Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.

Chapter 7 / Management of Oxaluria 103
103
From: Current Clinical Urology, Urinary Stone Disease:
A Practical Guide to Medical and Surgical Management
Edited by: M. L. Stoller and M. V. Meng © Humana Press Inc., Totowa, NJ
7
Management of Patients
With Hyperoxaluria
Ojas Shah, MD, Ross P. Holmes, PhD,
and Dean G. Assimos,
MD
CONTENTS
INTRODUCTION
OXALATE PHYSIOLOGY
SOURCES OF URINARY OXALATE
HYPEROXALURIA: DEFINITION
ENTERIC HYPEROXALURIA
PRIMARY HYPEROXALURIA
POTENTIAL FUTURE THERAPY
CONCLUSIONS
REFERENCES
Key Words: Nephrolithiasis; oxalate; absorption.
INTRODUCTION
Oxalate excretion is a prerequisite for the formation of calcium oxalate stones and the
development of hyperoxaluric states. In this chapter, we review the aspects of oxalate
physiology germane to these conditions, the clinical manifestations of these entities, as
well as therapy.
OXALATE PHYSIOLOGY
Oxalate is a simple dicarboxylic acid and is a major component of the most common
type of renal calculus. The oxalate concentration in urine affects its supersaturation with
calcium oxalate and an increase will promote crystal formation, a key event in cal-
culogenesis. The oxalate in urine is derived from two sources: the diet and endogenous
synthesis. Oxalate is a common component of plants and an unavoidable constituent of

104 Shah, Holmes, and Assimos
human diets. It is a metabolic end product in humans that is linked to a variety of vital
pathways, including gluconeogenesis, glycolysis, ureagenesis, pentose–phosphate path-
way, glyoxylate pathway, serine pathway, and xylulose pathway (1).
Oxalate is freely filtered through the glomerulus and secretory fluxes may also occur
(2–5). Oxalate transport in proximal tubular cells may be complex because it seem to
plays a role as a recycling substrate that functionally links the transcellular absorption
of chloride to that of other anions such as bicarbonate and sulfate (6,7). At the basolateral
membrane, oxalate enters the cell in exchange for sulfate or bicarbonate (6–8). At the
luminal brush border membrane, oxalate can be transported out of the cell in exchange
for chloride and may be transported back into the cell in exchange for sulfate (6, 7).
There is also evidence that oxalate exchange may occur in the distal tubule (9). Various
anion exchange proteins mediate these processes (10,11).
Oxalate is absorbed all along the gastrointestinal tract, including the stomach (12).
Absorptive and secretory pathways for oxalic acid regulated by substances that direct the
net oxalate ion flux have been identified in the proximal and distal segments of rat colon
(13–16). Cations such as calcium and magnesium complex with oxalate in the alimen-
tary tract and limit its absorption. The free anionic form of oxalate is thought to be the
one that is absorbed. Recent oxalate loading studies in healthy volunteers have demon-
strated that oxalate absorption varies tremendously, with 2–18% of a dietary oxalate load
in normal individuals being absorbed (17). In addition, the time sequences of absorption
studies suggest that a significant amount of oxalate is absorbed in the small intestine in
humans. Oxalate is also degraded in the colon by oxalate-degrading bacteria such as
Oxalobacter formigenes and other organisms (18). The actual role that this bacterium
plays in altering intestinal oxalate content and oxalate absorption has not been well
characterized.
A role for the intestinal absorption of dietary oxalate in stone formation was suggested
by the studies of Curhan and associates (19,20). A low-calcium intake was shown to be
a significant risk factor for stone development. The explanation for this finding is that
calcium complexes with oxalate in the alimentary tract thus limiting oxalate absorption.
Metabolic studies in humans support this theory. We demonstrated a 34% increase in
urinary oxalate excretion in normal adult subjects when dietary calcium is reduced from
1002 mg to 391 mg/d while other dietary constituents are unchanged, which is consistent
with this theory (21). In addition, Liebman and Chai found that supplemental calcium
decreased the absorption of an oxalate load in humans by more than 50% (22).
