at atmospheric pressure necessary to cause nucleation at all active
sites. Data: At 1 atm,
ρ
= 958 kg/m
3
,
ρ
g
= 0.593 kg/m
3
, h
fg
= 2.257E3 kJ/kg, k
f
=
0.68 W/m·C,
σ
= 0.059 N/m,
β
= 0.75E-3 C
-1
, v = 0.292E-6 m
2
/s, Pr = 1.73.
Solution: We first find the thickness of the thermal sub-layer in terms of ∆T
s
, the
surface temperature minus the saturation temperature:
Gr =
β
g∆T
s
D
3
/v
2
= [0.75E-3 × 9.81 ∆T
s
D
3
/(0.292E-6)
2
] = 8.63E10 ∆T
s
D
3
hD/k
f
= 0.14Gr
1/3
Pr
1/3
= 0.14 [8.63E10 ∆T
s
D
3
]
1/3
(1.73)
1/3
= 743 (∆T
s
)
1/3
D
Since
δ
= k
f
/h, we find:
1/
δ
= 743 (∆T
s
)
1/3
. Therefore,
δ
= 1.35E-3(∆T
s
)
–1/3
m. (1)

2. Convective Boiling, Analytical Solutions 645
Next, we find the required surface superheat, ∆T
s
= T
s
– T
sat
from Equation Vb.2.1:
∆T
s
= 2
σ
T
sat
/(h
fg
ρ
g
r
e
) = 2 × 0.059 × (100 + 273)/(2.257E6 × 0.593 × r
b
) = 3.3E-5/r
b
(2)
Substituting for r
b
= r
c
=
δ
/2 into (2) yields: ∆T
s
= 6.6E-5/
δ
. Substituting ∆T
s
into
(1) to find
δ
as:
δ
= 1.35E-3[6.6E-5/
δ
)
-1/3
. We, therefore, find
δ
= 6 mm. Thus, ∆T
s
= 0.019 C.
Comment: It is seen when cavities of all sizes are present, the required degree of
superheat is very small.
Let’s assume only cavities of 8
µ
m exist. In this case, r
c
= 8E-6 m and (∆T
s
)
required
= 3.3E-3/8E-6 = 4 C.
Heterogeneous nucleation formulation: We now want to quantify our quali-
tative argument regarding the vapor temperature and the thermal sub-layer tem-
perature. For this purpose we find the equation for liquid temperature in the ther-
mal sub-layer and set it equal to the vapor temperature in the bubble as given by
Equation Vb.2.1. This method was originally suggested in 1962 by Hsu. Since
the thermal sub-layer is thin, we use a linear temperature profile in this region
which must satisfy the following boundary conditions:
At y = 0, T(y = 0) = T
s
and at y =
δ
, T(y =
δ
) = T
f
where T
s
and T
f
are the surface and the free stream temperatures of the bulk liquid,
respectively. The profile is obtained as:
δ
δ
y
TT
TT
fs
fl
−
=
−
−
Vb.2.2
We make a change of variable from y with r
c
(See Figure Vb.2.3) to obtain:
y = c
1
r
c
= (1 + cos
φ
)r
b
Vb.2.3
We now set Equation Vb.2.1 equal to Equation Vb.2.2, while substituting for y
from Equation Vb.2.3.
