Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.


Evaporation 151
4.4 Theory and Mechanisms
4.4.1 Vacuum Evaporation
Reference to the various steps in the formation of a deposit enumerated in the previous section
shows that the theory of vacuum evaporation involves thermodynamic considerations, i.e.
phase transitions from which the equilibrium vapor phase pressure of materials can be derived,
as well as the kinetic aspects of nucleation and growth. Both of these are of obvious
importance in the evolution of the microstructure of the deposit.
The transition of solids or liquids into the gaseous state can be considered to be a macroscopic
or an atomistic phenomenon. The former is based on thermodynamics and results in an
understanding of evaporation rates, source-container reactions and the accompanying effect of
impurity introduction into the vapor state, changes in composition during alloy evaporation,
and stability of compounds. An excellent detailed treatment of the thermodynamic and kinetic
bases of evaporation processes is given by Glang [6]. He points out that the application of
kinetic gas theory to interpret evaporation phenomena resulted in a specialized evaporation
theory. Such well-known scientists as Hertz, Knudsen, and Langnuir were the early workers in
evaporation theory. They observed deviations from ideal behavior which led to refinements in
the theory to include concepts of reaction kinetics, thermodynamics, and solid state theory.
From the kinetic theory of gases, the relationship between the impingement rate of gas
molecules and their partial pressure, p,isgivenby
dN
i
A
e
dt
= (2 πmkT )
−
1
2
p (4.2)
where N
i
is the number of molecules striking a unit area of surface, and A
e
is the area of the
surface.
Hertz [77], in 1882, first measured the evaporation rate of mercury in high vacuum and found
that the evaporation rate was proportional to the difference between the equilibrium vapor
pressure of mercury, p*, at the evaporant surface and the hydrostatic pressure, p, acting on the
surface, resulting from the evaporant atoms or molecules in the gas phase. Thus, the
evaporation rate based on the concept of the equilibrium vapor pressure (i.e. the number of
atoms leaving the evaporant surface is equal to the number returning to the surface) is given by:
dN
i
A
e
dt
(2πmkT )
−
1
2
(p* − p)cm
2
s
−1
(4.3)
such that dN
e
, the number of molecules evaporating from a surface area A
e
in time dt, is equal
to the impingement rate of gas molecules based on the kinetic theory of gases with the value of
p* inserted therein, minus the return flux corresponding to the hydrostatic pressure p of the
evaporant in the gas phase. In the above equations, m is the molecular weight, k is
152 Chapter 4
Boltzmann’s constant, and T is the temperature in K. The maximum possible evaporation rate
corresponds to the condition p = 0. Hertz measured evaporation rates only about one-tenth as
high as the theoretical maximum rates. The latter were subsequently measured by Knudsen
[78] in 1915. Knudsen postulated that some of the molecules impinging on the surface were
reflecting back into the gas phase rather than becoming incorporated into the liquid. As a
result, there is a certain fraction (1 − α
v
) of vapor molecules which contribute to the evaporant
pressure but not to the net molecular flux from the condensed phase into the vapor phase. To
this end, he postulated the evaporation coefficient, α
v
, which is defined as the ratio of the real
evaporation rate in vacuum to the theoretically possible value defined by Eq. (4.3). This then
results in the well-known Hertz–Knudsen equation:
dN
e
A
e
dt
= α
v
(2πmkT )
−
1
2
(p* − p) (4.4)
The value of α
v
is very dependent on the cleanliness of the evaporant surface and can range
from very low values for dirty surfaces to unity for clean surfaces. In very high-rate evaporation
with a clean evaporant surface, it has been found that the maximum evaporation given by Eq.