Studies have shown that supraphysiologic concentrations of oxalate can damage
cultured renal tubular cells through free-radical-induced oxidative stress (23–27). Expo-
sure of a line of human renal epithelial cells, HK-2, to oxalate resulted in a programmed
sequence of events that led to an increase in membrane permeability, alterations in cell
morphology and viability, and the re-initiation of DNA synthesis (23). It has been
hypothesized that this concentration-dependent damage promotes a release of lipid-
rich cellular membranes that act as a nidus for crystal nucleation and retention, which
likely leads to lithogenesis (28–31). Although there is currently no evidence that such
events occur in human stone formers, stones do contain lipids in their matrix (32).
The roles of oxalate and calcium in the lithogenic process have nearly always been
attributed to their impact on the supersaturation of urine with calcium oxalate. Some
studies have demonstrated that supersaturation of calcium oxalate in stone formers does
not differ significantly from that in nonstone formers (33,34). However, Borghi et al.
have shown that oxalate-loading in calcium oxalate stone formers was associated with

Chapter 7 / Management of Oxaluria 105
an increased potential for crystal nucleation as compared to controls (35). Supersatura-
tion and nucleation of calcium oxalate are insufficient to explain the formation of stones
alone, but an increased tendency to nucleation may precipitate subsequent events that are
responsible for the formation of stones (36). Additionally, the upper limit of metastabil-
ity in urine samples and the ability of urine components to inhibit crystal growth may be
more sensitive indices in providing discrimination between the urine of stone formers
and normal individuals (34).
SOURCES OF URINARY OXALATE
It was previously thought that the contributions of urinary oxalate were 40–50%
hepatic synthesis, 40–50% breakdown of ascorbic acid (vitamin C), and the remaining
10–20% dietary (37). However, we and others have shown that dietary oxalate is a major
contributor to urinary oxalate excretion in most humans and may account for 50% or
more of the daily urinary oxalate excreted. We have demonstrated that when normal
adults are placed on an oxalate-free formula diet, oxalate excretion decreases about 50%
from the levels on self-selected diets, indicating that dietary oxalate is a major contribu-
tor of urinary oxalate in most individuals (21,22). Dietary oxalate originates almost
exclusively from plant-derived foods (Table 1).
Table 1
Oxalate Content of Various Foods
Oxalate, Oxalate per serving,
mg/100 g mg (serving size, g)
Spinach 645.0 645.0 (100)
Fibre One cereal 142.0 43.0 (30)
Bran flakes 141.0 42.0 (30)
Green beans (steamed) 33.0 33.0 (100)
Potato (raw) 27.1 27.1 (100)
Snack bar (Butterfinger) 53.5 24.0 (45)
Peanut butter 95.8 19.2 (20)
Tea (brewed) 7.5 18.8 (250)
Celery 61.2 18.4 (30)
Chocolate (American) 42.5 13.0 (30)
Ravioli 6.5 13.0 (200)
White bread 14.3 8.0 (56)
Carrots (raw) 5.7 5.7 (100)
Potato chips 9.4 3.0 (30)
White rice (steamed) 2.1 2.1 (100)
Broccoli (steamed) 1.8 1.8 (100)
Strawberry jelly 5.3 1.1 (20)
Corn flakes 1.9 0.6 (30)
Mustard 12.1 0.6 (5)
Apple (raw) 0.5 0.5 (100)
Peaches (canned) 0.3 0.3 (100)
Grape jelly 1.5 0.3 (20)
106 Shah, Holmes, and Assimos
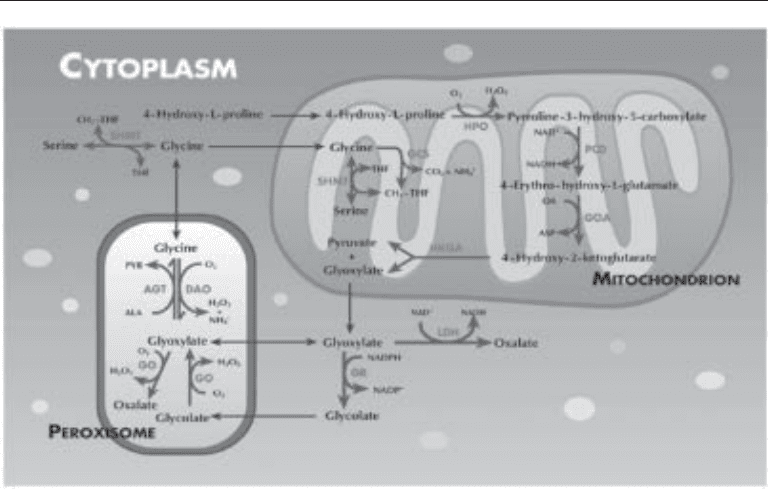
