Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.

70
Table 1
The Chemical Name, Composition, Mineral Name, and Abbreviation
of the Most Common Components of Human Urinary Tract Calculi
Chemical name Mineral name Chemical formula Abbreviation
Oxalates
• Calcium oxalate monohydrate Whewellite CaC
2
O
4
·H
2
OWH
• Calcium oxalate dihydrate Weddellite CaC
2
O
4
·(2+x)H
2
OWE
Phosphates
• Basic calcium phosphate Apatite Ca
5
(PO
4
)
3
(OH) AP
• Calcium hydrogen phosphate Brushite CaHPO
4
·2H
2
OBR
• Magnesium ammonium phosphate hexahydrate Struvite MgNH
4
PO
4
·6H
2
OST
Purines
• Uric acid C
5
H
4
N
4
O
3
UA
• Monosodium urate monohydrate NaC
5
H
3
N
4
O
3
·H
2
O MSU
Other
•
L
-Cystine (-SCH
2
CHNH
2
COOH)
2
CY
Chapter 5 / Kidney Stones 71
stones suggests multiple physiologic conditions that must be unraveled in the process of
defining the optimal medical management and the avoidance of stone recurrence.
The major crystalline components of human urinary tract stones are listed in Table 1.
Prevalence of crystalline admixtures from multiple studies of multicomponent stones is
presented in Table 2. Currently, there are two analysis methods that are routinely used
for stone analysis, namely X-ray powder diffraction crystallography (XRD) and Fourier
transform infrared spectroscopy (FTIR). These two analysis methods differentiate stone
crystalline composition based on either the uniqueness of crystalline structures or sub-
tle energy differences associated with the motion of atoms in chemical bonds found in
the various stone components. It is worthwhile to first address the chemistry of stone
components and aspects of various crystalline structures seen in stones before discussing
the unique aspects and capabilities of the two primary methods of stone analysis.
CHEMISTRY AND STRUCTURES FOUND IN STONES
The primary component of human stones, calcium oxalate, crystallizes in two differ-
ent chemical and crystallographic forms: a monohydrate and a dihydrate structural com-
plex. Calcium oxalate is a calcium salt of a dicarboxylic acid, oxalic acid. The differences
in the degree of water of crystallization included in the crystalline lattice results in two
different crystalline structures, leading to different growth morphologies, different
atomic structures on the crystal surfaces of the two hydrated structures, and different
effective biologic activities.
Table 2
Relative Frequency of Occurrence of Admixed Stones
a
Country USA (13) Glasgow (15) Berlin (11) USA (5)
No. Stones 1,000 149 10,000 3,833
WH 13.7 4 24.9 23.4
WE 0.4 1 0.6 4.7
WH/WE 18.6 21 33.2 29.4
WH/WE/AP 22.4 45
b
13.5 5.2
WH/AP 7.2 45
b
2.9 2.7
WE/AP 4.7 45
b
0.3 1.3
AP 3.4 5 1.1 6.6
ST/AP 15.5 14 7.0 6.4
ST 0.4 — 0.3 4.3
WH/ST/AP — 3 1.4 0.0
UA 4.7 — 2.4 5.2
UA/UD — 1 3.9 1.6
WH/UA — — 2.9 1.5
WH/UA/UD — — 1.8 0.2
BR — 2 0.4 1.2
CY 2.2 1 0.2 0.4
a
No. stones represents number of samples included in study.
b
Frequency of occurrence of both whewellite and weddelite mixed with
apatite reported as a single group.

72 Mandel and Mandel
The second major class of components of stones includes phosphate salts. Four
different calcium phosphate salts and two magnesium phosphate salts are found in
stones. The calcium phosphate salts vary in calcium-to-phosphate ratio, hydroxyl-vs-
hydrogen ion content, and the degree of water of crystallization included in the crystal-
line lattice structure. The magnesium phosphate salts differ by inclusion of either
ammonium or hydrogen ions and the degree of water of crystallization in the crystalline
lattice structure. Similarities in chemistry, but differences in crystalline structures, often
create challenges in composition analysis using infrared spectroscopic analysis that are
focused at differentiating chemistry.