(4.4) has been exceeded by a factor of 2–3 for the evaporation of a light metal such as
beryllium [21] using e-beam heating. The reason for this is that the high power input results in
considerable agitation of the liquid evaporant pool resulting in a real surface area much larger
than the apparent surface area. The directionality of evaporating molecules from an
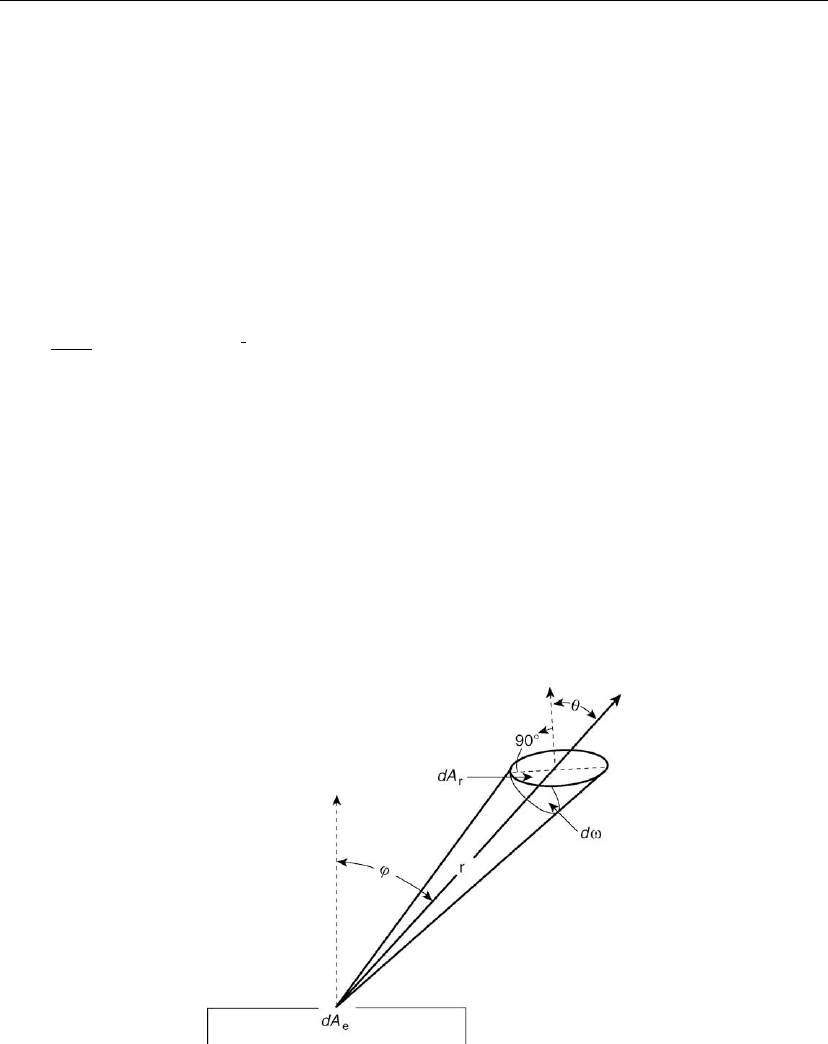
evaporation source is given by the well-known cosine law. Figure 4.11 shows a small surface
Figure 4.11: Surface element dA
r
receiving deposit from a small area source dA
e
. (From
Handbook of Thin Film Technology [7]. © 1970, McGraw-Hill. Used with permission of
McGraw-Hill Book Company.)

Evaporation 153
element dA
r
receiving deposit from a small area source dA
e
. The mass deposited per unit area
dM
r
(σ, θ )
dA
r
=
M
e
πr
2
cosϕ cosθ (4.5)
where M
e
is total mass evaporated.
For a point source, Eq. (4.5) reduces to:
dM
r
dA
r
=
M
e
πr
2
cosϕ (4.6)
For a uniform deposit thickness, the point source must be located at the center of the spherical
receiving surface such that r is a constant and cosθ =1.
In high-rate evaporation conditions, e.g. using a high-power e-beam-heated source, the
thickness distribution is steeper than with a point or small area source discussed above. This
has been attributed by some authors [33, 34, 79] to the existence of a virtual source of vapor
located above the molten pool. On the other hand, at high power, the e-beam impact area on
the surface of the molten pool is not flat but pushed down into an approximate concave
spherical segment which, as Riley shows [80], can equally well account for the steeper
thickness distribution.
