Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

Al(OH)
3
]. Most of the metals used in microelectronics are susceptible to electrolytic
dissolution.
Cathodic Alkalization (Cathodic Corrosion) This degradation mechanism
is specific to active/passive, amphoteric metals such as aluminum. In the absence
of chloride or other aggressive contamination, the leakage current through the
conductive path causes anodization (oxide buildup) at the anode instead of
localized dissolution. The potential drop across the oxide will consume part of the
applied potential and tend to reduce the leakage current. However, while current
is flowing, proton and water reduction reactions will take place at the cathodically
biased line, causing an increase in local pH by the following reactions:
2H
+
+ 2e
–
→ H
2
or 2H
2
O + 2e
–
→ 2OH
–
+H
2
. Because the metal is amphoteric,
the increase in pH results in dissolution of the protective oxide on the surface of the
cathodic line. The metal can then dissolve rapidly, even with a net negative current
at the surface. Analogous to the case of electrolytic dissolution at anodic lines, devices
fail by an open circuit, except now on the cathodic line (Fig. 4c). Similarly, a
corrosion product such as Al(OH)
3
will precipitate near the dissolution region.
Electrolytic Migration In this degradation mechanism, metallic ions dissolved
from the anode (electrolytic dissolution) are transported through the surface
electrolyte film and are electrolytically reduced at the cathode [45]. Metal
dendrites grow from the cathode toward the anode and lead to failure via a short
of the adjacent conductors (Fig. 4d). Important factors that affect dendritic
growth include (1) properties of the metal (some metals such as Ag and solder
readily electroplate with a dendritic morphology), (2) local current densities that,
if high enough, deplete cations in the surface electrolyte film in the vicinity of the
cathodic lines, and (3) mass transport–limited conditions that promote the
stability and propagation of dendrites. Notably, metals such as aluminum that
have a deposition potential that is outside the stability limit of water are not
vulnerable to this mechanism. Steppan et al. [45] listed the order of susceptibility
to electrolytic migration for many commonly used metals: Ag > Mo > Pb >
solder > Cu > Zn > bronze. A small volume of metal dissolved from the anode
can result in comparatively large dendrites, so the lines may short long before
an open would have formed in the anodic line [45]. As dimensions of micro-
electronics shrink, this failure mechanism becomes more important. Warren,
Wynblatt, and co-workers performed a series of studies on electrolytic migra-
tion of Cu [47–51]. Their work, which developed a good understanding of the
phenomenon, showed that contamination greatly accelerates electrolytic migra-
tion. The phenomenon of electrolytic migration should be distinguished from
another failure mechanism with a similar name, electromigration, which is not
electrochemical in nature [45].
Open Circuit (without Electrical Bias)
Although deleterious environmental interactions normally occur in microelectronics
under electrical bias, more traditional atmospheric corrosion mechanisms
certainly do take place in unbiased regions during the powered-off state and/or
during storage. As noted previously in this section, the power-off condition for ICs
may actually be more aggressive in some circumstances because a greater amount
of water may exist at the metal surface. Relevant mechanisms, with examples given in
654 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.
parentheses, include uniform attack (Cu contacts), pitting (Al lines and bondpads),
crevice corrosion (Al under wirebonds and under passivation layers), and intergranular
corrosion (Al bondpads). Under these unbiased conditions, chemical incompatibility
is the primary driver. Given the wide variety of metals that are present in intimate
contact with each other (e.g., Au/Al wirebonds), all of these mechanisms can be
enhanced in specific configurations by galvanic interactions. To help characterize the
potential galvanic effects, Griffin and co-workers [52] developed a galvanic series
specifically for the common thin-film metallization and barrier layers used in
microelectronic devices. The corrosion potential in 2000 ppm NH
4
C1 solution
decreased in the following order: Au-40%Pd, Au, Ag, Cu, Al-0.5%Cu-l%Si, CVD W,
W-10%Ti, Al-2%Cu-l%Si, Al-2%Cu, Al-0.4%Pd-l%Si, Si, Al-l%Si on CVD W, Al
on CVD W, Al on W-10%Ti, Al, Mg.