Endogenous oxalate synthesis occurs in the liver. A variety of precursor molecules,
including serine, glycine, hydroxyproline, ethylene glycol, and several carbohydrates,
can lead to production of oxalate via pathways that converge on glycolate and glyoxylate
(38). The key steps are depicted in Fig. 1. This includes the oxidation of glycolate to
glyoxylate catalyzed by glycolate oxidase and oxidation of glyoxylate to oxalate pri-
marily by lactate dehydrogenase. Alanine glyoxylate aminotransferase (AGT), a very
important enzyme located in the hepatic peroxisomal compartment in normal humans,
diverts glyoxylate from oxalate synthesis by a transamination process, with alanine
converted to pyruvate as glyoxylate is converted to glycine (1).
There is still an ongoing debate on whether ascorbic acid metabolism contributes
significantly to the urinary oxalate pool. Ascorbic acid is converted into oxalate by a
process in the liver involving diketogluconic acid as an intermediate, with two carbons
of ascorbic acid being converted into oxalate (39). This conversion of diketogluconate
to oxalate is nonenzymatic, although the extent to which it occurs is controversial. Little
is known about the factors controlling this metabolism. The intake of high doses of
ascorbic acid has been demonstrated to increase urinary oxalate excretion in normal
adult individuals and stone-formers (41).
Fig. 1. Pathways associated with oxalate synthesis in hepatocytes. Transport processes are
shown as the thin, dark arrows and enzymatic reactions as the thicker, lighter arrows. AGT,
alanine:glyoxylate aminotransferase; DAO,
D
-amino acid oxidase; GCS, glycine cleavage sys-
tem; GO, glycolate oxidase; GOA, glutamate oxaloacetate aminotransferase; GR, glycolate
reductase; HKGA, 4-hydroxy-2-ketoglutarate lyase; HPO, hydroxyproline oxidase; LDH, lac-
tate dehydrogenase; PCD, pyrroline-3-hydroxy-5-carboxylate dehydrogenase; SHMT, serine
hydroxymethyltransferase.
Chapter 7 / Management of Oxaluria 107
HYPEROXALURIA: DEFINITION
The definition of hyperoxaluria has varied in reports in the literature. We analyzed
urinary oxalate excretion in 100 normal adults and 100 adult stone-formers on self-
selected diets. We found a fairly distinct difference at 30 mg of oxalate/g of excreted
creatinine (42). We therefore define hyperoxaluria as anything greater than this level.
The use of creatinine in this ratio limits inaccuracy caused by collection error and
corrects for differences in body size. Other investigators have used different criteria for
defining hyperoxaluria; e.g., greater than 43 mg/d in men and 32 mg/d in women (43).
There are also varied reports of the prevalence of this metabolic disorder in stone form-
ers, with estimates ranging from approx 8–30% (42,44).
There are three types of hyperoxaluria: enteric, primary, and idiopathic. We will
discuss the etiology, manifestation, and treatment options for each.
ENTERIC HYPEROXALURIA
Enteric hyperoxaluria is caused by a variety of functional and anatomic small bowel
problems (Table 2) (37,45–50). It is encountered in approx 5% of patients evaluated in
specialized metabolic stone clinics and should be suspected in any patient with
hyperoxaluria and small bowel abnormality (1). These patients typically also have low
urine volume and diminished calcium, magnesium, and citrate excretion.
Various theories about the mechanism of enteric hyperoxaluria have been postulated.
