Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.


124 Chin
bicarbonate, the resultant pH can be predicted by the Henderson–Hasselbalch equation
(Eq. 7).
The amino acids aspartate and glutamate are metabolized to a neutral end product plus
bicarbonate molecules. Individuals on very strict vegetarian diets can consume propor-
tionately more proteins containing these amino acids than proteins with acidic metabolic
products, resulting in a lesser degree of acidosis than nonvegetarians (5).
With the endogenous acid load from protein breakdown, each H
+
consumes an equal
amount of HCO
3
–
. The body is left with a deficit of HCO
3
–
. To maintain homeostasis,
new bicarbonate must be formed. In regenerating bicarbonate, acid is excreted, a process
commonly referred to as renal net acid excretion (NAE). The contributions of nonrenal
mechanisms of acid–base regulation are also significant.
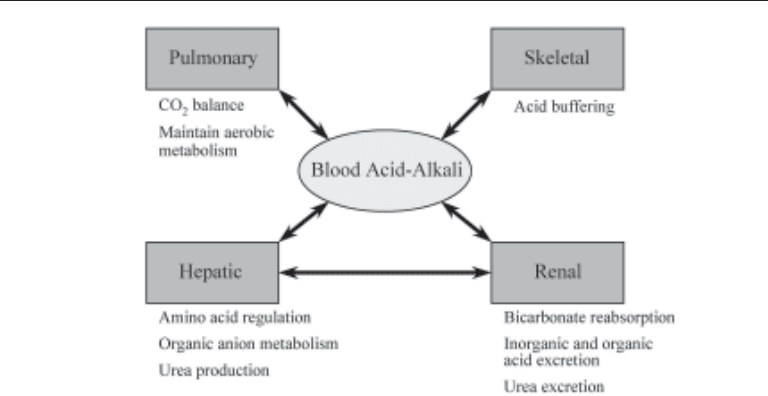
PULMONARY AND OTHER NONRENAL ACID–BASE REGULATION
Pulmonary
The pulmonary system provides two important processes in the regulation of acid–
base balance: removal of carbon dioxide and maintaining oxygenation for aerobic me-
tabolism. The production of CO
2
in the metabolism of carbohydrates and fats leads to
production of carbonic acid (H
2
CO
3
) via the enzyme carbonic anhydrase, which is found
in numerous cell types. The buildup of carbonic acid is a potentially enormous acid
burden. However, there is constant removal of CO
2
with pulmonary ventilation, keeping
the [H
2
CO
3
] extremely low. Even a small rise in the partial pressure of CO
2
(pCO
2
) is
a powerful stimulant for increased minute ventilation. When ventilation becomes inad-
equate, the pCO
2
rises quickly with a drop in pH: ↓pH = 6.10 + log {[HCO
3
–
]/(0.03
↑pCO
2
)} as predicted by the Henderson–Hasselbalch equation. With long-standing
elevations of pCO
2
, the renal system will increase the production of HCO
3
–
to a higher
baseline steady-state level to bring the pH closer to 7.40. Renal adjustments of HCO
3
–
serve as adaptive mechanisms to compensate for nonrenal disturbances in acid–base
balance and will not be further discussed.
The other important, although indirect, role of the pulmonary system in maintaining
acid–base homeostasis is the oxygenation of tissues to allow aerobic metabolism.
Anaerobic cellular metabolism from lack of intracellular oxygen produces lactic acid, an
organic acid. With return of aerobic metabolism, lactic acid is usually quickly and
efficiently metabolized, consuming a proton (and thus regenerating HCO
3
–
) in the pro-
cess: lactate + H
+
= glucose + CO
2
. Because this anion represents potential bicarbonate,
the loss of lactate before its metabolism by the liver and muscles can result in a nongap
acidosis.
Hepatic
Hepatic function is indirectly tied to acid–base regulation by its processing of amino
acids, as well as by its processing of organic anions such as lactate, citrate, and ketones.