All of the calcium and magnesium salts have different crystalline structures and are
easily differentiated by XRD methods of analysis, but sensitivity of detection can com-
plicate and challenge a complete analysis. Similarly, the purine structural moieties
include subtle chemical and structural variations of the uric acid-like six/five-member
aromatic ring structure. Addition of carbonyl groups to the purine ring moiety separates
related structures such as hypoxanthine, xanthine, and uric acid. The salts of the purine
organic acids and variations in the degree of water of crystallization induce additional
chemical and structural variation in the identification criteria for definitive composi-
tional analysis. The preceding description regarding the method of analysis for the
oxalates and phosphates in stones is also applicable to the purines and their salts. Similar
chemistry leads to very subtle differences in infrared spectra, but they lead to significant
differences in XRD data. The miscellaneous components are sufficiently unique that
analyses by either the XRD or the FTIR method is straightforward, assuming accurate
standards are available.
Need for instrumentation sensitivity and more importantly accurate standards for
comparison are critical for successful stone analysis using either XRD or FTIR analysis
methods. The two methods of analysis used separately have unique capabilities depend-
ing on the type of stone being analyzed. It is clear that the two analysis methods used
collectively yield the highest degree of certainty in compositional analysis of urinary
tract stones.
CRYSTALLURIA AND STONE MORPHOLOGY
Determination of crystalluria and probable crystalline composition must be accom-
plished using freshly voided urine and a polarizing microscope. The different crystalline
components found in crystalluria reveal different growth morphologies when viewed
with a polarizing microscope (1,2). Many of the crystals demonstrate birefringence so
observation of growth morphology is often more definitive. Calcium oxalate monohy-
drate can be observed as ovals or dumbbells and calcium oxalate dihydrate as bipyramids.
Apatite crystals usually appear as an amorphous precipitate, and frequently grow as
clumps of very small crystallites. Struvite crystals grow in a characteristic coffin lid
shape. Uric acid crystals appear as flat parallelepiped plates, and cystine crystals appear
as hexagonal plates. The most common growth morphologies of mature stones com-
posed of components listed in Table 1 are described in the following sections.
Calcium Oxalate Monohydrate
These stones are frequently hard, dark brown, and often have a dull gray exterior.
When sectioned, the stones often appear to grow in radial fashion from a nidus with
wedges rounding off at their extremities creating a generally smooth exterior.

Chapter 5 / Kidney Stones 73
Calcium Oxalate Dihydrate
Pure dihydrate stones are usually small and spherical consisting of a tan or yellow
cluster of platelets. The platelets are sharp and are arranged in various orientations.
Admixed monohydrate/dihydrate stones frequently have many of the characteristics
of a dihydrate stone because dihydrate most frequently appears on the exterior of the
admixed stone. These stones are normally larger than pure dihydrate stones, are often
spherical, and have a cluster of yellow platelets surrounding a hard dark brown interior.
The platelets are sharp and in various orientations. Occasionally, the interior is light
brown in color and has a granular consistency.
Apatite
Pure apatite stones are usually small, white in color with a very fine granular surface,
and they have a soft white chalklike interior. Occasionally, these stones are also light
brown with a smooth shiny surface.
The most frequently occurring stone admixture of apatite or calcium oxalate mono-
hydrate and calcium oxalate dihydrate is generally smooth, spherical, and has light
brown platelets on the surface. The interior is normally layered with both white and
light brown sections. Frequently, papillary casts are observed on one side of the stone
with apatite located in the cast because it is often the first crystalline substance depos-
ited. The dihydrate plates are normally observed on the opposing side from the papillary
casts.
Struvite
Pure struvite stones are usually off-white to light brown in color with a rough textured
surface. When sectioned, the interior of the stone contains white concentric rings, and
occasionally the interior contains white porous granulated material. Struvite stones fre-
quently grow in a staghorn shape.
Admixed struvite/apatite stones are usually light brown in color with a coarse, granu-
lar surface. The interior is normally intermixed with white and light brown layers.
Brushite
Pure brushite stones are normally clusters of beige, nodular material surrounding a
crystalline interior with a cauliflower-like growth pattern. Occasionally, the surface has
a yellow or white tinge.