Oxalate normally complexes with calcium to form an insoluble salt or crystal that is
excreted in feces. The saponification of calcium and magnesium with excess intralumi-
nal fatty acids, secondary to their malabsorption, leaves more free oxalate to be absorbed
by the intestine. Gregory et al. demonstrated increased oxalate absorption in a group of
patients who underwent small bowel bypass surgery for treatment of morbid obesity
(46). These patients absorbed an average of 38% of an oxalate load, compared with 6%
in normal controls. In addition, there is an increased delivery of bile acids and salts to
the colon, which has been demonstrated to increase oxalate uptake in this bowel segment
Table 2
Diseases Associated With Enteric Hyperoxaluria
Diseases involving bowel mucosa
• Crohn’s disease
• Ulcerative colitis
• Celiac sprue
Surgical interruption
• Jejunoileal bypass
• Ileal resection
Other causes of malabsorption and steatorrhea
• Pancreatic insufficiency or resection
• Biliary obstruction or diversion
• Primary biliary cirrhosis
• External biliary drainage
• Bacterial overgrowth
• Blind loop syndrome

108 Shah, Holmes, and Assimos
in animal models (51). The mechanism of this phenomenon has not been identified.
Patients subjected to contemporary bariatric surgery may also develop this problem.
Treatment
If correction or treatment of the underlying bowel pathology is possible, this should
be the initial approach for treatment of patients with enteric hyperoxaluria (Table 3).
These individuals should be encouraged to increase their fluid intake because they are
frequently dehydrated. They should also limit consumption of fat and high oxalate
containing foods.
The administration of calcium salts with meals is recommended for most patients. The
supplemental calcium promotes enteric complexation with oxalate thus limiting oxalate
absorption. Calcium citrate (Citracal, 250 mg), at an initial dose of one to two tablets
three times a day with meals, is the preferred agent as it will also help correct hypo-
citraturia (45,46,52,53). The dose may need to be escalated based on follow-up 24-h
urine assessments.
Table 3
Treating Hyperoxaluria With Medical Therapies
Enteric
• Fluid therapy:
50 mL/kg body weight every 24 h in adults; higher relative intake in children
• Low-fat, low-oxalate diet
• Calcium citrate supplementation
• Magnesium oxide or magnesium gluconate can be substituted or added
• Cholestyramine therapy
• Potassium citrate to correct associated hypocitraturia
• Correct underlying bowel pathology when possible
Primary
• Type 1:
— Fluid therapy (as described above for enteric hyperoxaluria)
— Pyridoxine therapy
— Orthophosphate therapy (not in patients with renal insufficiency)
— Magnesium oxide therapy (select cases)
— Potassium citrate therapy (select cases)
— Sodium citrate therapy (select cases)
— In patients with progressive renal damage leading to failure, combined renal
and liver transplantation
• Type 2:
— Medical therapy similar to type I
— Role of transplantation not well defined
Idiopathic
• Fluid therapy (as described above for enteric hyperoxaluria)
• Low-oxalate diet
• Adequate calcium consumption
• Pyridoxine therapy for patients who do not respond to diet restrictions

Chapter 7 / Management of Oxaluria 109
Magnesium oxide or magnesium gluconate may be substituted or added to this regi-
men. This should be considered in patients who have high normal or increased urinary
calcium excretion and in those with concomitant hypomagnesemia (54–56).
Cholestyramine therapy can be used when there is bile acid malabsorption to bind
fatty acids, bile acids, and oxalate (57,58). Other citrate salts, preferably potassium
citrate, can also be given to correct associated hypocitraturia (59,60). The liquid form
of potassium citrate is preferred as these patients typically have rapid gastrointestinal
transit.
Specific therapies of steatorrhea in certain conditions can be used: gluten-free diet
in celiac sprue, pancreatic enzyme replacement in pancreatic insufficiency, and antibi-
otics in bacterial overgrowth.
PRIMARY HYPEROXALURIA
The primary hyperoxalurias result from endogenous overproduction of oxalate. There
are two main types of primary hyperoxaluria, type I (PH1) and II (PH2). Both are rare
autosomal recessive disorders caused by defects in hepatic enzyme systems important
in the metabolism of glyoxylate. The marked oxalate production in both disorders may
result in calcium oxalate stone formation, nephrocalcinosis, systemic oxalosis, and, in
some, end-stage renal disease.