Conversion of nitrogen-containing substances, such as amino acids, to urea is the job
of the liver. Urea formation, however, consumes bicarbonate. The alteration of the
urea cycle in different states of acid–base balance is well described, but whether or not
this represents true adaptability to acid–base disturbances remains controversial. None-
theless, hepatic uptake of amino acids is decreased in the setting of metabolic acidosis,
allowing a greater availability of amino acids for renal ammonium production and
enhanced acid excretion (6,7). In addition, the liver plays a major role in the metabo-
Chapter 8 / Renal Acid–Base Balance 125
lism of organic anions. The breakdown of some organic anions results in the utilization
of protons, and thus formation of bicarbonate. Other anions such as citrate are metabo-
lized to more that one bicarbonate molecule, and its processing by the liver is important
in converting this potential bicarbonate source into usable buffer. Conversely, the liver
is the primary organ for urea formation, and in doing so bicarbonate is consumed. In
the setting of liver function abnormalities, either acute or chronic, the handling of these
anions can be significantly impaired, leading to a metabolic acidosis.
Bone
The skeletal system serves as a large buffer for acids. The ability of phosphates in the
bone to buffer protons allows pH to be regulated at the expense of bone minerals such as
calcium. The short-term consequences of acidosis on the skeletal system are unclear, but
chronic acidosis may play a role in the development of bone disease (3). The destructive
effect of acidosis on bone formation is most commonly seen in children with chronic
acidemia. Long-term acidosis inhibits both matrix formation as well as bone mineraliza-
tion, and correction of the acidosis usually leads to dramatically improved skeletal growth
in these children (8). The contribution of various organ systems is illustrated in Fig. 1.
RENAL ACID–BASE REGULATION
The role of the kidneys in acid–base regulation can be simplified to two basic tasks:
the reclamation of filtered bicarbonate, and the excretion of accumulated, endogenous
noncarbonic acid. The two processes are frequently described as functionally separate,
but there is substantial overlap in the physiology.
Reclamation of Filtered Bicarbonate
One of the first, and arguably most crucial, functions of the kidneys in acid–base
regulation is the reclamation of the filtered bicarbonate, which is freely filtered by the
glomeruli. Assuming a normal glomerular filtration rate of 100 mL/min and a serum
Fig. 1. Contributions of renal and nonrenal organs to acid homeostasis.

126 Chin
bicarbonate concentration of 24 mmol/L, approx 3500 mmol of bicarbonate are filtered
in a day (100 mL/min 1440 min/d 1L/1000 mL 24 mmol/L = 3456 mmol/d). This
filtered bicarbonate needs to be actively transported back into the serum. The reclama-
tion process does not generate any new bicarbonate, yet defects in this vital function can
lead to profound buffer losses and a resulting metabolic acidosis.
P
ROXIMAL TUBULE
The proximal tubule, ascending limb of the loop of Henle and collecting tubules are
all involved in this task. The proximal tubule is responsible for the bulk (80–85%) of the
reabsorption. In normal circumstances, essentially all of the filtered bicarbonate is
recovered by the time the urine leaves the collecting tubules, with normal fractional
excretion of bicarbonate (FE
HCO3-
) <0.1%. In all segments, the reclamation of bicarbon-
ate involves a proton-secreting event rather than the direct removal of an intact bicar-
bonate molecule from the filtrate.