Uric Acid
Uric acid stones are spherical with a smooth yellow-orange surface. When sectioned,
the interior of the stone appears as orange concentric rings with little to no macroscopic
substructure.
Uric Acid Dihydrate
The surface of uric acid dihydrate stones is often dark orange and the stone is com-
posed of small spherical regions. When sectioned, the stones have a well-defined nidus
with thick concentric rings composed of small needles that radiate from the center of the
stone.

74 Mandel and Mandel
L-Cystine
Pure L-cystine stones are homogeneously composed of very small yellow spheroids.
Matrix
Matrix stones are noncrystalline and take on a variety of shapes and colors. The stones
are composed of a variety of organic molecules including urinary macromolecules and
membrane fragments (3).
Other
Other substances that have been reported in stones include the drugs sulfamethoxaz-
ole, crixivan, guaifenesin, triamterene and 5-fluorocytosine, xanthine, 2,8-dihydroxy-
adenine, gypsum, and silicates following antacid therapy. Growth morphology for these
components is often variable.
STONE ANALYSIS
XRD (1–5) and FTIR (6–8) are the most frequently used methods of stone analysis.
Both XRD and FTIR methods require a very comprehensive and accurate library of
standard diffraction or spectroscopic patterns for comparative analysis. The focus of
these methods is the identification of the crystalline mineral composition of the stone
with little emphasis on the macromolecular or other noncrystalline entities that may be
present. XRD should be viewed as a semiquantitative method of analysis. XRD analysis
should be presented in rank order of relative amounts of the stone components in mul-
ticomponent stones. FTIR spectral libraries allow for the reporting of relative percentage
compositional analyses in multicomponent stones using computer algorithms that math-
ematically admix spectra of pure components at specified percent admixtures. Computer
addition of spectra from components with significantly different infrared absorption
characteristics can artificially and inappropriately skew spectral standard comparison
methodology.
Virtually all crystal structures are unique is some structural aspect and their diffrac-
tion patterns can be differentiated from other structures and diffraction patterns. Highly
sensitive and accurate XRD instruments are often necessary to differentiate some of the