y
φ
T
f
r
b
r
c
Figure Vb.2.3. Depiction of bubble height, radius, and cavity radius
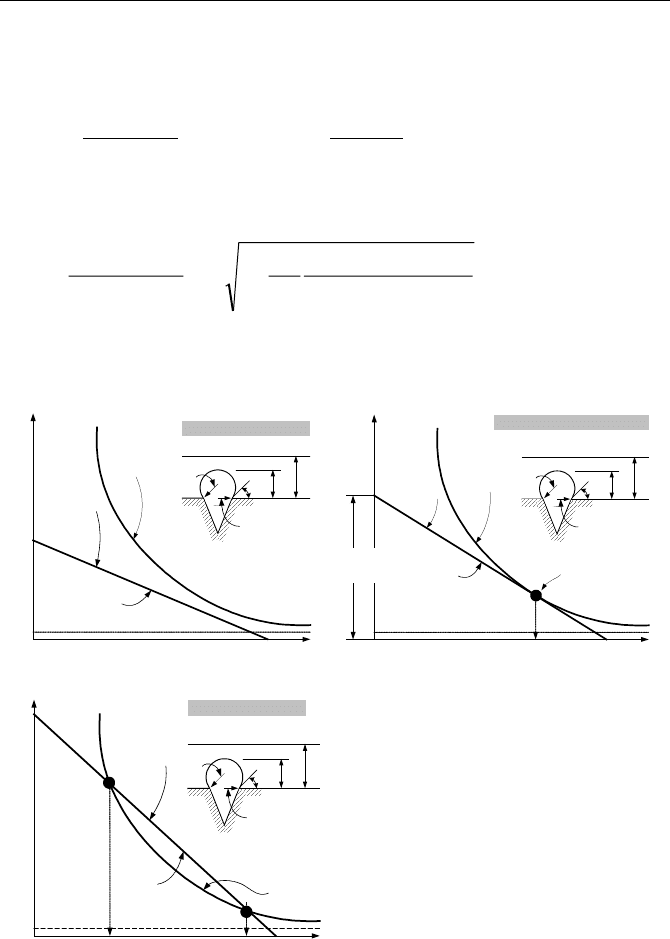
646 Vb. Two-Phase Flow and Heat Transfer: Boiling
Depending on the boiling condition, the curves representing the two temperature
profiles may a) not meet, b) be tangent to each other, or c) intersect at two loca-
tions. These conditions are obtained from the solution to the following equation:
δ
δ
ρ
σ
c
fsf
cgfg
sat
sat
rc
TTT
rch
T
T
1
2
)(
2 −
−+=+
This results in a second order algebraic equation for r
c
. The solution is found as:
»
»
¼
º
«
«
¬
ª
−
−
−±
−
−
=
fggsats
satfs
fs
sats
c
hTT
TTT
c
c
TTc
TT
r
δρ
σ
δ
2
2
1
1
)(
)(
8
11
)(2
)(
Vb.2.4
The results are plotted in Figure Vb.2.4.
T
sat
y
Liquid
temperature
profile
Bubble
temperature
profile
δ
r
c
r
b
δ
y
φ
r
c
T
f
1
q
′′
No Nucleate Boiling
T
sat
y
Liquid
temperature
profile
Bubble
temperature
profile
δ
r
c
r
b
δ
y
φ
r
c
T
f
2
q
′′
Boiling
Inception
∆T
ONB
Onset of Nucleate Boiling
T
sat
y
Liquid
temperature
profile
Bubble
temperature
profile
δ
r
c
r
b
δ
y
φ
r
c2
r
c1
T
f
3
q
′′
Nucleate Boiling
Figure Vb.2.4. Comparison of the vapor bubble and liquid temperature profiles
These concepts are further developed in Chapter VIe.