These traditional corrosion mechanisms are described in detail in standard
corrosion textbooks [53] and elsewhere in this book. However, care must be
exercised during the application of this conventional understanding to
microelectronics because of their very small dimensions. This caution can be
demonstrated by considering the atmospheric pitting of aluminum. A standard
technique for characterizing the atmospheric corrosion susceptibility of Al
involves a salt fog test (ASTM B117-90). An associated military specifications
(mil-spec) (Mil-C-5541E) states that no more than five pits can exist on a panel
and normal practice is not to count a pit that is less than 125 μm in diameter. As
noted previously, many IC feature are < 1 μm. Clearly, even small metastable pits in
the wrong place can be disastrous.
Device-Specific Corrosion Behavior and Concerns
Integrated Circuits
Aluminum Metallization Because of the predominant use of aluminum alloys
in integrated circuits and their vulnerability to atmospheric corrosion, the majority of
the environmentally induced problems that have historically occurred have involved
aluminum. As introduced in the section on contamination, corrosion has been
observed even during manufacture due to exposure to aggressive processing
chemicals. During the early phases of the microelectronic industry, yield loss from
in-process corrosion was a costly problem. Chemical and RIE processes have been
the most damaging. The preferred use of Al-Cu alloys exacerbates the problem
because Cu is enriched in the subsurface region during RIE due to the lower volatility
of Cu chlorides compared with Al chlorides [36]. At the high etching
temperatures, Al
2
Cu θ-phase particles precipitate and accelerate the subsequent
corrosion of the Al matrix. Several postetch process steps have been developed
to reduce corrosion, including CF
4
plasma cleaning, O
2
plasma cleaning, DI
water rinsing, wet etching, and heat treatments [30–36]. Brusic et al. [30] evaluated
the efficacy of several of these cleaning steps. By simply rinsing in water before
stripping the photoresist, the impurity level and associated corrosion rate were
reduced by more than two orders of magnitude compared with parts cleaned only
with an O
2
plasma. Elimination of CHCl
3
from the RIE gas in the last step resulted
in still lower corrosion rates and, unlike those with etching in CHCl
3
, the
properties did not degrade during subsequent storage in air. Any in-process
Corrosion of Data Storage Devices 655
Copyright © 2002 Marcel Dekker, Inc.
corrosion or staining that does take place on bondpads during manufacture can
adversely affect long-term device reliability because the integrity of the subsequent
wirebond can be reduced [54].
During service, biased Al metallization features most commonly fail at
defects or breaks in the passivation layer (upper right photograph in Fig. 4). If
chloride contamination is present, electrolytic dissolution occurs in the positive
(or anodically) biased lines [55–57]. As implied in this photograph, the production of
corrosion products causes more passivation layer defects and, in turn, more
corrosion. As with pitting corrosion of aluminum, the presence of a halide is very
important because it induces breakdown of the Al oxide film and permits rapid
dissolution. Biased Al metallization that is not exposed to chloride-contaminated
environments may still exhibit extensive corrosion and fail by cathodic corrosion
[58–64]. This phenomenon is promoted for structures having a top passivation
layer of phosphosilicate glass (PSG) with a high P content. The addition of P to
SiO
2
improves coverage by lowering the stress in the oxide and reducing cracks.
However, cracks along the sidewall and lack of coverage at steps and pinholes can
still occur. When PSG containing more than about 5% P is exposed to moisture,
phosphate dissolves to form a highly conductive surface layer. Cathodic corrosion is
promoted because of the high surface conductivity and the absence of chloride ions.
The anodic aluminum anodizes rather than dissolves. The activation energy for
cathodic corrosion has been found to be similar to that for dissolution of Al in an
NaOH solution [62]. Other ions besides phosphate can cause this form of corrosion,
even those that do not form a strong base [62]. The effect of P in PSG was identified
in the early 1980s and has been addressed by keeping the P content low or by using
silicon nitride passivation, which has been found to be more protective [65–67].
Even in the absence of an applied bias, galvanic-induced electrochemical
driving forces for corrosion can exist because unpassivated wirebonds often contain
couples of Al and Au [57,68]. In the presence of moisture and chloride contamination,
the galvanic driving force due to the Au can be sufficient to cause pitting
and inergranular attack of Al and eventually produce device failure. Several
researchers have studied the corrosion of Au/Al wirebonds [69,70]. Recent work
at Sandia National Laboratories has focused on galvanic corrosion under dormant,
unpowered storage conditions and has specifically been exploring the role of Al-Au
intermetallic compounds that form during encapsulant curing operations. This study
has shown that, under mildly accelerating conditions, unbiased Al bondpad corrosion
occurs only in the presence of an Au wire and that it initiates under the wirebond
and propagates along an Al/intermetallic interface (Fig. 5). Also, considerable
variability exists in the distribution and structure of the intermetallic phases (Fig. 5).