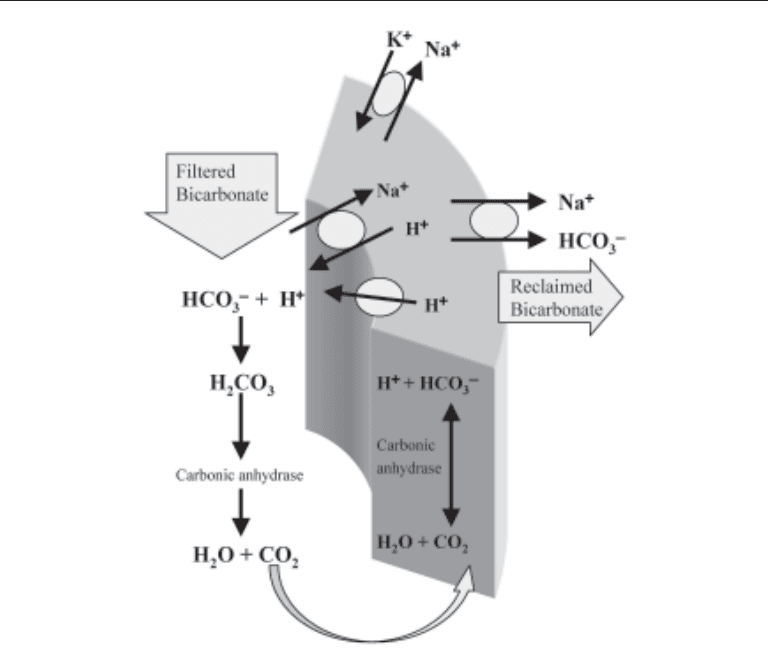
The proximal tubule performs the vast majority of the bicarbonate reclamation. Car-
bonic anhydrase found on the luminal side of the tubular cell, specific luminal surface
Na
+
/H
+
exchangers, H
+
-ATPase and basolateral Na
+
/HCO
3
–
cotransporters give this
tubular segment the ability to remove large amounts of bicarbonate from the filtrate
(Fig. 2). The driving force for this process is the electrochemical gradient produced by
the basolateral Na
+
/K
+
-ATPase, which produces a relatively electronegative, low [Na
+
]
environment within the proximal tubular cell. This favors the movement of Na
+
from the
filtrate into the cell via the antiporter, with subsequent transport of H
+
out of the cell into
the luminal space. Energy-requiring ATPase also pumps H
+
out of the cell. H
+
combines
with the HCO
3
–
in the filtrate, forming H
2
CO
3
. Because carbonic anhydrase (isoform 4)
is present on the luminal surface of the proximal tubule (9,10), the H
2
CO
3
is rapidly
converted to H
2
O and CO
2
, which pass easily into the cell. Once inside, the two are
combined again to form H
2
CO
3
via another isoform (isoform 2) of carbonic anhydrase
(9–11), and then dissociates to H
+
and HCO
3
–
. The proton goes back out into the luminal
space via the Na
+
/H
+
exchanger for the next cycle. Finally, the HCO
3
–
remaining in the
cell is transported out by the basolateral Na
+
/HCO
3
–
cotransporter, into the peritubular
fluid, and eventually into the serum as one molecule of reclaimed bicarbonate. This
mechanism in the proximal tubule reclaims about 85% of the filtered HCO
3
–
load in
normal conditions.
The molecular biology of the proximal tubule transporters has been recently eluci-
dated. Two important channels have been described in detail: the luminal Na
+
/H
+
exchanger (NHE) and the basolateral Na
+
/HCO
3
–
cotransporter (NBC). The proximal
tubular Na
+
/H
+
exchanger (NHE3) is in a family of transmembrane transporters found
in various tissues, of which seven types have been identified so far. These transporters
are important for cellular volume control as well as for intracellular pH regulation
(12,13). The NHE3 channel function can be controlled by cytosolic C-terminal alter-
ations, including phosphorylation and binding by other proteins (13). The gene for
human NHE3 is now determined to be on chromosome 5p15.3 (14).
The Na
+
/HCO
3
–
cotransporter (NBC) is also in a family of transporters important for
pH regulation. The kidney type of the cotransporter (kNBC1) is predominantly found in
the basolateral side of proximal tubular cells and cotransports one Na
+
molecule with
three HCO
3
–
molecules. kNBC1 is mapped to chromosome 4p21 (15). Defects in these
membrane channels are the basis for some forms of proximal renal tubular bicarbonate
handling problems, discussed in the section on renal tubular acidosis.
Chapter 8 / Renal Acid–Base Balance 127
LOOP OF HENLE AND COLLECTING DUCT
The thick ascending limb of Henle’s loop (TALH), the distal tubule and the collecting
duct are responsible for the remaining 15% of filtered bicarbonate not reclaimed by the
proximal tubule. Recent evidence supports the TALH as being important in the reabsorp-
tion of the majority of the remaining bicarbonate coming out of the proximal segment.