Primary Hyperoxaluria 1
PH1 is caused by a defect in glyoxylate metabolism attributable to low or absent
activity of the liver-specific peroxisomal enzyme AGT (61). Except for the AGT defect,
peroxisomes in PH1 are normal in almost every respect (62). The functional deficiency
of AGT in PH1 results in a failure to detoxify glyoxylate within the peroxisomes (Fig. 1).
Instead of being transaminated to glycine, glyoxylate is oxidized to oxalate and reduced
to glycolate (10,61,62). As a result, urinary excretion of glycolate and oxalate is signifi-
cantly increased.
The gene encoding for AGT is located on chromosome 2q37.3 (10,63,64). Several
mutations and polymorphisms have been identified thus far and there is considerable
molecular heterogeneity among patients. Half of the patients exhibit no detectable AGT
activity, whereas the other half exhibits residual (2–48%) AGT activity (10,62). How-
ever, even patients with residual activity can become ill and do not differ clinically from
patients without AGT activity. Danpure et al. demonstrated that this occurs because
AGT is mistargeted from the peroxisomes to the mitochondria (10,62,64). Such patients
exhibit residual enzymatic activity, with only 10% or less of the AGT localized in
peroxisomes. This mistargeting defect is observed in approx 30% of all patients with
PH1 (10,65). A number of mechanisms have been suggested to explain the lack of
catalytic activity in those with immunochemically detectable AGT. These include: (1)
presence of a common P11L polymorphism, which decreases the specific activity of
AGT; (2) the presence of the P11FL polymorphism in conjunction with the PH1 specific
mutation, which leads to AGT protein destabilization and aggregation into inclusion
bodies; (3) naturally occurring amino acid substitutions that lead to peroxisome-to-
mitochondrion AGT mistargeting; and (4) interference with cofactor binding (66). These
may be caused by synergism between certain disease-associated mutations and the
common nondisease causing AGT polymorphism, P11L.

110 Shah, Holmes, and Assimos
PH1 typically manifests in patients early in life and should be suspected when oxalate
excretion is greater than 1–2 mmol/1.73 m
2
/24 h (normal reference range 0.11–0.46
mmol/24 h/1.73 m
2
) and elevated glycolate excretion (normal range 21–168 µmol per
mmol of creatinine) (1,10,65,67). Glycolate levels are elevated in approximately two-
thirds of patients with PH1. Therefore findings of normal values do not exclude this
diagnosis (10,65). Liver biopsy can be performed to establish a definitive diagnosis. The
AGT activity and immunoreactivity in hepatic tissue are measured (10,65). A search for
disease associated AGT mutations can also be undertaken. The latter approach can be
used for prenatal diagnosis using chorionic villous sampling (68). The diagnosis can now
be established in some patients with polymerase chain testing of blood to screen for
certain AGT gene mutations associated with PH1.
Recurrent urolithiasis and nephrocalcinosis are the main manifestations, and the
combination of the two conditions is characteristic for PH1 and may lead to progres-
sive loss of renal function. Half of the patients with PH1 exhibit their first symptoms
by age 5 (71,72). Approximately 40% of PH1 patients will eventually develop end-
stage renal disease (ESRD) (1). The calculi of PH1 patients almost exclusively consist
of calcium oxalate monohydrate (whewellite) (69,70).
Systemic oxalosis occurs when the critical saturation point for plasma oxalate (levels
>30 µmol/L) is reached, typically early in renal insufficiency (73). Deposition occurs in
every organ and tissue except the liver and can lead to significant morbidity. The skel-
eton is the most crippling site of calcium oxalate deposition. The lesions are character-
istic both in X-rays (radiodense metaphyseal bands, a “bone-within-bone” appearance,
and diffuse demineralization with a coarse trabecular pattern) and in histologic assess-
ments (intraosseous tophi of calcium oxalate and granulomas replacing bone marrow)
(74). Clinical manifestations of oxalate osteopathy are pain, spontaneous fractures, and
erythropoietin-resistant anemia (74,75). Additional sites of oxalate deposits are the
retina, media of the arteries (with subsequent ischemia and gangrene), peripheral ner-
vous system (neuropathy), the heart, thyroid gland, and skin (livedo reticularis) (76).