This latter finding suggests that the often-observed stochastic nature of corrosion
may actually be the result of manufacturing process variability. If the galvanic
influence of Au is not present, θ-phase particles in Al-Cu alloys can still initiate
pitting or intergranular corrosion on an unbiased surface via local galvanic action
[38,71,72]. Pits associated with θ phase may be limited in size but are large
enough to cause failure of micrometer-sized features.
Gold Metallization Anodically biased gold metallization can fail in the
absence of a contaminating salt by electrolytic dissolution if the ionic pathway
contains an effective gold complexing agent. The dissolution is accompanied by
656 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.
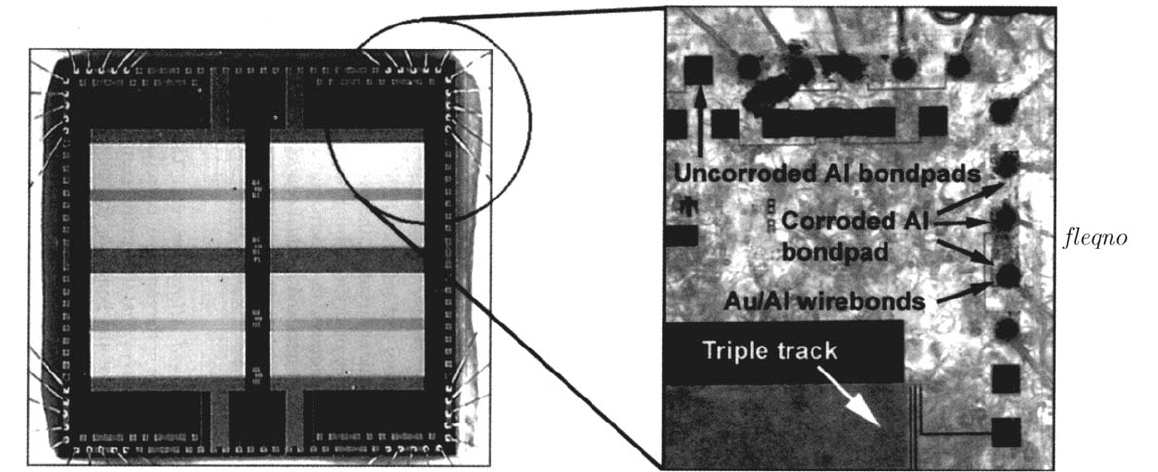
Corrosion of Data Storage Devices 657
Figure 5 Corroded aluminum-bondpad metallization layer taken under back-lit conditions. A photograph of the entire test device prior to any
corrosion is shown on the right. The photograph of the corroded structure shows the importance of galvanic interactions and the existence of
crevice corrosion (under the passivation layer) and was taken after underlying silicon and glass insulation layer were etched away.
Copyright © 2002 Marcel Dekker, Inc.
the formation of a voluminous Au(OH)
3
. However, examples of this type of failure
have not been widely reported [73]. In the presence of a halide contaminant, failure
can also result from electrolytic migration because gold metallization can form
dendrites [73]. Der Marderosian [74] found a humidity threshold below which no
migration of Au occurred. The threshold value was a function of the type and
amount of purpose applied halide salt contamination but ranged from about 30 to
50% RH.