The mechanism, including luminal carbonic anhydrase, Na
+
/H
+
exchangers, and
basolateral Na
+
/HCO
3
–
cotransporters, is similar to that of the proximal tubule (16).
Although proportionally not large, the actual amount of remaining bicarbonate in the
filtrate is quite substantial, making the reclamation function of these distal segments
important in maintaining acid–base homeostasis. The molecular biology of the ion trans-
porters in the TALH is similar to that of the proximal tubule. NHE3 channels and kNBC1
are also sparingly found on the TALH (17).
Although the collecting duct is predominantly important for net acid excretion, this
segment performs bicarbonate reabsorption to a small degree. Importantly, luminal
carbonic anhydrase (isoform 4) is generally not found in this segment.
Acid Excretion
The second major task of the renal system in maintaining acid–base homeostasis is the
excretion of accumulated noncarbonic acid. As discussed, the reclamation of bicarbon-
Fig. 2. Proximal tubular cell: basic mechanisms for bicarbonate reclamation.

128 Chin
ate from the filtrate does not regenerate any previously consumed HCO
3
–
from the
buffering of daily acid production. To maintain equilibrium, bicarbonate regeneration
must be equivalent to the net amount of endogenous acid produced, approx 50–100
mmol for someone eating an average Western diet. Net acid excretion (NAE) essentially
results in bicarbonate formation.
U
RINARY BUFFERS
As in the serum, buffering in the urine, mainly by phosphates and ammonia, maintains
the urine pH within a specific physiologic range. Although bicarbonate is found in large
amounts in the filtrate, the bicarbonate reclamation process usually removes the vast
majority of this buffer by the time the urine reaches the distal tubular segments. Thus,
bicarbonate is usually not a major urinary buffer. The H
+
from acid production cannot
be excreted as free protons; the number of free protons—which determines urinary pH—
is trivial compared with the number excreted bound to urinary buffers. NAE requires an
adequate amount of nonbicarbonate urinary buffers to bind the secreted protons. The
amount of phosphate, or “titrateable” acid, can increase a few-fold if increased acid
excretion is required (18). However, the amount of H
+
that can be excreted in the form
of ammonium can be increased dramatically when more acid excretion is required (19).
Each proton excreted with a nonbicarbonate urinary buffer represents a regenerated
bicarbonate molecule.
A
MMONIAGENESIS AND THE AMMONIA CYCLE
Ammonia (NH
3
) production begins within the proximal tubular cells. The amino acid
glutamine is broken down to ammonium (NH
4
+
) and glutamate by glutaminase within
the cell: Glutamine → NH
4
+
+ Glutamate. Subsequently, the glutamate is broken down
by glutamate dehydrogenase in the reaction: Glutamate → NH
4
+
+ α-ketoglutarate. The
α-ketoglutarate is eventually metabolized within both the tubular cell as well as the liver
to form two molecules of HCO
3
–
. Thus, the net result of one mole of glutamine is 2 mol
of NH
4
+
and 2 mol of HCO
3
–
(19). Some of the ammonium is transported back into the
blood and metabolized to urea via the liver. In this process, H
+
is produced, consuming
HCO
3
–
, resulting in no net gain or loss of acid. However, when the ammonium produced
by the proximal tubule is excreted, there a net loss of acid.
The route of ammonium excretion is quite circuitous and demands contributions from
various tubular segments. The movement of NH
4
+
from within the proximal tubule to the
collecting tubule is commonly referred to as the “ammonia cycle” (Fig. 3). Ammonium
produced by the proximal tubule moves out of the proximal tubular cell by substituting
for H
+
in the Na
+
/H
+
antiporter. The pKa of NH
4
+
↔ H
+
+ NH
3
is approx 9.0. Therefore,
at usual urine pH of 5.0–6.0, most of the H
+
remains bound to NH
3
in the tubular lumen.