The above discussion points out one of the problems with evaporation technology, i.e. the
variation in thickness of the deposit on a flat substrate. Numerous solutions are possible which
involve either moving the substrate in a manner so as to randomly sample the vapor flux, the
use of multiple sources, or sources of special shapes. These have been discussed in some detail
by Holland [8] as well as by Bunshah and Juntz [81].
Models have also been presented for calculating the deposit temperature [82] and thickness
distribution [83] during high-rate evaporation and verified against experimental data. In a more
recent paper, Szekely and Poveromo [84] have given a more general formulation describing
the net rate of vapor deposition from a molten source onto an initial cold surface, making
allowance for both molecular transport and diffusion effects.
With PVD metal can be also be evaporated, vaporized, and then condensed in the form of
nanoparticles. Okuyama et al. reported production of ultrafine metal oxide aerosol particles by
thermal decomposition of metal alkoxide vapors [85]. A comprehensive study on the growth
dynamics of the particles is presented by Flagan et al. [86]. The temperature required for
vaporization can be achieved by Joule heating, although thermal plasmas [87], laser ablation
[88], and spark ablation sources [89] operate in the same manner. In these processes vapors
migrate from the hot source into a cooler gas by a combination of convective flows and
diffusion. Although dilution resulting from the latter transport mechanism decreases the partial

154 Chapter 4
pressure of the vapor from the saturation condition at the source, the decreasing temperature
leads to a far more rapid decrease in the equilibrium vapor pressure and correspondingly high
supersaturation. Nanostructured materials can be synthesized by condensation from the vapor
phase in the presence of inert carrier gas, most often in a buoyant plume above a hot vapor
source. Particle growth in these condensation processes can be classified into three stages as
nucleation, coalescent coagulation, and agglomerate growth. The growth characteristics are
determined by both the probability for interparticle collisions due to Brownian motion and the
velocity of interparticle coalescence. Furthermore, agglomeration is characterized by the
equality of the characteristic collision and coalescence times, denoted by τ
col
and τ
coa
,
respectively. The coalescence time is mainly determined by the diffusivity and, thus, depends
strongly not only on the temperature, but also on the activation energy and the diffusion
constant. If we consider surface diffusion the predominant sintering mechanism, the following
equations for the coalescence and collision times can be obtained [86, 90, 92]:
τ
coa
∝ d · T exp
E
a
k
B
T
τ
col
∝ d
5
2
T
−
1
2
(4.7)
where d, T, E
a
, and k
B
denote the particle diameter, the temperature, the activation energy for
diffusion, and the Boltzman constant, respectively.
The metal atoms effused from the source will rapidly lose their energy by collision with carrier
gas atoms. The nucleation process is performed homogeneously in the vapor phase and the
collision mean free path is very short, i.e. in the order of 100 nm. If one further assumes a
steady-state cluster formation and distribution is considered, the nucleation rate depends on
the supersaturation of metal vapor in the gas phase, S [86]:
J ∝ exp
−σ
3
ln
2
S
(4.8)
where σ is the surface tension. The free energy barrier against nucleation is also a function of
σ and S as follows [92, 93]:
G =
16
3
π
σ
2
(ρRT lnS)
2
(4.9)
where ρ is the particle density, R is the gas constant, and T is temperature. Since the
supersaturation of metal vapor is caused by the collision of the metal atoms with the gas
atoms, the amount of undercooling determines the supersaturation and thus the nucleation
process. It is known that the value of vapor phase undercooling depends on the evaporation
temperature. This model has been applied on Ag nanoparticles prepared by IGC [92, 93].
Evaporation 155
4.5 Evaporation Process and Apparatus
4.5.1 The System
A schematic of the evaporation apparatus has been illustrated in Figure 4.1. It consists of the
following: chamber, vacuum pumps, vacuum gauges, including total and partial pressure
gauges on sophisticated systems, evaporation sources, substrate holders, rate monitors, process
controller, etc.
