Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

133. M. Walsh and R. Mitchell, Microbial influence on hydrogen uptake by metals,
Proceedings Biologically Induced Corrosion (S. C. Dexter, ed.), NACE-8, Houston,
1986, pp. 201–208.
134. D. Walsh, J. Seagoe, and L. Williams, Microbiologically influenced corrosion of
stainless steel weldments; attachment and film evolution, CORROSION 92,
Nashville, paper 165, NACE, 1992.
135. O. Wanner, Modelling of biofilms. Biofouling 10(1–3):31–41 (1996).
136. B. J. Webster, R. J. Kelly, and R. C. Newman, The electrochemistry of SRB corro-
sion in austenitic stainless steel, Proceedings Microbially Influenced Corrosion and
Biodeterioration (N. J. Dowling, M. W. Mittleman, and J. C. Danko, eds.),
University of Tennessee, Knoxville, 1990, pp. 2/9–2/18.
137. B. J. Webster, R. J. Kelly, and R. C. Newman, SRB-induced localized corrosion of
stainless steels, CORROSION 91, Cincinnati, OH, paper 106, NACE, 1991.
138. B. J. Webster, D. B. Wells, and P. J. Bremer, Potable water biofilms, copper corro-
sion and copper by-products release, Proceedings 13th International Corrosion
Congress, Melbourne, 1996, paper 408.
139. D. C. White, A. A. Arrage, D. E. Nivens, R. J. Palmer, J. F. Rice, and G. S. Sayler,
Biofilm ecology: on-line methods bring new insights into mic and microbias
biofouling. Biofouling 10(1–3):3–16 (1996).
140. F. Widdel, Mikrobielle Korrosion, Jahrbuch Biotechnologie, Band 3 (P. Präve, M.
Schlingmann, W. Crueger, K. Esser, R. Thauer, and F. Wagner, eds.), Carl Hanset,
Munich, 1990, pp. 277–318.
141. K. M. Wiencek and M. Fletcher, Effects of substratum wettability and molecular
topography on the initial adhesion of bacteria to chemically defined substrata.
Biofouling 11(4):293–311 (1997).
142. J. H. Wolfram, R. D. Rogers, and B. J. Buescher, A summary of microbias influ-
enced corrosion in nuclear power plants, Proceedings of the 1988 ASME Pressure
Vessels and Piping Conference, Pittsburgh, 1988, pp. 99–23.
Microbially Influenced Corrosion 603
Copyright © 2002 Marcel Dekker, Inc.
17
Corrosion in Nuclear Systems:
Environmentally Assisted Cracking in
Light Water Reactors
F. P. Ford and Peter L. Andresen
General Electric Company, Schenectady, New York
INTRODUCTION
The phenomena of initiation and subcritical propagation of cracks in structural
materials due to the conjoint actions of stress, material microstructure, and
environment have been recognized for many years, and the mechanisms have
been extensively investigated. This is especially the case for stressed alloys in
environments containing high anionic concentrations of e.g., chlorides, phosphates,
and hydroxides, either in the bulk environment (e.g., in the paper, chemical,
petrochemical, and marine industries) or in localized environments where boiling or
gradients in electrochemical potential, temperature etc. can concentrate species.
However, it has been recognized that environmentally assisted cracking under static
or cyclic loading (i.e., stress corrosion or corrosion fatigue) can occur in
ultrahigh-purity water even when the concentration of non-OH
–
anions is < l0 ppb.
Although the cracking susceptibility is generally lower than in the concentrated
environments, it is sufficient to cause concern when extended lives or high
levels of plant availability are required, as in the power generation industry and
especially for light water reactors (LWRs). The objective of this chapter is to
discuss the development and use of mechanistically based models of
environmentally assisted cracking of structural materials in highpurity water, with
special emphasis on boiling water reactors (BWR) and occasional reference to
pressurized water reactors (PWR).