Plastic Encapsulated Microelectronics Because the plastic encapsulant
materials are relatively permeable to water, chip metallization in PEM devices is
vulnerable to corrosion when exposed to or operated in humid environments. Nguyen
and colleagues [75–77] used an in situ capacitance monitor to study moisture
permeation in plastic packages [5]. The uptake and transport of moisture in epoxy
were Fickian in nature and exhibited a diffusivity of about 2×10
9
cm
2
/s, meaning that
water can penetrate a polymeric package and reach the metals on the die relatively
quickly. Moisture can also migrate along defects in the lead frame–polymer interface
and condense in undesirable voids near metallization surfaces. Because the bulk
permeability of the plastic to typical external contaminants is very low, these lead
frame defects appear to be a primary contaminant transport path. Historically, the
plastic encapsulants themselves have been a source of significant contamination, with
liquid/glob materials having higher corrosion-enhancing levels than the injection
molded compounds. Once sufficient moisture and contamination reach the IC
metallization and a void for adsorption/condensation exists, corrosion can occur and
degradation may ensue by any of the mechanisms described previously, including
electrolytic, pitting, galvanic, and crevice corrosion. It should be noted again that
top-level conductor lines are normally under passivation layers that, if impermeable,
would prevent corrosion [78,79]. Historically, defects such as cracks, pinholes, and
inadequate edge coverage were commonly present [55]. During the past 10–l5years,
processes and materials have improved to the point that passivation defects are no
longer a primary concern. Now, the unpassivated bondpads themselves are the most
susceptible.
In practice, the key factors that influence PEM corrosion vulnerability are
probably the defects in the plastic encapsulant, the level of leachable contaminant in
the encapsulant, and the degree of drying during power-on cycles. The first two
factors have been adequately addressed in modern, best commercial practice
devices to the point that passivation detects and residual contamination are
essentially inconsequential. Pecht and co-workers [9,29] have now concluded that
modern, properly fabricated PEM devices are very reliable under a variety of service
conditions.
Ceramic Hermetic-Packaged Microelectronics The meaningful differences
between CHP and PEM devices in the context of corrosion are the following: (a) Al
wires are used instead of Au so that the prime corrosion vulnerability is the
ultrafine 25-μm wire instead of the bondpad, (b) the internal environment can
theoretically be controlled such that a benign environment will always exist, and
(c) the cavity containing the die is unfilled so encapsulation defects are not needed
as sites for water condensation. To achieve the environmental control advantage,
three factors must be satisfied: (a) a hermetic seal, (b) a clean and dry assealed internal
658 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.
environment, and (c) control of outgassing from internal materials. If they are
properly designed and manufactured, the reliability of CHP devices is not a concern.
However, in practice, all three of these aspects have produced problems as evidenced
by field-returned “hermetic” packages in which corrosion has occurred [80–82].
The specifications on hermetic seals are not always adequate to ensure total
isolation from the environment for extended periods of time. For example, as noted by
Pecht and Ko [83], hermeticity is defined by a maximum leak rate (e.g., 10
–7
atm cc/s).
If the device leaks at this particular maximum rate, the critical moisturecontent (three
monolayers of water) can diffuse through the seal in as little as 2500 h. Supporting this
finding is a study in which 20% of field failures of equipment sited in a tropical envi-
ronment were due to corrosion of interconnects in packages that met the hermeticity
specification [17]. Also, the seals and/or ceramic bodies can crack and create larger
leaks during handling, soldering, or qualification thermal cycling, which permits both
water and contamination to enter easily. If care is not exercised during fabrication,
moisture adsorbed on the inside walls of the package can desorb during subsequent
processing steps or during use. To address this possibility, mil-spec CHP parts are nor-
mally sealed in an atmosphere containing no more than 5000 ppm of water, ensuring
that no liquid phase will exist at temperatures down to 0°C, where ice forms. The seal-
ing glass used in some packages or a popular organic die-attach material can actually
be sources of moisture. Devitrifying glass is one type of sealant with a high moisture
content that is evolved during closure, whereas vitreous glass contains little moisture
and can result in a ceramic package with less than 500 ppm moisture in the cavity [84].
Moisture trapped inside a sealed cavity can leach ions from the sealing glass or other
sources to form a conductive electrolyte. Once an ionic path exists between conduc-
tors, corrosion and failure by any of the mechanisms described previously can ensue.
Finally, one researcher found that residual stresses in Al-containing wirebonds could
be an important factor in failure (e.g., by stress-induced corrosion) [85].
Macro Interconnects
Solder, copper conductors, and plated-copper connectors constitute the major metallic
components of second-level packaging for functional electronic circuits. These
metals are all susceptible to corrosion. Typically, interconnects are less protected and
more exposed to ambient environments. However, their dimensions are much larger
than those of chip metallization and more corrosion can therefore be tolerated before
failure is produced.