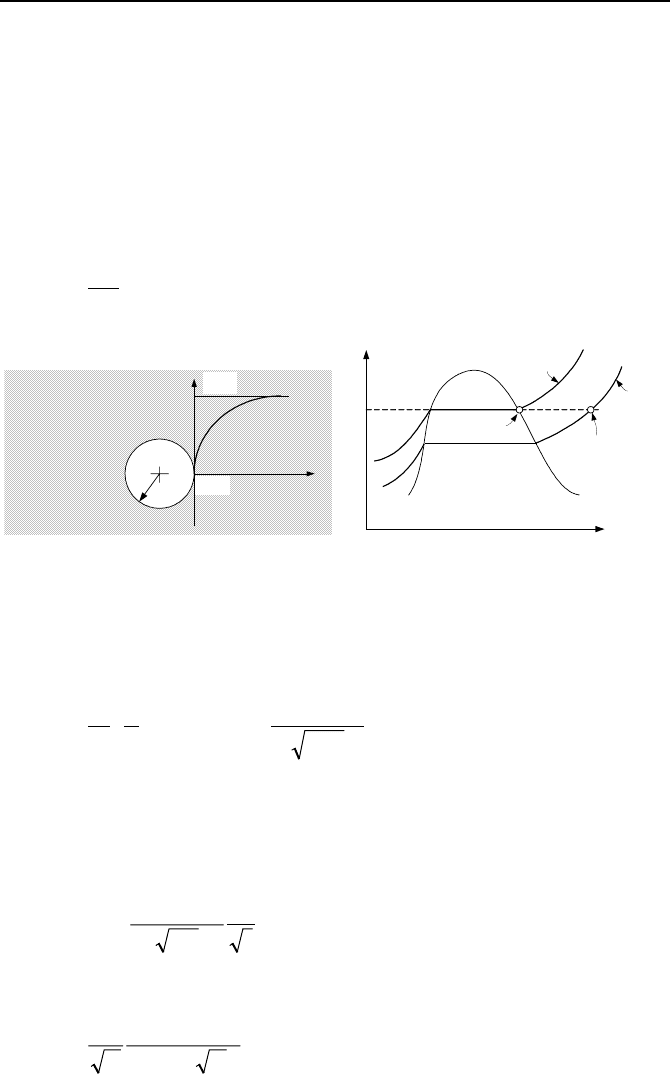
2. Convective Boiling, Analytical Solutions 647
2.3. Prediction of Bubble Growth
We can predict the growth rate of a vapor bubble rather accurately by treating the
surrounding superheated liquid as a semi-infinite body. In this case, the specified
boundary condition is heat flux at the interface between the liquid and the vapor
bubble, as shown in Figure Vb.2.5. Since liquid is being cooled at the interface
we can write the following energy balance:
Rate of increase in bubble internal energy = Rate of liquid cooldown at the inter-
face
()
qR
dt
d
h
fgg
′′
=
2
4
V
πρ
Liquid
T = T
sat
R
x
T
T
sup
T
sat
P
Bubble
P
Liquid
T
sat
T
sup
T
s
T
Bubble
T
Liquid
=
P
Bubble
>
P
Liquid
Figure Vb.2.5. Growth of a vapor bubble in the pool of superheated liquid
Substituting for heat flux at the interface from Equation IVa.9.10 and for volume
in terms of radius yields:
()
t
TTk
RR
dt
d
h
iS
fgg
πα
ππρ
)(
4
3
4
23
−
=
¸
¹
·
¨
©
§
Note in this case, the semi-infinite body is initially at T
i
= T
sup
when the interface
is suddenly cooled to T
s
= T
sat
. Subscripts sup and sat stand for superheated and
saturated, respectively. Carrying out the derivative, canceling similar terms
(4
π
R
2
) from both sides of the equation, and rearranging, we obtain:
t
dt
TTk
dRh
iS
fgg
πα
ρ
)( −
=
Using the initial condition of R = 0 at t = 0, we find:
(
)
2/1
sup
2
)( t
h
TTk
tR
fgg
sat
αρπ
−
=
Vb.2.5
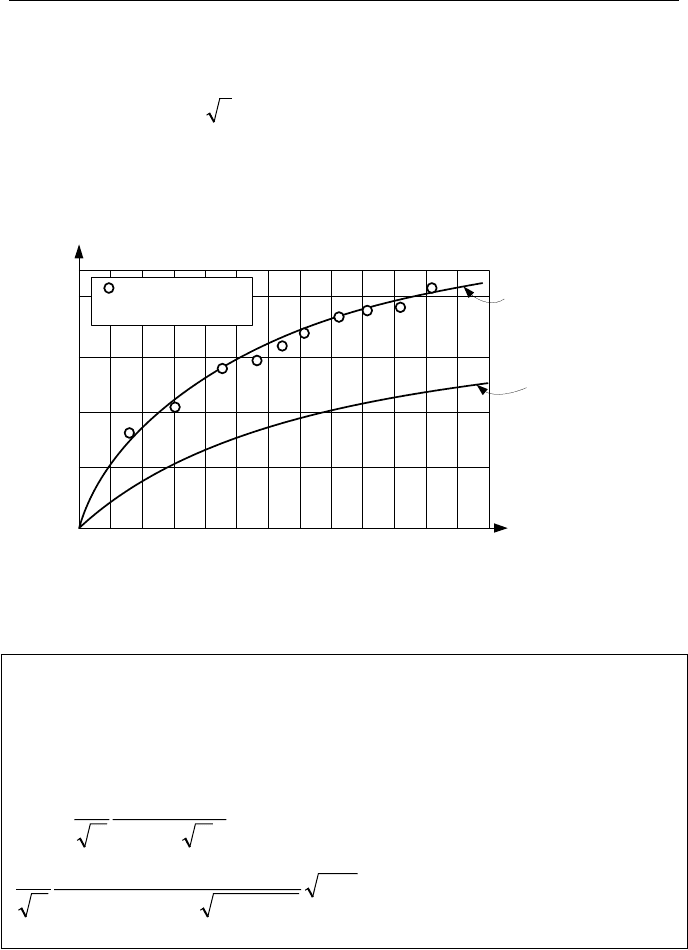
648 Vb. Two-Phase Flow and Heat Transfer: Boiling
As Lienhard describes, Jakob initially suggested the method that led to the deriva-
tion of Equation Vb.2.5. As shown in Figure Vb.2.4, this equation under-predicts
the data obtained by Dergarabedian. Hence, Scriven used a more rigorous method
and found that R
bubble
= 3 R
Jakob
, which closely matches the data.
Figure Vb.2.6 shows that the trend predicted by Jakob is as expected but the