THE PRACTICAL PROBLEM AND THE PROPOSED SOLUTION
There have been well-documented instances of environmentally assisted cracking in
high-temperature water of austenitic stainless steels, nickel-base alloys, low-alloy and
carbon steels, and their weld metals in various subcomponents of pressure vessels,
pressurizers, steam generators, piping, deaerators, etc. Consequently, there is a
strong driving force to derive design and life prediction codes which can account for
605
Copyright © 2002 Marcel Dekker, Inc.
the multitude of material, stress, and environmental combinations relevant to these
systems. This requirement has posed a difficulty for the experimentalist, since it
is not easy to control adequately the system conditions (e.g., water purity, loading)
and to measure crack propagation rates with a sensitivity that is relevant to a
typical 40-year design life. As a result, the current life prediction codes for
environmentally assisted cracking of structural materials in high-temperature
water usually represent upper bounds of the available data base and do not account
for the wide range of material and environment conditions that are possible for the
“nominal” system. This conservative “upper-bound” scenario can put unreasonable
constraints on the continued operation of specific plants which are operating under
far different system conditions than those represented by the design or life evaluation
code. In other cases, life prediction codes do not exist either because of wide scatter
in the data (e.g., stress corrosion of low-alloy pressure vessel steels) or because of
lack of relevant data (e.g., irradiation-assisted cracking of stainless steel).
This unsatisfactory situation is offset, however, by the increase in mechanistic
understanding of environmentally assisted cracking, which has accelerated in the
last decade because of the availability of experimental and analytical procedures
that allow quantification and validation of various cracking hypotheses. The
ultimate validation of any such mechanistically based model is its ability to
predict the extent of crack penetration as a function of the wide combination of
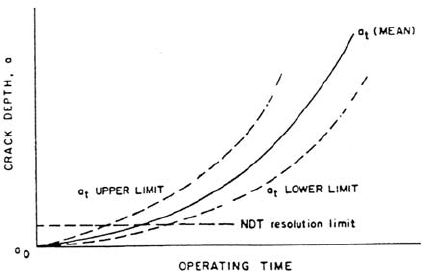
the material, stress, and environment factors relevant to LWR operation (Fig. 1).
Since the precise shape of the crack depth–operational time curve in Figure 1
must be a function of the specific material, environment, and stressing conditions, it
follows that if there is a range in the actual system conditions, there will be a
predictable range in the observed data, with the distribution of the range in cracking
mirroring the distribution of system conditions which affect the propagation process.
This provides a bridge between deterministic and statistical or probabilistic life
prediction methodologies [1,2].
606 Ford and Andresen
Figure 1 Schematic crack depth–time relationships for a range of relevant material,
environment, and stress conditions.
Copyright © 2002 Marcel Dekker, Inc.
CANDIDATE CRACKING MECHANISMS
Historically, environmentally assisted cracking has been divided into the “initiation”
and “propagation” periods. To a large extent this division is arbitrary, for in most
investigations initiation is defined as the time at which a crack is detected, or when
the load has relaxed a specific amount (in a strain-controlled test); in these cases such
a definition of initiation corresponds to a crack depth of significant metallurgical
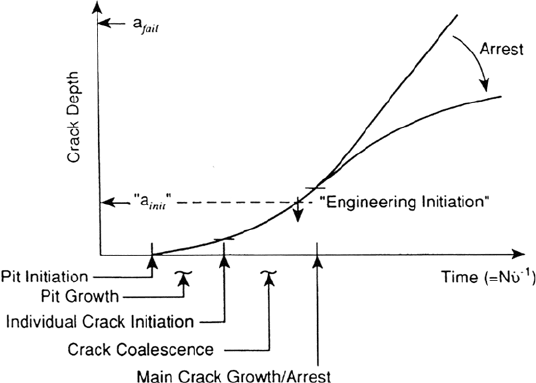
dimensions (e.g., ≥2 mm). Thus, for the purpose of lifetime modeling it is assumed
(Fig. 2) that, phenomenologically, initiation is associated with microscopic crack
formation at localized corrosion or mechanical defect sites generally associated with
pitting, intergranular attack, scratches, weld defects, or design notches. If it is further
assumed that the probability of such initiation sites existing or developing relatively
early in the life of the component is high, then the problem of life prediction devolves
to understanding the growth of small cracks from these geometrically separated
initiation sites and the coalescence of these small cracks to form a major crack, which
may then accelerate or arrest depending on the specific material, environment, and
stress conditions.
There has been relatively little fundamental work on the growth of such
microscopically short cracks. For brevity, this will not be reviewed in detail, apart from
mentioning that most work has been done under fatigue loading [3–7], with emphasis
on the microstructural interactions required for microscopic crack arrest or
propagation and modifications to linear elastic fracture mechanics analyses to account
for the observed behavior in this crack size range. In the area of environmentally
assisted cracking, the coalescence of microscopically small cracks has been
investigated for carbon steels in carbonate-bicarbonate solutions [8,9] and stainless
steels and nickel-base alloys [10] in high-temperature water. In these cases it is observed
that the crack growth rate increases as the small cracks coalesce and approaches a
Corrosion in Nuclear Systems 607
Figure 2 Proposed sequence of crack initiation, coalescence, and growth for steels
undergoing subcritical cracking in aqueous environments.