structures seen in stones as their chemistry and crystal structures can be similar.
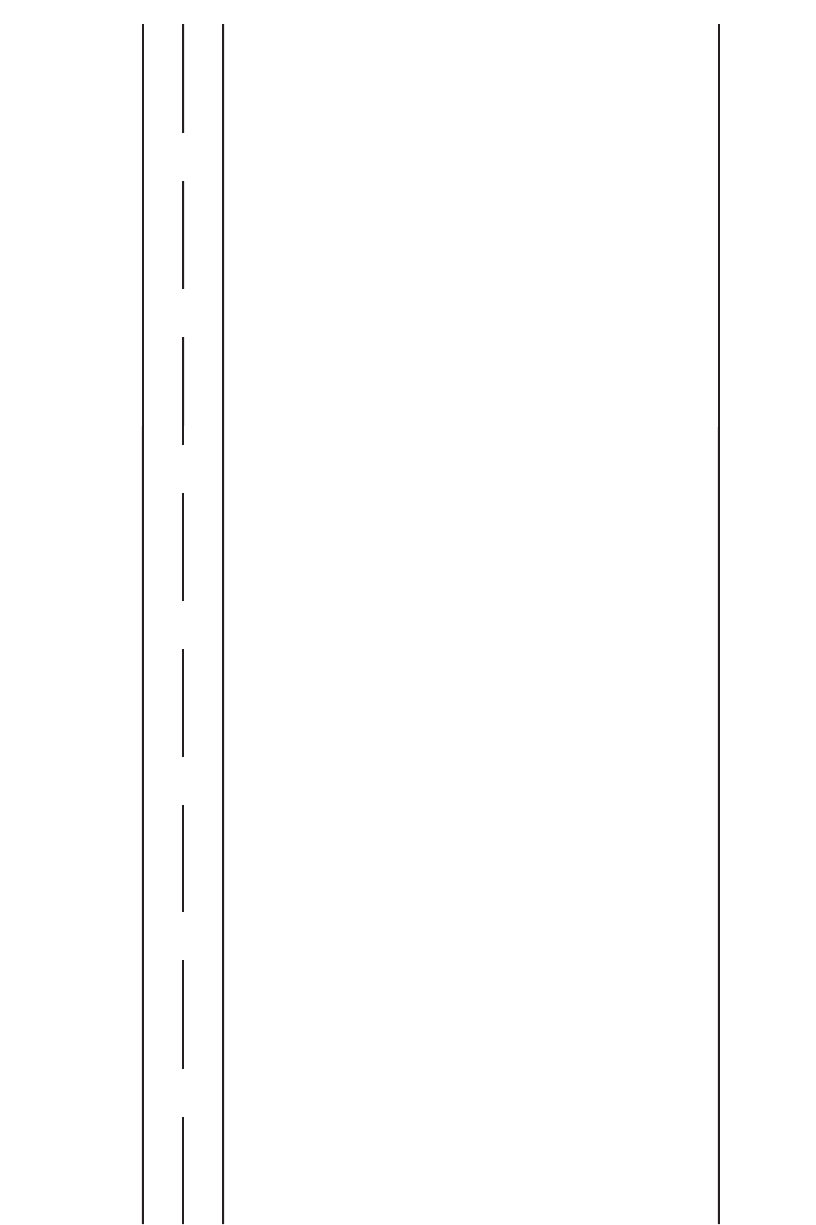
The XRD data for the most common components of human kidney stones are pre-
sented in Table 3. The diffraction data are presented in crystallographic language as
interplanar d-spacings in Ångstroms (d[Å]) associated with the distances between atoms
in the structure and as diffraction intensities either relative to a weak vs strong scale or
on a maximum of 100 scale (4,9,10).
In practice, the experimental XRD patterns are compared with those for the standard
patterns presented in Table 3. All diffraction lines of a given standard pattern, especially
the strongest lines, must be matched with diffraction lines in the sample pattern. If some
lines of a given intensity are thought to match a standard, then all lines with equal or
greater intensity must also match the standard lines. Remaining unmatched lines are used
to determine any other crystalline components in the sample. With the advent of high
resolution XRD cameras utilizing focusing monochromators and high flux X-ray gen-
erators, the ability to detect minor stone components has greatly increased as the diffrac-
tion patterns appear sharper and diffraction lines are easily differentiated from
Chapter 5 / Kidney Stones
75
Table 3
XRD Interplanar Spacings and Relative Intensities of the Components of Human Urinary Calculi
COM COD AP BR ST UA MSU CY
d (Å) I d (Å) I d (Å) I d (Å) I d (Å) I d (Å) I d (Å) I d (Å) I
5.93 100 8.70 12 8.15 29 7.59 100 5.90 38 6.56 38 10.60 8 4.70
5.79 25 6.31 5.26 6 4.24 100 5.60 45 5.63 20 9.30 30 4.68 100
4.64 7 100 4.08 8 3.80 8 5.38 22 4.91 48 7.50 25 4.63
4.52 6 6.15 3.89 10 3.04 75 4.60 6 4.76 6 5.28 10 4.45 5
3.78 4.40 45 3.44 45 2.93 70 4.25 100 3.86 4.88 22 4.20 7
13 3.89 14 3.17 10 2.85 15 4.14 34 + 55 4.63 4 3.32 8
3.65 100 3.09 2.81 100 2.60 30 3.29 24 3.70 5 3.45 15 3.18 19
3.00 10 18 2.78 62 2.56 6 3.02 10 3.28 14 3.38 20 3.13 30
2.97 46 3.07 2.72 58 2.96 3.18 70 3.26 5 3.06 16
2.91 12 2.81 20 2.63 31 + 16 3.09 100 3.12 100+ 2.71
2.89 10 2.77 2.53 5 2.95 2.87 3.01 1 2.70 32
2.84 14 85 2.40 7 2.92 46 + 25 2.87 12 2.68
2.48 30 2.75 2.26 20 2.80 26 2.86 2.84 8 2.60 17
2.41 5 2.41 14 2.15 9 2.72 8 2.80 2.78 5
2.34 90 2.39 14 2.06 7 2.69 37 + 10 2.63 40
2.33 10 2.00 5 2.66 37 2.79 2.59 5
1.94 30 2.57 18
1.89 15
1.87 5
1.81 20

76 Mandel and Mandel
neighboring diffraction lines separated by as little as 0.01–0.03 Å interplanar spacings.