absolute value under-predicts the data. The reason is that the bubble growth in-
creases the temperature gradient, which has been treated as constant in Jakob’s
model. Scriven accounts for this and practically matches the data.
0.005 0.01 0.013
0.8
0.4
0.0
0.0
Time (s)
Bubble Radius (mm)
Data
T
sup
- T
sat
= 3.1 C
R
Scriven
R
Jakob
Figure Vb.2.6. Comparison of R
Jakob
and R
Scriven
with data
Example Vb.2.2. Find the bubble diameter 0.01 s into the bubble growth for wa-
ter boiling at 1 atm and ∆T = 3 C. Data: k
f
= 0.68 W/m·K,
ρ
g
= 0.593 kg/m
3
,
α
f
=
1.68E-7 m
2
/s, and h
fg
= 2.257E6 J/kg.
Solution: From Equation Vb.2.5, we find R(0.01 s) as:
(
)
2/1
sup
2
)(
t
h
TTk
tR
fgg
sat
αρπ
−
=
=
4336.03E101.0
7E68.16E257.2593.0
1.368.02
=×
−×
×
π
mm
3. Convective, Boiling, Experimental Observation
Before discussing the two distinct modes of pool and flow boiling, we consider the
landmark experiment performed by Nukiyama in 1934, which led to the estab-
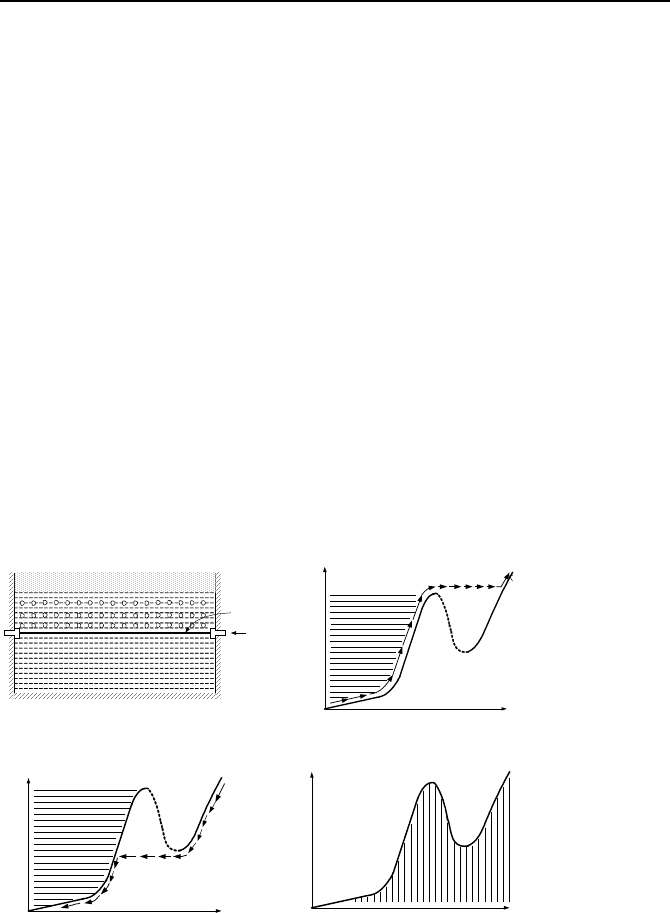
3. Convective, Boiling, Experimental Observation 649
lishment of the boiling curve. The importance of this curve is in its clear depiction
of various modes of heat transfer and demonstration of the effect of the method of
heat addition to the liquid. This was the first experiment for the measurement of
surface heat flux versus surface superheat (∆T
sl
= T
s
– T
sat
). As shown in Fig-
ure Vb.3.1(a) the experiment consists of an electrically heated wire in a water con-
tainer at atmospheric pressure. Nukiyama used a nichrome wire connected to an
electric voltage. Data were obtained by varying the electric power measuring wire
temperature after steady-state is achieved. This is referred to as power-controlled
or heat flux controlled heating where
s
q
′′
is the independent variable and surface
temperature (hence ∆T
sl
= T
s
– T
sat
) is the dependent variable. As power increased,
there was a sudden jump in the wire temperature and eventual burnout. The heat