Copyright © 2002 Marcel Dekker, Inc.
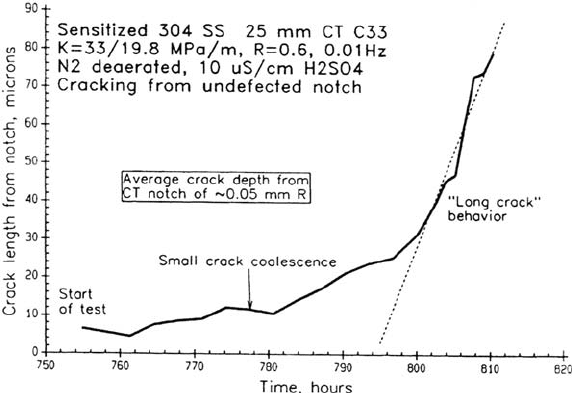
steady-state value when the mean crack depth is about 20 to 50 μm (Fig. 3);
thereafter the crack propagation rate may be analyzed in terms of linear elastic
fracture mechanics normally applicable to “deep” cracks. Thus, attention can be
focused on current mechanistic knowledge of crack propagation rates for deep
cracks (e.g., ≈1 grain diameter and greater), recognizing that the resultant life
prediction may be conservative because the microscopic crack initiation and crack
coalescence periods are not accounted for.
The basic premise for all of the proposed crack propagation mechanisms for
ductile alloys in aqueous solutions is that the crack tip must propagate faster than
the corrosion rate on the unstrained crack sides so that the crack does not degrade
into a blunt notch [11,12]. Based on this criterion, the material-environment
conditions for cracking can be defined based on the thermodynamic criteria for the
existence of a protective oxide, salt, or compound film on the crack sides. For
instance, cracking susceptibility of mild steel in hydroxide, carbonate-bicarbonate,
nitrate, phosphate, and molybdate solutions can be predicted to occur in the specific
potential-pH ranges where the protective film is thermodynamically stable [13].
Very similar thermodynamic arguments may be made for other systems, (e.g.,
brassammoniacal solutions [14]), and may be extended to kinetic arguments [15–17]
which require that the electrochemical reaction rates (e.g., dissolution or oxidation)
at the strained crack tip be significantly higher than those on the crack sides for an
“electrochemical knife” [15] to propagate. Indeed, the suppression of stress corrosion
and corrosion fatigue in many systems may be explained in terms of chemical
blunting of cracks during the early propagation stage. The cracking of mild and
low-alloy steels in aqueous environments offers ideal examples of this criterion.
For instance, low-alloy steels will not exhibit stress corrosion in acidic or concentrated
chloride solutions unless the general corrosion-blunting effect is counteracted with
608 Ford and Andresen
Figure 3 Crack depth–time relationship for intergranular cracks initiating, coalescing,
and growing in a notched 1T CT specimen of sensitized stainless steel in 288°C water.
(From Ref. 10.)
Copyright © 2002 Marcel Dekker, Inc.
chromium or nickel alloying additions [13,18]. Similarly, in high-purity water
systems, carbon steel will not undergo stress corrosion cracking in low-temperature,
oxygenated environments, since the embryo crack will be blunted by pitting;
however, at temperatures >150°C, cracking is possible because of the protective
nature of magnetite, Fe
3
O
4
[19], which constitutes the inner film of the duplex
surface oxide.
For systems in which chemical blunting is not an issue, numerous crack
propagation mechanisms were proposed in the period 1965–1979 [20–31], ranging
from preexisting active path mechanisms, to strain-assisted active path mechanisms,
to mechanisms that depend on various adsorption-adsorption phenomena (e.g.,
hydrogen embrittlement mechanisms). There was considerable debate concerning
the dominant mechanism in a given system, promulgated in part by the fact
that up to 15 years ago there were few analytical techniques to test the various
hypotheses quantitatively. Parkins [32] pointed out early on, however, that it was
likely that there was a “stress corrosion spectrum” which logically graded the
cracking systems between those that were mechanically dominated (e.g., hydrogen
embrittlement of high-strength steels) to those that were environmentally dominated
(e.g., preexisting active path attack in the carbon steel–nitrate system). Indeed, it
was suggested that two mechanisms may operate in one alloy-environment system
with a dominant mode being determined by relatively small changes in the material,
environment, or stressing condition. This was followed by the suggestion (e.g.,
[1,11,32–34] that a similar spectrum of behavior occurs between constant load
(stress corrosion), dynamic load (strain induced cracking), and cyclic load (corrosion
fatigue) conditions.