The increased sensitivity has allowed for the identification of smaller amounts of poorly
crystalline materials such as apatite. However, the most severe limitation of XRD is
sensitivity when a very limited amount of sample is available. Also, if the stone material
is a drug, or drug metabolite whose XRD pattern or single crystal structure has not been
published, XRD methods fail to definitively characterize the sample. In those cases, the
XRD method can only tell you what the stone is not composed of.
In FTIR spectral analysis, spectral data is related to the vibrational motions of atoms
in bonds (e.g., bond stretching, bond contracting, or bond wagging, etc.). Classically,
the powdered sample is admixed with powdered potassium bromide, compressed into
a nearly transparent wafer, and the IR beam is passed through the wafer. Recently,
advances in other sample preparation methods have allowed powdered samples to
simply be ground to ensure optimal sampling of a multicomponent stone and then the
IR beam is directed at the sample surface (attenuated total reflectance). The reflected
IR beam containing spectral data specific to the sample is then recorded. Standard IR
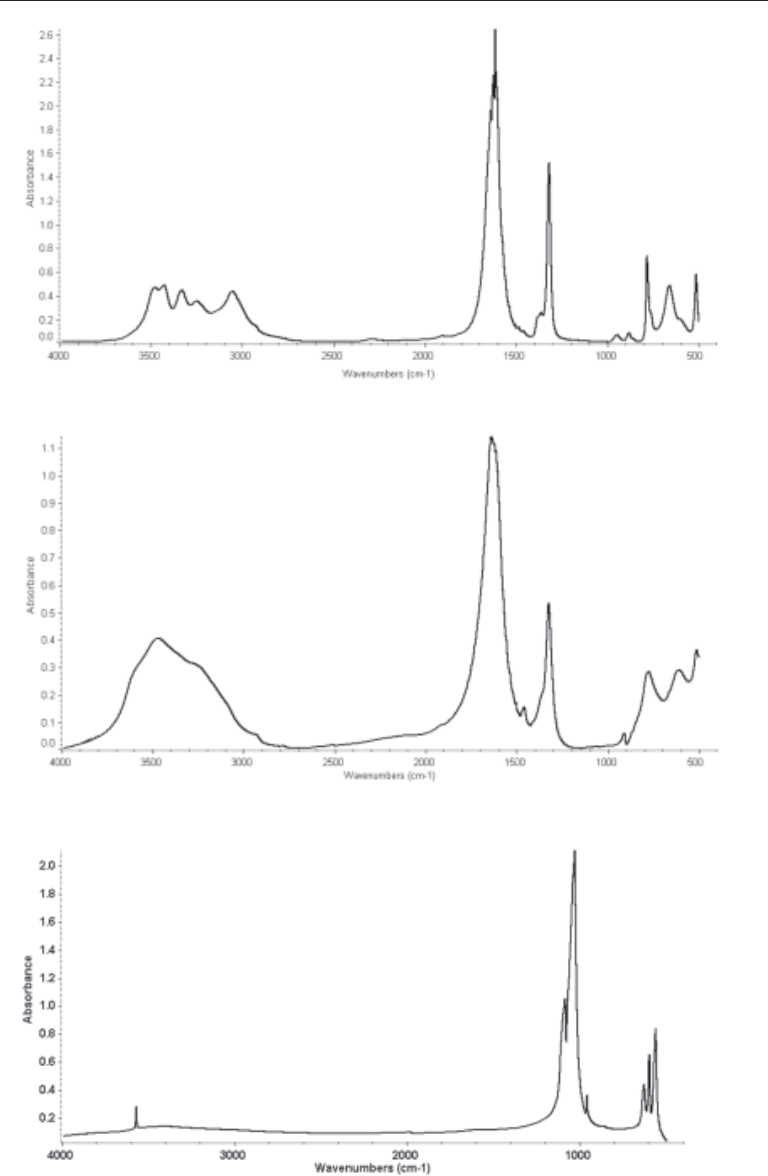
patterns for common stone components are presented in Figures 1–8. The IR pattern
contains absorption bands representing specific energies (presented as wavelengths in
units of cm-1, or more commonly known as wavenumbers) corresponding to molecular
motions in molecules. It is therefore possible to differentiate molecular motions in
similar organic groups. The IR pattern of a mixed component stone is frequently very
complex, but the advent of computer controlled IR spectrometers, especially modern
FTIR spectrometers has allowed for computer assisted pattern stripping and compara-
tive standards library matching. Frequently, the assignment of weak absorbance bands
in the spectra to specific structures is very difficult and often requires careful back-
ground and noise level adjustments in the computer data smoothing functions. The
specific choice of mathematical calculations used in the standards library search/match
routines can provide markedly different results, so operator training and experience is
critical.