up path is shown in Figure b with the arrows. The cool down path was obtained
by reducing electric power to the wire as shown in Figure c by the arrows. As
these figures indicate, on both heat up and cooldown paths, there is a jump from
one side of the curve to the other. This is typical of power-controlled heat up and
cooldown. Figure d shows how the entire boiling curve can be constructed if the
process is temperature-controlled. In this case, there is a specific heat flux for a
specific wall temperature. In practice, most processes such as production of heat
in the core of nuclear reactors are power-controlled. As a results, in such applica-
tions, care must be exercised no to exceed the maximum heat flux as damage to
the surface would follow.
I
Water
Vapor
Wire
∆T
sl
s
q
′′
(a) (b)
∆T
sl
s
q
′′
∆T
sl
s
q
′′
(b) (d)
Figure Vb.3.1. Nukiyama experiment for developing the boiling curve
650 Vb. Two-Phase Flow and Heat Transfer: Boiling
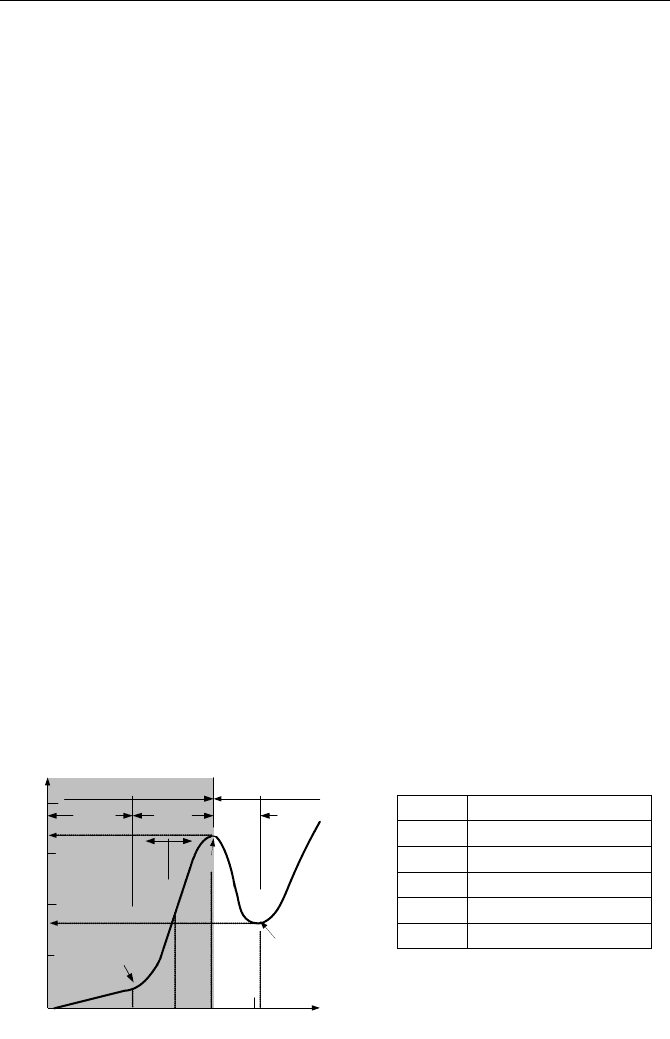
4. Pool Boiling Modes
The boiling curve for pool boiling heat transfer at atmospheric condition is shown
in Figure Vb.4.1. Temperature is in degrees centigrade. Since the bulk liquid is
quiescent, there is no heat flux when ∆T
sl
= 0. With an increasing degree of su-
perheat, the surface heat flux increases solely due to free convection. At about
∆T
sl
= 5 C, the bubbles begin to grow and some may depart the surface. The
buoyancy driven bubble causes agitation in the liquid. This mixing of liquid en-
hances heat flux. With increasing ∆T
sl
, more bubbles are formed and the rate of
carrying energy from the surface to the bulk liquid increases. Eventually, the rate
of bubble production becomes so great that at ∆T
sl
about 30 C, heat flux reaches
its peak value. Beyond this point, the bubble population is so dense that it pre-
vents liquid from reaching the surface. When this happens, heat transfer takes
place only by conduction through the layer of vapor, which has blanketed the sur-