T
REATMENT
The goals of therapy are to decrease oxalate production and increase the urinary
solubility of calcium oxalate. Without treatment, the outlook for patients with PH1 is
extremely poor. Latta and Brodehl reviewed 330 patients with PH1 and found that 50%
of 330 patients had ESRD by the age of 15 yr and 80% by the third decade (77). Dialysis
does not remove sufficient oxalate to match its production in these patients (78–80). If
initiated when renal function is satisfactory, treatment of PH1 may improve the long-
term outcome of these patients (67).
Fluid therapy is a key factor in the management of patients with PH1. It has been
recommended that adults with PH1 consume greater than 50 mL/kg body weight every
24 h; a higher relative intake is recommended for pediatric patients (67). It is extremely
important for close follow-up monitoring and to maintain preventative measures to
avoid episodes of dehydration.
Pyridoxine is an essential cofactor in the AGT enzyme pathway and approx 30% of
PH1 patients respond to pyridoxine therapy (81). Pharmacologic doses of pyridoxine
(dose ranges from 2.5 to 20 mg/kg/d) are able to significantly reduce (by at least 30%)
oxalate excretion in one-third of patients (67,83–87). To follow pyridoxine responsive-
ness, reliable baseline urinary oxalate values are needed. A trial of pyridoxine therapy
for at least 3 mo is warranted, although the effects usually occur in 1–2 wk in most cases

Chapter 7 / Management of Oxaluria 111
(76). Very high doses of pyridoxine should be avoided as this may cause peripheral
neuropathy (76). The mechanism of pyridoxine’s action has not been defined. Pyridox-
ine responsiveness is associated with the G17OR and F1521 mutations, the latter causing
mistargeting of AGT.
Orthophosphate therapy has been used in combination with pyridoxine to reduce
stone formation and attenuate calcium oxalate deposition in patients with PH1 (67). Its
mechanism of action is thought to be caused by a resultant increase in pyrophosphate
concentration, a known inhibitor of calcium oxalate crystallization, and perhaps to
decreased calcium excretion (1). A clinical trial of this combination in 25 patients lead
to a significant decrease in stone events and prevented renal deterioration in most
patients at a mean follow-up of 10.3 yr (67). Orthophosphate should not be used in
patients with renal insufficiency.
Magnesium oxide, sodium citrate, and potassium citrate therapy have also been used
in PH1, but the true efficacy of these agents has not been determined. Citrate not only
binds calcium but also is an inhibitor of calcium oxalate crystal nucleation and growth
(88). Although there is clinical and experimental evidence of the beneficial effects of this
therapy, it has been difficult to conclusively prove (89,90). Magnesium is another po-
tential inhibitor of calcium oxalate crystallization because it binds to oxalate.
Patients with PH1 who develop renal failure are best managed by combined liver and
renal transplantation. Renal transplantation alone does not correct the basic hepatic
enzyme abnormality and therefore recurrent disease is a potential problem. Broyer et
al. reviewed the European experience where primarily cadaveric renal transplantation
was performed. The 3-yr graft and patient survivals were 20% and 74%, respectively
(91). Saborio and Scheinman reported on the experience in the United States where
living-related renal transplantation was primarily undertaken. The 6-yr actuarial graft
and patient survivals were 51% and 84%, respectively. However the 10-yr graft sur-
vival rate was only 35% (92).
Because the metabolic defect is in the liver, total hepatectomy is required before liver
and renal transplantation. The first successful, combined liver and kidney transplant for
a patient with PH1 was performed in 1984 (93). The actuarial 5-yr graft and patient
survival rates for this approach in some series have been approx 70% and 80%, respec-
tively. However, recent studies demonstrated that patient survival is better after renal
transplantation. Poor prognostic factors for graft and patient survival include presence
of systemic oxalosis, young age (<5 yr), and history of >2 yr of dialysis. In addition, the
renal function of survivors remains stable over time, between 40 and 60 mL/min/1.73 m
2
after 5–10 yr (87, 94–96).