The real benefit of FTIR is the high sensitivity of the new computer controlled spec-
trometers that can take many repetitive spectra of the same sample and mathematically
enhance the sample signal to experimental noise ratio. It is often possible to obtain a
definitive FTIR spectra with less material than is needed for XRD. FTIR is the method
of choice for the characterization of noncrystalline samples or drug related samples
because the IR absorbance is related to independent chemical bonds and IR data related
to a majority of the molecule is still definitive. Metabolism of a drug resulting in an
altered chemical structure in a stone compared with the ingested molecule has far less
impact in FTIR analysis compared with XRD. The drawback to FTIR is that the differ-
entiation of spectral signals from more than one component with similar molecular
bonds can be difficult. All too often the operator of modern FTIR instruments may rely
too heavily on the computer-assisted analysis of the spectra as the computer algorithms
scan and interpolate data from computer based spectral libraries. In the case of weak
spectra or multicomponent stones, the computer often indicates a positive identification,
but in fact the component identification is false.
The analysis of stone composition with microscopic inspection (including polarizing
microscopy) is very inaccurate and unfortunately too frequently used for the routine
analysis of stones (3,11,12). The assumption is that all components of stones always
appear the same regardless of unique urine chemistry and different admixtures with other
Chapter 5 / Kidney Stones 77
Fig. 3. The FTIR spectra of hydroxyapatite.
Fig. 2. The FTIR spectra of calcium oxalate dihydrate.
Fig. 1. The FTIR spectra of calcium oxalate monohydrate.
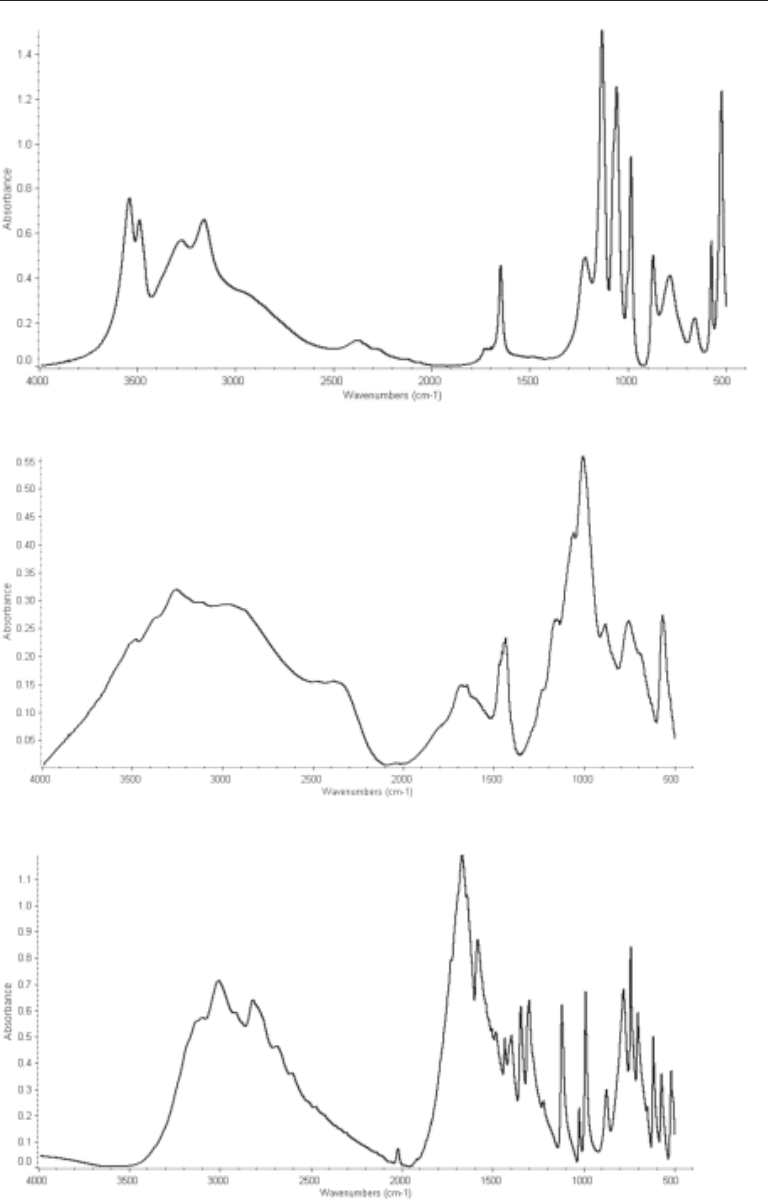
78 Mandel and Mandel
Fig. 6. The FTIR spectra of uric acid.