Solder PbSn alloys, ranging from Pb-rich to Sn-rich compositions, are the
most solder materials used in electronic applications. Pure Sn forms a protective
oxide film, but Pb forms an oxide that is not stable and can easily react with
chlorides, borates, and sulfates [86]. Frankenthal and Siconolfi [87,88] found that
both Sn- and Pb-rich PbSn alloys form an Sn oxide, most likely SnO, during the
initial exposure of oxide-free surfaces to oxygen. Lead is oxidized on the surface
of PbSn and a mixed oxide results only after all the metallic Sn is totally depleted
from the surface. Similarly, the corrosion product formed on Pb-50In solder during
exposure to an aggressive gaseous environment was found to be rich in In [89].
Not surprisingly, the corrosion resistance of PbSn in various aqueous solutions
and gaseous environments depends strongly on the alloy composition, improving
greatly as the Sn content increases above 2wt% [86].
Corrosion of Data Storage Devices 659
Copyright © 2002 Marcel Dekker, Inc.
In the presence of moisture and contamination, the most common degradation
mechanism of solder is electrolytic migration (shown previously in lower right
photograph in Fig. 4). Thus, the cleanliness of fabrication steps and the effective
removal of flux residues are critical factors. Some IC package types (e.g., surface
mount) are very hard to clean effectively. Manko [43] summarized the corrosion
behavior of PbSn solders in various chlorides that are typically used as activators in
fluxes and noted their deleterious effect in destroying the native passivating oxide
layers. He also noted that corrosion related to flux use might result from flux or flux
residue that is trapped in inaccessible locations or from fumes liberated during
soldering and subsequent condensation in uncleaned regions. Specifically, water-
white rosin flux has been found to leach Sn from the solder and therefore decrease its
corrosion resistance [90]. Soldering traditionally uses nonaqueous fluxes that require
cleaning with Freon-based solvents. In an effort to reduce chlorofluorocarbon
usage, water-soluble fluxes have been substituted in some applications. Cleaning of
water-soluble fluxes can normally be accomplished in deionized water. Sn-Pb-In
solders corrode in water-soluble fluxes containing chloride-based activators but are
not attacked in fluxes containing phosphate-based activators [91]. Finally, in-process
corrosion can result in poor quality solder joints or decreased thermal fatigue life.
This latter result is especially significant for C4 solder connections [90].
Copper Conductors Although precautions are taken to protect the copper
conducting lines in printed wiring boards from environmental exposure, defects exist
and corrosion does occur under many atmospheric conditions. For most
environments that are encountered, copper is not thermodynamically stable.
However, its native cuprous oxide surface film does offer some limited protection.
Atmospheric corrosion of copper is briefly described in the chapter on atmospheric
corrosion. Examples of typical industrial atmospheric pollutants that are harmful to
copper include SO
2
, H
2
S, COS, NO
x
, Cl
2
, and CO
2
.
Contamination of copper surfaces with atmospheric particulate matter can
accelerate the corrosion process. For example, the corrosion of copper in 100°C
air in the presence of submicrometer ammonium sulfate particles has been found
to depend strongly on RH [40]. Below the critical RH, Cu
2
O formed uniformly on
the Cu surface. At the critical RH of 75%, Cu
4
(SO
4
)(OH)
6
formed in the region
where the (NH
4
)
2
SO
4
particles had been deposited. At 85% RH, a thick corrosion
product covered the entire surface. Particulate contamination such as this is typically
believed to be an issue during manufacturing. However, it can also cause field
failures in electronic installations, such as telecommunications centers [92].
The formation of conductive anodic filaments (CAFs) has been observed
during accelerated aging testing to cause failure in epoxy-glass printed circuit
boards containing copper conductors, a process clearly related to electrolytic
dissolution [93]. Here, a conductive ionic path consisting of precipitated corrosion
products presumably forms between the oppositely biased conductors. These fibres
grow from the corrosion-producing anodic line to the cathodic line. The degradation
was found to be most severe between two nearby through-holes. Because CAF has not
been observed in field-returned parts, it may be only an artifact of the test conditions.