4.5.1.1 Vacuum Chamber
This ranges from a simple bell jar or rectangular box for experimental or batch type production
to more complex gear for production applications. The latter may consist of a deposition
chamber with loading and unloading chambers attached to the deposition chambers by
manifolds with isolation high-vacuum valves. These are called fast cycle coaters. Alternate
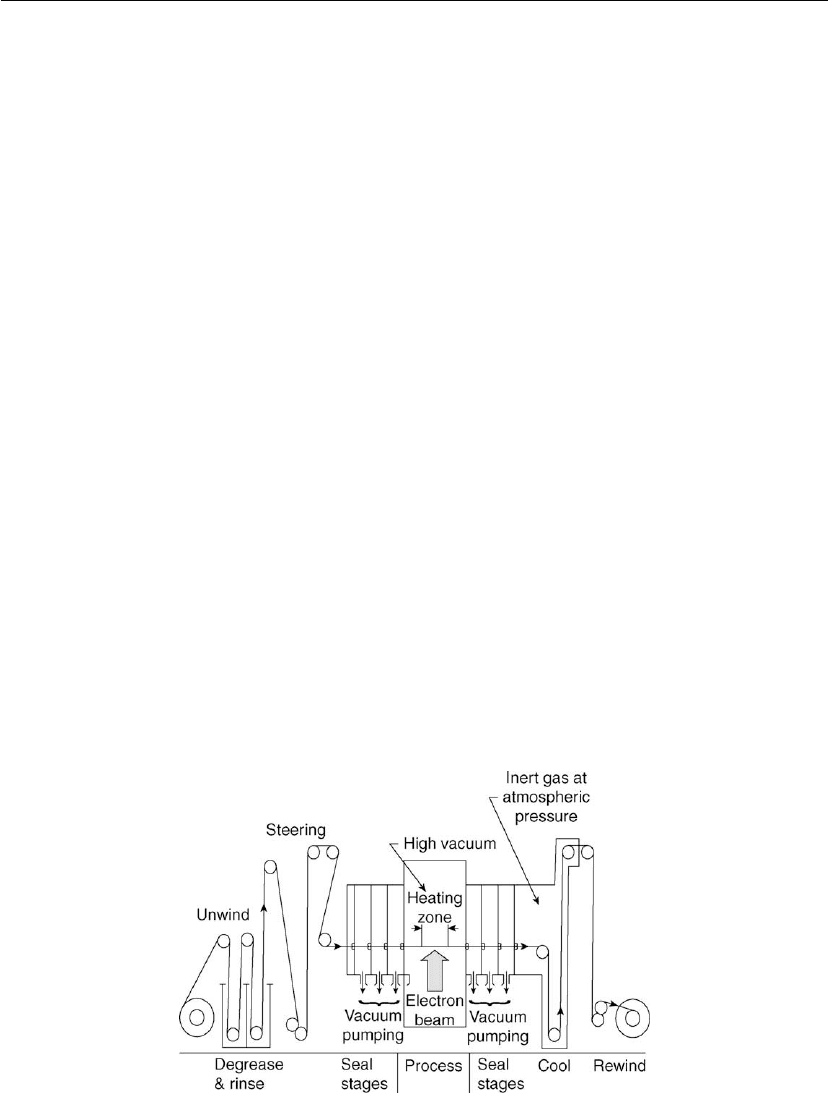
approaches are semi-continuous in-line systems where a strip substrate stored in the vacuum
chamber can be fed continuously over the source (Figure 4.12) or a continuous system where
the strip or sheet substrate is inserted and removed from the deposition chamber through
air-to-air seals [4, 94] (Figures 4.13 and 4.14).