Fig. 5. The FTIR spectra of struvite.
Fig. 4. The FTIR spectra of brushite.
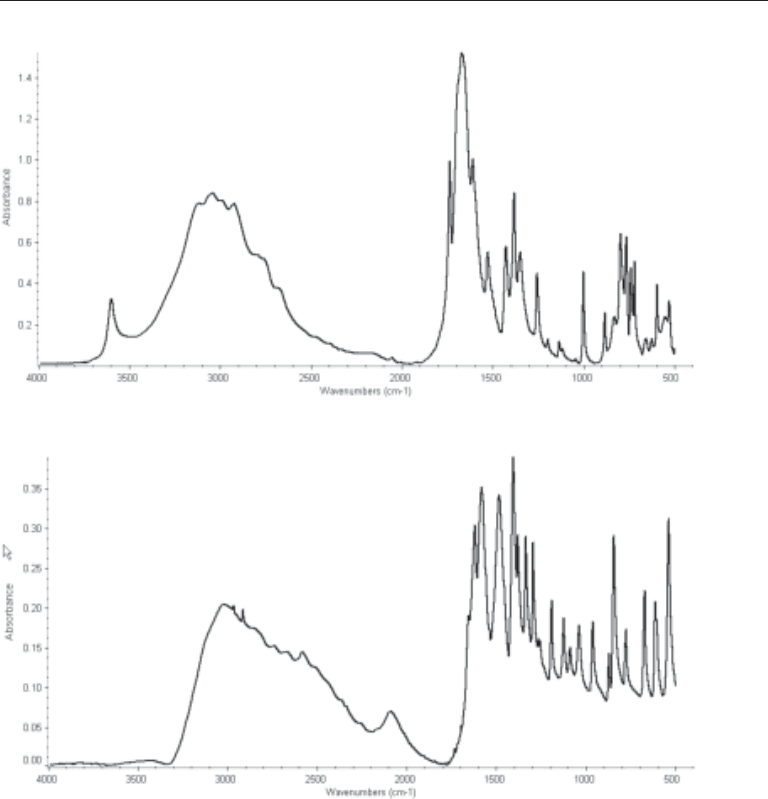
Chapter 5 / Kidney Stones 79
stone components. This is not a sound assumption. After fracturing the calculi to reveal
the internal structure, samples are taken from various portions of the stone and are
visually inspected and identified using a polarizing microscope. This technique is not
capable of identifying small amounts of crystalline materials in admixed samples. A
significant contribution to the potentially low level of accuracy using this method is that
the accuracy is entirely dependent on the level of sophistication and experiences of the
technicians conducting the analyses (12).
CONCLUSIONS AND RECOMMENDATIONS
Numerous treatises have been written on the crystalline composition of renal calculi
and the frequency of occurrence of the varied crystalline components in stones observed
in industrialized countries (3,5,13–17). The majority of urinary stones are admixtures of
Fig. 8. The FTIR spectra of cystine.
Fig. 7. The FTIR spectra of monosodium urate monohydrate.