Some investigators have performed pre-emptive hepatic transplantation in those
afflicted with PH1. However this approach is controversial. If liver transplantation was
a simple procedure with minimal risks and there were no shortage of organs, preemptive
transplantation would probably be an acceptable treatment option in patients with PH1.
However, there are ethical issues including the risk of graft loss and complications
related to the transplant in a patient who might have done well for several years longer
without such an aggressive intervention. There is also concern about the potential long-
term effects of immunosuppressive drugs including renal toxicity and malignancy.
Isolated liver transplantation can only be successful if renal function is adequate (glom-
erular filtration rate greater than the 40–50 mL/min) (76). If the glomerular filtration
rate is <40 mL/min/1.73 m
2
, there is a significant risk of eventual renal failure (97).
Results of preemptive liver transplantation have thus far been reasonable (97).

112 Shah, Holmes, and Assimos
Other approaches have been considered for patients with PH1. We evaluated the
short-term efficacy of (
L)-2-oxothiaolidine-4-carboxylate (OTZ) therapy. OTZ is a cys-
teine precursor that is converted to cysteine within the cell and forms a stable adduct
with the aldehyde group of glyoxylate, which could potentially decrease oxalate synthe-
sis. Administration of OTZ has been demonstrated to reduce oxalate excretion in rats
and normal human subjects (98,99). However, it did not result in a reduction in two
patients with PH1 perhaps caused by the extremely large glyoxylate pool. The utiliza-
tion of agents that inhibit glycolate oxidase, a key enzyme in oxalate synthesis, has been
proposed as another therapeutic approach.
The identification and characterization of the AGT gene has stimulated interest in
gene therapy for those afflicted with PH1 (63). Intraportal infusion of recombinant
viruses or macromolecular DNA as gene vectors has been suggested (1). The other
approach involves the surgical removal or ablation of hepatic tissue, isolation of hepa-
tocytes or stem cells, ex vivo transfection of these cells, and infusion of the transfected
hepatocytes into the portal vein (100–102). A major hurdle to overcome is the large size
of the hepatic target which makes vector delivery quite difficult. The ability to maintain
the corrective gene will also need to be established. Surgical or pharmacologic strategies
to reduce the targeted hepatic mass will be necessary to facilitate adequate gene delivery;
another impediment.
Primary Hyperoxaluria (PH2)
PH2 results from a deficiency in glyoxylate reductase (GR) activity which leads to
an increased oxalate synthesis and urinary excretion (103–106). The responsible gene,
GRHPR, is mapped to the centromeric region of chromosome 9 (107). Although the
liver has the highest GR activity, the enzyme is ubiquitously expressed in all tissues,
perhaps indicating that all tissues synthesize glyoxylate. As with PH1, a range of
different mutations has been identified, although one mutation predominates in Cau-
casians (108). Clinical manifestations are not as severe as in PH1, although ESRD can
result (109). The metabolic features of PH2 are increased urinary excretion of L-
glycerate and oxalate. The conservative stone prevention treatments used with PH1
patients can also be applied to PH2 patients. However the efficacy of pyridoxine
therapy and liver transplantation has not been established. Owing to the ubiquitous
expression of GR activity, liver transplantation may not be as effective.
Another group of patients with hyperoxaluria in the primary hyperoxaluria range has
been identified which do not have the metabolic characteristics of PH1 and PH2 (110).
The mechanisms of this novel form of hyperoxaluria have not yet been defined.
Idiopathic Hyperoxaluria
Idiopathic hyperoxaluria is the most common type of hyperoxaluria. Patients fre-
quently have other coexistent metabolic abnormalities that should also be addressed.
Dietary modifications should be initially considered for treatment (Table 3). Given
that a significant amount of urinary oxalate is derived from dietary sources, patients
should refrain from consuming high oxalate containing foods (Table 1). Approximately
one-third of patients with idiopathic hyperoxaluria are protein sensitive, with increased
animal protein consumption leading to increased urinary oxalate excretion (111,112).
Therefore, animal protein restriction should be considered for this reason as well as the