The ammonium then moves with the filtrate to the thick ascending limb of the loop of
Henle (TALH) where approx 50% of the ammonium is reabsorbed. Some of the ammo-
nium moves by way of paracellular channels, whereas the majority substitutes for potas-
sium on the Na
+
/K
+
/2 Cl
–
pump, moving into the cell reliant on the energy consuming
effort of the basolateral Na
+
/K
+
-ATPase (20,21). At this point, some of the ammonium
is reabsorbed by the capillaries, and in doing so, effectively cancels any net acid–base
change; urea formation from ammonium by the liver consumes bicarbonate. The remain-
ing ammonium dissociates to NH
3
and H
+
in the renal medulla.
This transport by the TALH is the primary method of medullary concentration of
ammonia. Some to the ammonia diffuses back into more proximal segments where it is
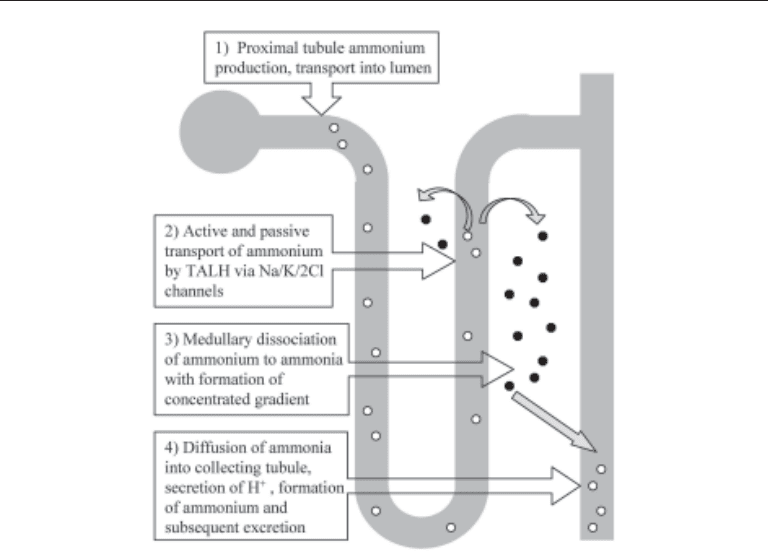
Chapter 8 / Renal Acid–Base Balance 129
again converted to ammonium and “cycles” through to the TALH. The medullary con-
centration gradient provides the driving force for ammonia diffusion into the cortical
collecting duct. Once in the distal luminal nephron segment, free H
+
, if present, can bind
to the ammonia, given the favorable pKa of the reaction, forming ammonium again. The
NH
4
+
is now “trapped” in the tubule because of the molecule’s polarity and lack of
luminal transport channels in the collecting duct. Excretion into the urine, along with
chloride or other anions to maintain electro negativity, is the final step in this aspect of
NAE.
C
OLLECTING DUCT IN NET ACID EXCRETION
The collecting ducts are responsible for the secretion of H
+
, and if ammonia is present,
will allow the formation of ammonium. In doing so, one new bicarbonate molecule is
effectively added back into the circulation. Bicarbonate, consumed by previous serum
buffering of endogenously produced acids, is now regenerated.
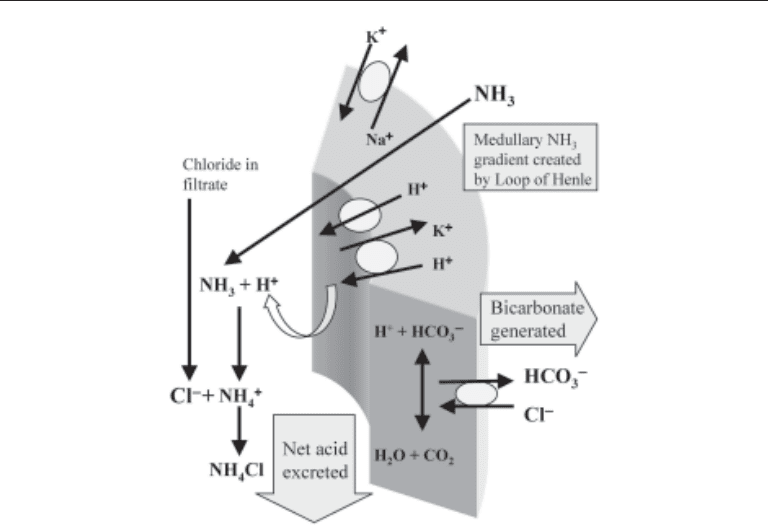
Collecting duct cellular mechanisms are highly specialized for this purpose and in-
volve a few important types of cells. There is varied distribution of these cell types
among the cortical collecting duct and the inner and outer medullary collecting ducts.