Au-Plated Connectors and Contacts As noted earlier, the subtrates of
connectors and contacts are normally fabricated from copper or copper alloys and
660 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.
then plated with a noble metal. The presence of the thin plating that almost always
contains some level of physical defects (e.g., cracks and pores) enhances the
corrosion susceptibility of the substrate and enables other processes to take
place. Slade [22] and Abbott [23] recently published a comprehensive review of
connector/contact corrosion. One form of observed degradation occurs when
corrosion products creep across a noble metal surface. When Au-plated Cu is
exposed to a sulfide-containing atmosphere, a tarnish film composed of copper
oxide and copper sulfide forms in the plating pores and cracks where the Cu layer
is exposed [94,95]. With time, a predominately copper sulfide tarnish film migrates
over the Au surface, resulting in increased contact resistance. This phenomenon,
termed creep corrosion by Tierney [94], is becoming increasingly important as the
thickness of the gold plating is reduced for economic reasons and as the edges of
exposed Cu become closer to contact points as a result of miniaturization. A second
relevant degradation mode is often referred to as pore corrosion and simply consists
of accelerated corrosion of locally exposed regions of the substrate. Two important
processes are probably responsible: (a) capillary condensation of water in cracks,
pores, and between mated surfaces and (b) galvanic interactions with a potentially
large cathode-to-anode area. Ming et al. [14] showed that contact force and
electrical loading can also affect corrosion. For example, contact force can
improve performance in stationary contacts and applied voltage can accelerate
growth of surface films and decrease contact service life. Finally, in recent years, a
few special contact lubricants have been developed that can also inhibit corrosion [23].
Product Qualification/Reliability Testing and Analysis
A significant effort has been made over the past quarter century to develop effective
techniques to test and assess the effect of corrosion on the performance and life of
electronic devices. Such techniques are desired for two specific uses: (a) as a means
of rapidly characterizing product quality during manufacturing (acceptance testing)
and (b) to provide customers and users with an accurate estimate of expected service
life. In this context, corrosion degradation is primarily characterized in terms of
reliability, which is the probability that a device will not perform its intended
function (i.e., electrically fail). Such device-level studies are required because the
direct measurement of corrosion is very difficult or even impossible in actual
operating environments using standard corrosion characterization techniques (e.g.,
electrochemical and gravimetric). A device manufacturer wants to be certain that a
product will have at least a certain functional lifetime in service with a minimum
number of failures. A typical historical requirement is 100 failures in 10
9
device hours
(100 FIT) or 0.01% failure in 1000 hours [96]. In modern practice, most electronic
devices are expected to function reliably for at least 10 years [17], although
obsolescence can certainly set in much sooner. Therefore, reliability engineers are
forced to perform accelerated aging exposures on large numbers of parts. For general
reference, Nelson [97] has published a comprehensive overview of accelerated
aging, and Osenbach [17] describes factors and issues associated with accelerated
aging of microelectronics. A clearly recognized deficiency of the reliability
approach is that such tests do not permit a fundamental understanding of the corrosion
mechanisms and processes to be easily identified. Truly predictive reliability
Corrosion of Data Storage Devices 661
Copyright © 2002 Marcel Dekker, Inc.
assessments must have this type of physical basis. Nevetheless, accelerated reliability
testing is of tremendous practical importance because susceptible materials, design
flaws, and processing problems can be identified in a timely manner and a qualitative
understanding of the degradation processes can be generated.
The underlying principle behind the use of accelerated aging is that the
functional behavior of real devices or the response of test structures with similar
materials and dimensions can be characterized using various combinations
of environmental stress factors that include temperature, humidity, bias, and
contamination. Each of these variables is typically used at levels more severe than
actual operating conditions. Osenbach also notes that all of the accelerated aging
strategies include two significant assumptions: (a) the parameters that characterize
failure under high-stress conditions (where failure occurs in short time periods of
days to months) can be extrapolated to actual use conditions where failure occurs
after years of exposure, and (b) the test population is representative of the entire
population. This section contains brief descriptions of (a) the aging techniques
commonly being used, (b) resultant models of acceleration factors, and (c) the
concerns and potential deficiencies associated with this subject.