4.5.1.2 Vacuum Pumping System
The gas loads in evaporation processes are fairly high owing to outgassing from chamber walls
promoted by the heat load from the evaporation source and substrate heaters, particularly for
high deposition rate conditions. Therefore the pumping system is usually based on a diffusion
pump with a liquid nitrogen cooled anticreep type baffle backed with a mechanical pump or a
Roots blower/mechanical pump combination for large systems. For very high-purity,
Figure 4.12: Schematic representation of a 24 inch continuous high vacuum strip processing line.
156 Chapter 4
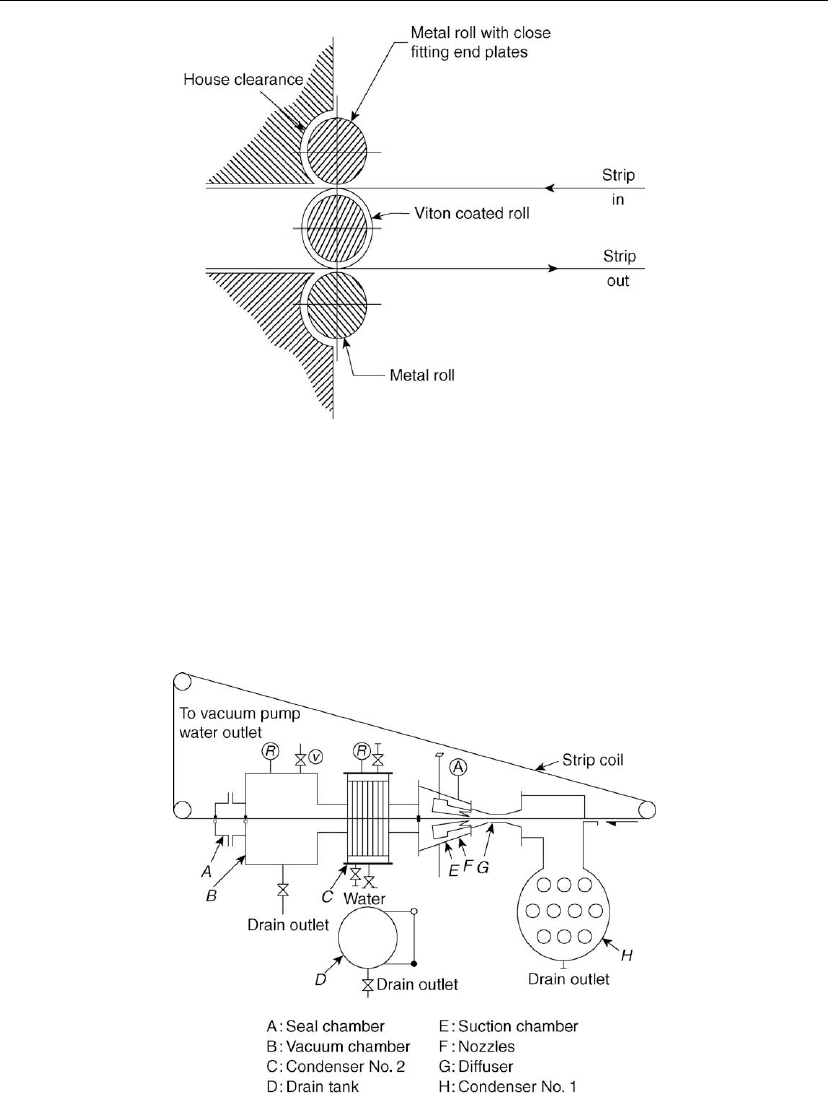
Figure 4.13: Three-high roll seal arrangement for stripline.
low-deposition rate, low-heat flux conditions, ion pumped systems backed with cryosorption
rough pumping are used, since a base pressure of 10
−9
–10
−10
torr is needed. More recently,
turbomolecular and cryogenic pumps have been used instead of diffusion pumps where desired
(e.g. oil-free systems). This is particularly true for molecular beam epitaxy where extreme
Figure 4.14: Vacuum seal using steam jet or curtain.

Evaporation 157
control over composition and layer thickness is essential and deposition rates can be quite low.
In such cases, the chamber and pumps are to be baked as with any other ultrahigh vacuum
operation.