However, for purposes here, the collecting duct cell types will be generalized. Type A
intercalated cells (Fig. 4) have apical energy-requiring H
+
-ATPase as well as H
+
/K
+
-
ATPase. These pumps push intracellular protons out into the lumen to create an H
+
gradient. Intracellular carbonic anhydrase (CA2) is necessary for supplying these pro-
tons, and defects or deficiencies in this enzyme may lead to acidification defects to be
Fig. 3. The ammonia cycle.
130 Chin
discussed later. The formation of an H
+
gradient also requires an intact luminal mem-
brane to keep the protons from leaking back into the cell. The basolateral HCO
3
–
/Cl
–
exchanger (AE1 or band 3 protein) is important for the transport of the bicarbonate out
of the cell and into the capillaries. Recent discoveries have implicated defects in both the
proton pump and the AE1 as causes of inherited forms of distal renal tubular acidosis
(22,23). The importance of the H
+
/K
+
-ATPase is also being recognized as a potential
pathologic defect in some cases of distal renal tubular acidosis (RTA).
The β-intercalated cell is found in the collecting ducts as well. The arrangement of
transporters is inverse to that found on the α-intercalated cell, with mechanisms for
pumping HCO
3
–
out of the cell and H
+
into the circulation (Fig. 5). Currently there is not
a known pathologic defect in this cell to account for renal acidosis. Theoretically, over-
activity in the β-intercalated cell can create a RTA.
The principal cell is the second cell type found adjacent to intercalated cells in the
collecting ducts (Fig. 6). Although mainly responsible for sodium reabsorption and
potassium excretion, the principal cell influences proton secretion by its active sodium
reabsorption, which provides the electronegative luminal environment favoring H
+
secretion by the type-A intercalated cells. In other words, increased rates of distal
sodium absorption will facilitate distal proton secretion.
In summary NAE requires: (1) functional distal H
+
secretion, including an appropriate
lumen electronegativity; (2) functional CA2; (3) adequate urinary buffer (i.e., ammonia)
to bind the protons; and (4) intact distal luminal membrane to allow formation of a H
+
gradient. Defects in one or more of these steps result in either deficient NAE or problems
with distal urine acidification.
Fig. 4. α-Intercalated cell in the cortical collecting duct: ammonium excretion.
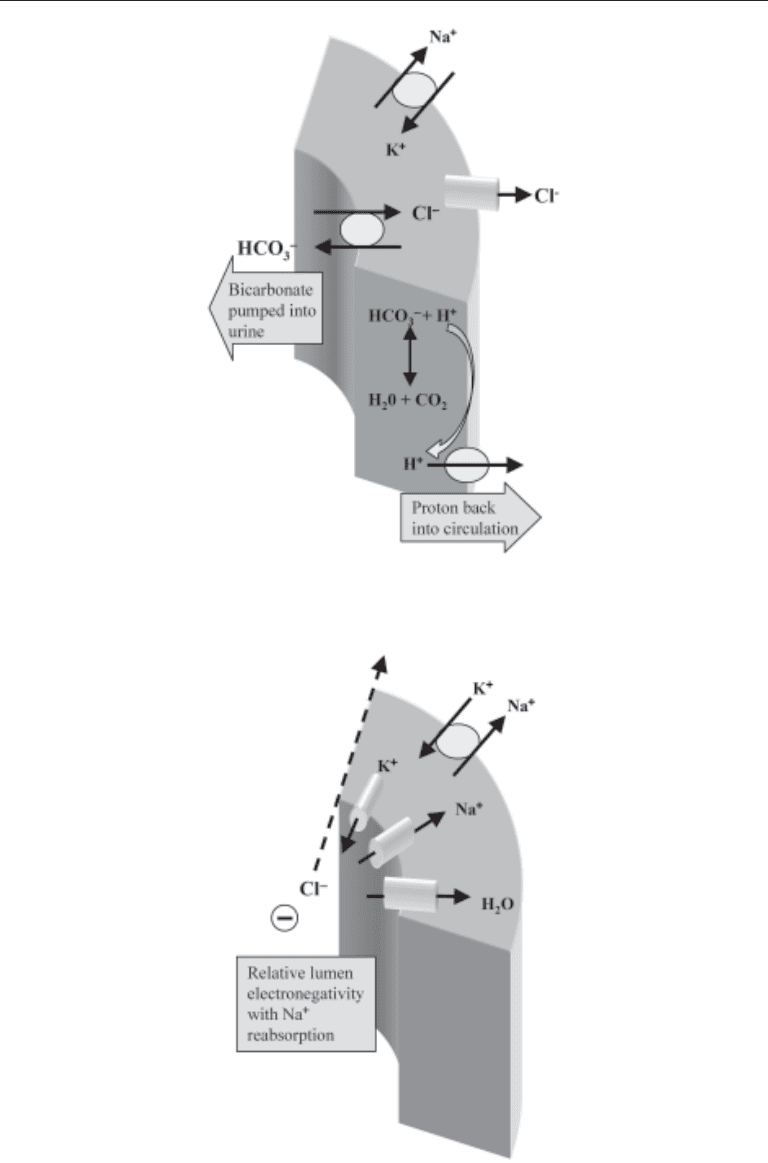
Chapter 8 / Renal Acid–Base Balance 131
Fig. 5. β-Intercalated cell in the cortical collecting duct: bicarbonate secretion.