face. With the surface being deprived of an efficient means of heat transfer by
boiling bubbles, surface temperature jumps to elevated values. With heat flux
maintained at its peak value, the jump in the surface temperature compensates for
the sudden drop in the heat transfer coefficient. The heat transfer regime with va-
por blanketing the surface is referred to as film boiling. The peak heat flux is re-
ferred to as the critical heat flux (CHF). A modest increase in heat flux beyond
the CHF is due to both conduction through the vapor film and radiation due to the
surface elevated temperature. On the cool down path, the reverse process occurs.
When ∆T
sl
reaches around 100 C, the vapor production is not vigorous enough to
keep liquid away from the surface. With liquid in contact with the surface, the ef-
ficient heat transfer resumes. The point at which liquid contacts the surface again
is known as the minimum stable film boiling (MSFB) or the Leidenfrost point. In
1756 Leidenfrost observed droplet boil off on hot surfaces. For surface tempera-
ture-controlled processes, the path between CHF and MSFB can be constructed.
In this path, liquid and surface contact intermittently. This mode is known as
transition boiling.
∆T
sl
(C)
MSFB
CHF
ONB
max
q
′′
min
q
′′
s
q
′′
Post-CHFPre-CHF
Forced
Convection
Nucleate
Boiling
TB
Film
Boiling
5
1
30
100
SB BB
1E3
10
1E4
1E5
1E6
MSFB Min. Stable Film Boiling
CHF
ONB
TB
Critical Heat Flux
Onset of Nucleat Boiling
Transition Boiling
BB Bulk Boiling
SB
Subcooled Boiling
Figure Vb.4.1. The boiling curve for water at 1 atm and various heat transfer regimes
4. Pool Boiling Modes 651
4.1. Nucleate Pool Boiling
Rohsenow, in 1952, obtained the nucleate pool boiling correlation in the form of
Ja = f(Re, Pr). The Reynolds number was defined for the bubbles as Re = G
b
D
b
/
µ
b
where G
b
is bubble mass flux, D
b
is bubble diameter, and
µ
b
is liquid viscosity.