4.5.1.3 Pressure Measurement
The vacuum gauges used depend again on specific applications, such as a combination of
high-pressure gauges (such as the Pirani or Thermocouple Gauge) for monitoring the roughing
of the system and high-vacuum gauges (such as the hot cathode ionization gauge and/or
capacitance manometer). A partial pressure gauge is highly desirable, particularly for
ultraclean applications, as well as for leak hunting.
4.5.1.4 Evaporation Sources
These are discussed separately in Section 4.6.
4.5.1.5 Substrate Holders and Heaters
Substrate holders may be very simple for stationary flat substrates or can incorporate quite
complex motions as illustrated by planetary or rotating devices. The reason for this is to ensure
deposition thickness uniformity and control over a large number of small parts such as lenses
or silicon wafers. Substrate heating can be accomplished by radiant heaters with refractory
wires or quartz lamps acting as the heat source. Occasionally, substrates are directly heated by
a scanning or diffuse e-beam.
4.5.1.6 Deposition Rate Monitors
These are discussed in Section 4.7.
4.6 Evaporation Sources
4.6.1 General Considerations
Evaporation sources are classified by the mode of heating used to convert the solid or liquid
evaporant to the vapor phase. Thus one talks of resistance, arc, induction, electron beam, arc
imaging, lasers, and exploding wire types of sources. It is very important to note that we
cannot evaporate every material from any of the types of sources listed above, for the
following reasons:
chemical interaction between the source material and the evaporant would lead to
impurities in the deposit. For example, evaporation of titanium from a MgO source
would cause oxygen and magnesium contamination of the deposit; the titanium would
reduce the MgO. Therefore, for the evaporation of reactive metals such as titanium or
zirconium, water-cooled copper crucibles are used
158 Chapter 4
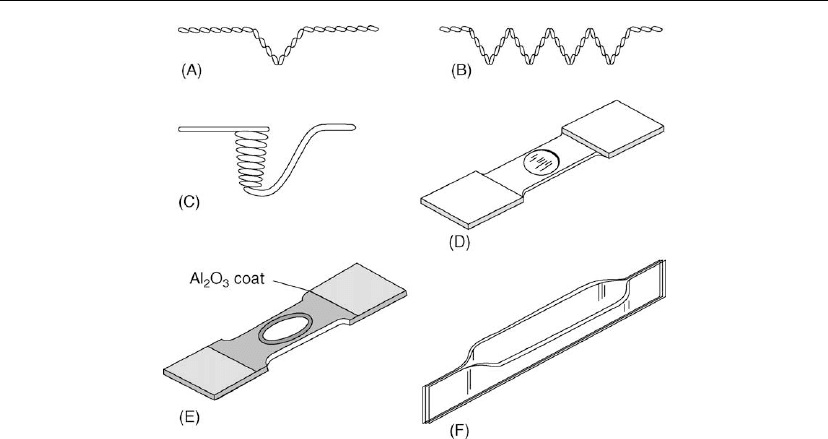
Figure 4.15: Wire and metal-foil sources: (A) hairpin source; (B) wire helix; (C) wire basket; (D)
dimpled foil; (E) dimpled f oil with alumina coating; (F) canoe type. (From Handbook of Thin Film
Technology [7]. © 1970, McGraw-Hill. Used with permission of McGraw-Hill Book Co.)
reaction between metallic source (such asaWorTaboat) and evaporant (Ti) could
occur. In many cases at high temperatures two metals can mutually dissolve in each
other, leading to destruction of the source
the power density (i.e. W/cm
2
) varies greatly between the various heat sources.
Table 4.2, from the article by Glang [6], lists the temperature and support materials to be used
in the evaporation of elements. Similar tables are found in the literature of many of the
manufacturers.
Evaporation of alloys and compounds poses additional problems, and they are considered in
Section 4.9.
4.6.2 Resistance-Heated Sources
The simplest vapor sources are resistance-heated wires and metal oils of various types shown
in Figure 4.15.