Fig. 6. Principal cell in the collecting duct: sodium chloride reabsorption with luminal
electronegativity.

132 Chin
CONTROL OF NET ACID EXCRETION
Net acid excretion relies on the workings of many tubular segments: proximal tubule
for ammonia production, the TALH for medullary concentration of ammonia, and the
collecting tubule for H
+
secretion. Regulation of this process involves local, hormonal,
and neurological controls at each of these tubular segments.
Intracellular pH and potassium levels regulate proximal tubular ammoniagenesis. A
metabolic acidosis (but not a respiratory acidosis) with decreased intracellular pH will
stimulate ammonium production (24). Hypokalemia is a potent stimulator for proximal
ammonium production (25,26). On the other hand, hyperkalemia may not reduce ammo-
nium production, but may decrease ammonium transport from the proximal tubule to the
collecting duct (19,27). Increased dietary protein intake, which increases the amount of
acid to be excreted, will stimulate proximal tubule ammonium production (28). Aldos-
terone also plays a role in its positive control of ammoniagenesis. In addition, chronic
acidosis also increases the synthesis of transporters, such as Na
+
/H
+
exchangers in the
proximal tubule (29).
Intracellular pH and aldosterone levels influence distal regulation of proton secretion.
This is accomplished through mechanisms that affect transport kinetics, modulate mem-
brane transporters, and alter the number of transporters by inserting or removing them
from the membranes. First, the optimum environment for proton secretion is partially
reliant on the luminal electronegativity provided by sodium reabsorption in the principal
cells; this is under the control of aldosterone. Second, aldosterone can increase both
apical H
+
secretion as well as basolateral Cl
–
/HCO
3
–
exchange (30,31).
Intracellular pH of the cortical collecting ducts influences the pattern and number of
H
+
-ATPase in the membrane. Experimentally, there is an increase in intracellular cal-
cium with acidosis, causing increased exocytosis of intracellular vesicles containing
preformed H
+
-ATPase channels, via a calmodulin/microtubule system (32). In addition,
basolateral AE1 (Cl
–
/HCO
3
–
exchanger) protein synthesis may increase during aci-
demia, as evidenced by animal studies showing increased transporter mRNA in the
intercalated cells (33).
Other hormonal modulators of collecting duct acid–base control include vasopressin,
glucagon, prostaglandin E
2
, and calcitonin (34). There is also evidence that locally
produced mediators, such as nitric oxide (NO), may play a role in acid regulation by
affecting H
+
-ATPase activity (35).