Therefore, c
p,f
∆T/h
fg
= C
s,f
(G
b
D
b
/
µ
b
)
r
Pr
n
. Rohsenow introduced C
s-,f
, r, and n so
that nucleation on a variety of heated surfaces and liquids can be represented by
the same relation. Expressing the bubble diameter in terms of contact angle, sur-
face tension, and fluid density as D
b
= 1.48
φ
[2g
c
σ
/(g∆
ρ
)]
0.5
and the bubble mass
flux in terms of G
b
= q
′′
/h
fg
, we find:
0.33
,
()
/
()Pr
ssat
pf
c
sf
n
g
ffg ffg f
cTT
g
QA
C
hgh
σ
µρρ
−
ªº
«»
«»
¬¼
−
=
−
Vb.4.1a
Solving for heat flux:
3
,
5.0
Pr
)()(
¸
¸
¹
·
¨
¨
©
§
−
»
¼
º
«
¬
ª
−
=
′′
n
ffgsf
satsfpgf
fgf
hC
TTcg
hq
σ
ρρ
µ
Vb.4.1b
The values for coefficient C
sf
and n for various surfaces and liquids are given in
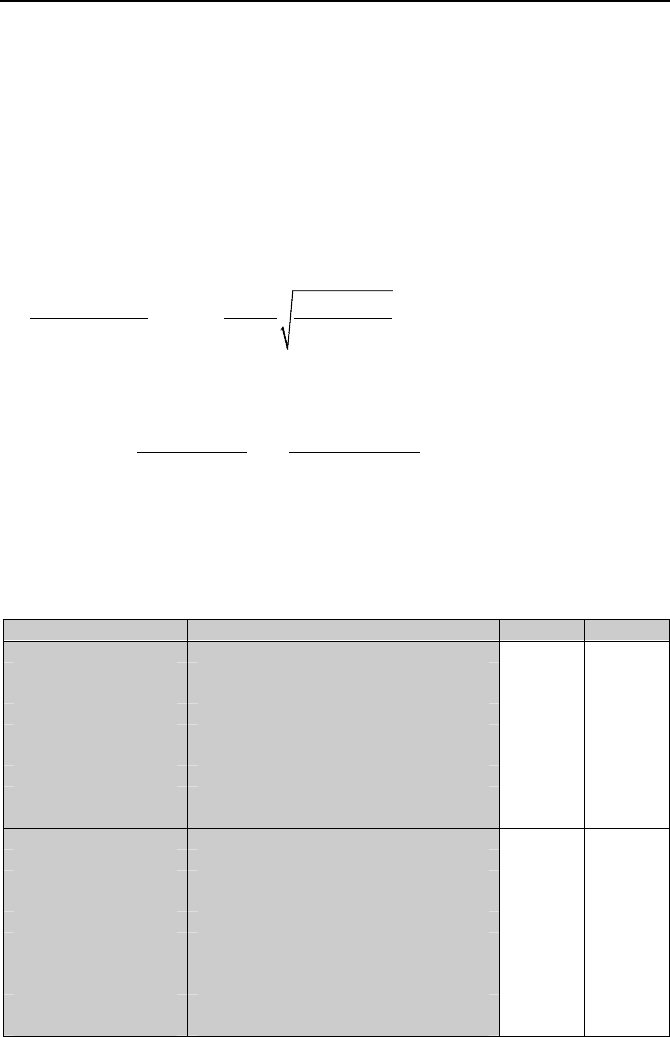
Table Vb.4.1.
Table Vb.4.1. Values for coefficients C
sf
and n for various liquid and surfaces
Fluid Surface C
sf
n
Benzene Chromium 0.1010 1.7
Carbon tetrachloride Copper, polished 0.0070 1.7
Ethyl alcohol Chromium 0.0027 1.7
Isopropyl alcohol Copper 0.0025 1.7
n-Butyl alcohol Copper 0.0030 1.7
n-Pentane Copper, polished 0.0154 1.7
Nickel, polished 0.0127 1.7
Copper, emery-robbed 0.0074 1.7
Chromium 0.0150 1.7
Water Brass 0.0060 1.0
Copper, polished 0.0128 1.0
lapped 0.0147 1.0
scored 0.0068 1.0
Nickel 0.0060 1.0
Stainless steel, ground & polished 0.0080 1.0
, Teflon pitted 0.0058 1.0
, chemically etched 0.0133 1.0
, mechanically polished 0.0132 1.0
Platinum 0.0130 1.0