Techniques
Microelectronics Peck and Zierdt [98] led the way for adopting elevated
humidity and temperature as the primary stress factors. The most common
accelerated test used over the past 25 years is referred to as THB (temperature,
humidity, and bias). In this test, parts are exposed for extended periods (> 1000 h)
at 85°C, 85% RH, wih the conductor lines biased at, or sometimes above, the
operating voltage [68,99]. As PEM device reliability improved during the 1980s
due primarily to a reduction in residual chloride contamination and molding
compound cleanliness, exposure times under THB conditions required to generate
failures started approaching 10,000 h. In order to formulate predictive relationships,
failure during accelerated testing must be observed. The extremely long times
needed in conventional THB tests led to the development of tests with more severe
conditions to reduce needed test time. A pressure-cooker test at 100% RH and
temperatures above 100°C has been used for this purpose [99–102]. Its efficiency
can be demonstrated by the observation that for one specific device, results
equivalent to those obtained in 1000 h at 85°C, 85% RH could be produced in 20 h
at 140°C, 100% RH [99]. However, artifacts associated with condensation and
difficulties in applying voltage in saturated atmospheres complicated widespread
adoption of this procedure. Because of attractive equipment cost and availability,
many manufacturers still use this type of testing and the standard conditions are
121°C, 100% RH and no bias. More recently, the highly accelerated stress test
(HAST) has become the standard. HAST tests typically use temperatures up to
150°C and humidity levels less than 100% [63,103,104]. Several other variations
of the standard THB aging techniques have been developed that involve additions
of internal and external contamination and/or cyclic application of the environmental
parameters to better simulate actual use [28,105–109]. Failure analysis techniques
to supplement the reliability information have also been reported [110].
Although most accelerated aging studies are performed on actual functional
devices and the time to failure is directly measured, some studies have used test
662 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.
structures designed specifically to better address particular degradation modes.
For corrosion under electrical bias, common configurations include two interdigitated
combs, a triple track with three parallel but meandering lines, and combinations of
tracks and bondpads/wirebonds [8,65,66,103,111,112]. Example of triple-track
structures are shown in Figure 6 [8]. The two outer track conductors are normally
biased positively and the center one is negative or grounded. The portion of the
triple-track structure in Figure 6b is contained within the test device previously
shown in Figure 5. To assess wirebond degradation, the design permits conventional
four-point resistance measurements to be made. Such test devices can be tested
bare or as packaged.
Macro Interconnects Because connectors and contacts are usually exposed
without protection to the environment during operation, testing typically has been
performed in the context of atmospheric corrosion [113]. Various forms of accelerated
tests for indoor office environments have been developed for this purpose
[26,114–117]. For example, procedures have been developed at IBM [the G1(T)
test] [26,114,117], Battelle Institute (Flowing Mixed Gas Class II or FMG II)
[23,115,116], and in Europe by the International Electrotechnical Commission
(IEC Test 68-2-60) [118] for simulating an indoor office environment. These
techniques vary slightly but involve exposure to an atmosphere containing a
combination of dilute pollutants such as H
2
S, SO
2
, NO
2
, and Cl
2
and humidity
(Table 1) and are usually performed at near-ambient temperatures because of
complications arising from reactions between the various components at higher
temperatures. Acceleration factors from 10 to 1000 are possible depending on the
chosen conditions [115]. These aging environments are now used to test and qualify
many electronic parts other than connectors and contacts, including printed circuit
boards. The reliability of PCBs is also routinely tested using THB testing as discussed
in the previous paragraph.
Mathematical Relationships for PEM Aging
When plastic-encapsulated microelectronic devices are exposed to aggressive
environments, there is often a bimodal distribution in the time to fail resulting from
Corrosion of Data Storage Devices 663
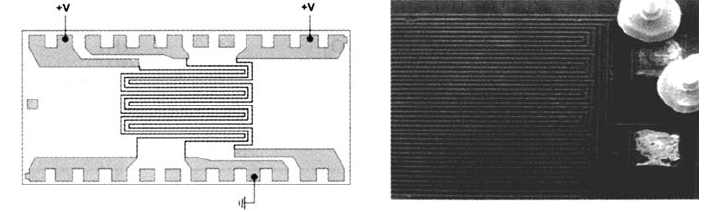
Figure 6 Schematic diagram (left) and an SEM photograph (right) of two triple-track test
structures that are used to study electrolytic corrosion mechanisms along with the
effectiveness of passivation layers. The structure shown on the right was encapsulated and
then exposed to HAST conditions until failure. This particular structure is part of the integrated
test device shown previously in Figure 5. These test devices contain eight triple-track sections
(the left set with windows in the passivation layer) and exposed wirebond pads.
Copyright © 2002 Marcel Dekker, Inc.