URINARY PH AND ASSESSMENT OF ACID EXCRETION
Determinants of Urinary pH
Urinary buffers in normal conditions consist predominantly of inorganic anions and
ammonia. The inorganic anions, often referred to as titrateable acids, consist predomi-
nantly of phosphates. Under situations of increased acidemia, the inorganic anion avail-
ability can increase by about twofold. Ammonia production, on the other hand, can
increase 10-fold if needed to excrete a large acid lead.
Physiologic urinary pH ranges from 4.5 to 7.5, implying urinary [H
+
] ranging from
32 µmol/L to 32 nanomol/L. Urinary pH provides information only about the availability
of free protons in the urine. This amount of free H
+
, even at the lowest physiologic urine
pH, represents a trivial fraction of the total number of protons excreted to maintain acid–
base balance. The bulk of daily H
+
excretion occurs when protons are bound to the

Chapter 8 / Renal Acid–Base Balance 133
excreted urinary buffers. An isolated urine pH does not give information about the
adequacy of acid excretion or the availability of urinary buffers.
To illustrate this point, take the example of an alkaline urine pH (pH >6.0) with an
underlying metabolic acidosis. This scenario can be seen when excessive buffers are
delivered to the distal tubules and relatively more H
+
is bound to the buffer. This results
in a more alkaline urine pH even with intact distal tubular proton secretion. Clinically,
bicarbonaturia during the bicarbonate-losing stage of proximal tubular renal tubular
acidosis will give this set of findings. On the other hand, a defect in distal nephron proton
secretion with adequate buffer formation, as in classic or type I distal renal tubular
acidosis, will provide the same disturbances of acidosis and elevated urine pH, but
through a completely different set of defects. Conversely, an acidic urine pH (pH <5.5)
with a metabolic acidosis may be owing to inadequate urinary buffer formation and
delivery to the distal nephron, despite adequate distal nephron acidification. Here, net
acid excretion is impaired because of a lack of buffer. The various causes of type 4 RTA
with decreased ammoniagenesis are examples of this process. Thus, urinary pH in iso-
lation provides useful but limited information in discerning the underlying cause of renal
acid–base disturbance.
Assessment of Net Acid Excretion
Measurement of net acid excretion (NAE) relies on additional information beyond the
urine pH. NAE, which must be equal to net acid production to maintain homeostasis, can
be theoretically viewed as the difference between urinary excretion of acids and bases:
NAE = (phosphate + ammonium) – (bicarbonate + organic anions). Organic anions are
potentially important factors in NAE because the loss of these anions, such as β-hydro-
xybutyrate and citrate, is a loss of a potential bicarbonate source. Additionally, there is
evidence that the kidney will preferentially excrete nonorganic acids and limit organic
acid losses, such as citrate, when an acid load is placed on the system, in an attempt to
save these potential bicarbonate substrates (36). Therefore, some investigators include
the organic anion variable in their evaluation of NAE (4). In normal circumstances,
bicarbonate and organic anion losses are minimal, so the term can be simplified to: NAE
= phosphate + ammonium. The ability to increase phosphate in the urine is limited, and
with a large acid burden, it is the large increase in ammonium excretion that permits the
increased acid excretion. Therefore, measurement of urinary ammonium excretion would
be the most important assessment of NAE. Ammonium measurement is, unfortunately,
not conveniently available. Alternative methods must be used to indirectly estimate
NAE.
U
RINARY ANION GAP
One method of indirectly determining the adequacy of NAE is to calculate a Urinary
Anion Gap (UAG) using the concentrations of the primary urinary electrolytes: UAG
= [Na
+
] + [K
+
] – [Cl
–
]. The usual UAG is a negative number (usually < –10), indicating
that there is the presence of unmeasured cations, presumably ammonium. Conversely,
a positive UAG would signify inadequate ammonium excretion. This is illustrated in
Fig. 7.
There are limitations to the use of the UAG. In some situations, the finding of a
positive UAG does not indicate a decreased amount of ammonium being excreted. For
example, when other nonchloride anions, such as bicarbonate, ketoacids or some an-