They are available in a variety of sizes and shapes and at sufficiently low prices so that they
can be discarded after one experiment if necessary. They are usually made from the refractory
metals, tungsten, molybdenum, and tantalum, which have high melting points and low vapor
pressure so as not to contaminate the deposit. Their properties are given in Table 4.3.
Evaporation 159
Table 4.2: Temperatures and support materials used in the evaporation of the elements
Element and
predominant
vapor species
Temp. (
◦
C) Suppor t materials Remarks
mp p*=10
−1
torr Wire, foil Crucible
Aluminum (Al) 650 1220 W C, BN, TiB
2
-BN Wets all materials readily and tends to
creep out of containers. Alloys with W
and reacts with carbon. Nitride crucibles
preferred
Antimony (Sb
4
,
Sb
2
)
630 530 Mo, Ta, Ni Oxides, BN,
metals, C
Polyatomic vapor, ˛
2
= 0.2. Requires
temperatures above mp. Toxic
Arcenic (As
4
,
As
2
)
820 ∼ 300 – Oxides, C Polyatomic vapor, ˛
2
=5.10
−6
–5.10
−2
.
Sublimates but requires temperatures
above 300
◦
C. Toxic
Barium (Ba) 710 610 W, Mo, Ta, Ni,
Fe
Metals Wets refractory metals without alloying.
Reacts with most oxides at elevated
temperatures
Beryllium (Be) 1283 1230 W, Mo, Ta C, refractory
oxides
Wets refractory metals. Toxic,
particularly BeO dust
Bismuth (Bi, Bis) 271 870 W, Mo, Ta, Ni Oxides, C,
metals
Vapors are toxic
Boron (B) 2100 ± 100 2000 – C Deposits from carbon supports are
probably not pure boron
Cadmium (Cd) 321 265 W, Mo, Ta, Fe,
Ni
Oxides metals Film condensation requires high
supersaturation. Sublimates, Wall
deposits of Cd spoil vacuum system
Calcium (Ca) 850 800 W Al
2
O
3
Carbon (C
2
,C,
C
2
)
∼ 3700 ∼ 2600 – – Carbon-arc or electron-bombardment
evaporation. ˛
2
<1
(Continued)

160 Chapter 4
Table 4.2: (Continued)
Element and
predominant
vapor species
Temp. (
◦
C) Suppor t materials Remarks
mp p*=10
−1
Torr Wire, foil Crucible
Chromium (Cr) ∼ 1900 1400 W, Ta – High evaporation rates without melting.
Sublimation from radiation-heated Cr
rods preferred. Cr electrodeposits are
likely to release hydrogen
Cobalt (Co) 1495 1520 W Al
2
O
3
, BeO Alloys with W, charge should not weigh
more than 30% of filament to limit
destruction. Small sublimation rates
possible
Copper (Cu) 1084 1260 W, Mo, Ta Mo, C, Al
2
O
3
Practically no interaction with refractory
materials. Mo preferred for crucibles
because it can be machined and
conducts heat well
Gallium (Ga) 30 1130 – Beo, Al
2
O
3
Alloys with refractory metals. The oxides
are attacked above 1000
◦
C
Germanium
(Ge)
940 1400 W, Mo, Ta W, C, Al
2
O
3
Wets refractory metals but low solubility
in W. Purest films by electron-gun
evaporation
Gold (Au) 1063 1400 W, Mo Mo, C Reacts with Ta, wets W and Mo. Mo
crucibles last for several evaporations
Indium (In) 156 950 W, Mo Mo, C Mo boats preferred
Iron (Fe) 1538 1480 W Beo, Al
2
O
3
,
ZrO
2
Alloys with all refractory metals. Charge
should not weigh more than 30% of W
filament to limit destruction. Small
sublimation rates possible
Lead (Pb) 328 715 W, Mo, Ni, Fe Metals Does not wet refractory metals. Toxic
Magnesium
(Mg)
650 440 W, Mo, Ta, Ni Fe, C Sublimates