With the advent in the last 15 years of more sensitive experimental and
analytical capabilities, many of the earlier cracking hypotheses have been shown
to be untenable, and the candidate mechanisms for environmentally assisted crack
propagation (stress corrosion and corrosion fatigue) have largely been narrowed
down to slip dissolution, film-induced cleavage, and hydrogen embrittlement.
These mechanisms are briefly described below, followed by a discussion of the
development of the slip dissolution model for life prediction for austenitic
stainless steels in BWR coolant. Modifications to the basic model to explain
changes in cracking susceptibility due to (a) irradiation for reactor core components,
(b) material (e.g., nickel-base or low-alloy alloys), and (c) environment (e.g., BWR
vs PWR) are then outlined.
Slip Dissolution Mechanism
Various crack advance theories have been proposed to relate crack propagation to
oxidation rates and the stress-strain conditions at the crack tip, and these theories
have been supported by a correlation between the average oxidation current
density on a straining surface and the crack propagation rate for a number of
systems [12,35]. There have been various hypotheses about the precise atom-atom
rupture process at the crack tip—for example, the effect that the environment has
on the ductile fracture process (e.g., the tensile ligament theory [36], the increase
in the number of active sites for dissolution because of the strain concentration
[37], the preferential dissolution of mobile dislocations because of the inherent
chemical activity of the solute segregation in the dislocation core [38]).
Corrosion in Nuclear Systems 609
Copyright © 2002 Marcel Dekker, Inc.
Experimentally validated elements of these earlier proposals have been
incorporated in the current slip dissolution model, which relates the crack
propagation to the oxidation that occurs when the protective film at the crack tip is
ruptured [39–44]. Different types of protective films have been proposed, including
oxides, mixed oxides, salts, or noble metals left on the surface after selective
dissolution of a more active component in the alloy. As discussed below, quantitative
predictions of the crack propagation rate via the slip dissolution mechanism are
based on the faradaic relationship between the oxidation charge density on a surface
and the amount of metal transformed from the metallic state to the oxidized state
(e.g., MO or M
+
aq
).
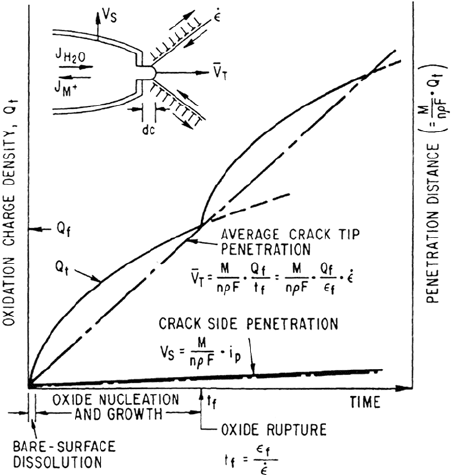
The change in oxidation charge density with time following the rupture of a
protective film at the crack tip is shown schematically in Figure 4. Initially, the
oxidation rate and, hence, crack advance rate will be rapid, typically controlled by
activation or diffusion kinetics as the exposed metal dissolves, although availability
of the balancing cathodic reduction current is also clearly necessary. However, in
most LWR cracking systems, a protective oxide re-forms at the bared surface and
the rate of total oxidation (and crack tip advance) decreases with time. Thus, crack
advance can be maintained only if the film rupture process is repetitive. Therefore,
for a given crack tip environment, corrosion potential, and material condition, the
crack propagation rate will be controlled by the change in oxidation charge density
with time and the frequency of film rupture at the strained crack tip. This latter
parameter will be determined by the fracture strain of the film, ε
f
, and the strain rate
at the crack tip,
⋅
ε
ct
. By invoking Faraday’s law, the average environmentally
610 Ford and Andresen
Figure 4 Schematic oxidation charge density–time relationship for a strained crack tip
and unstrained crack sides. (From Ref. 11.)
Copyright © 2002 Marcel Dekker, Inc.
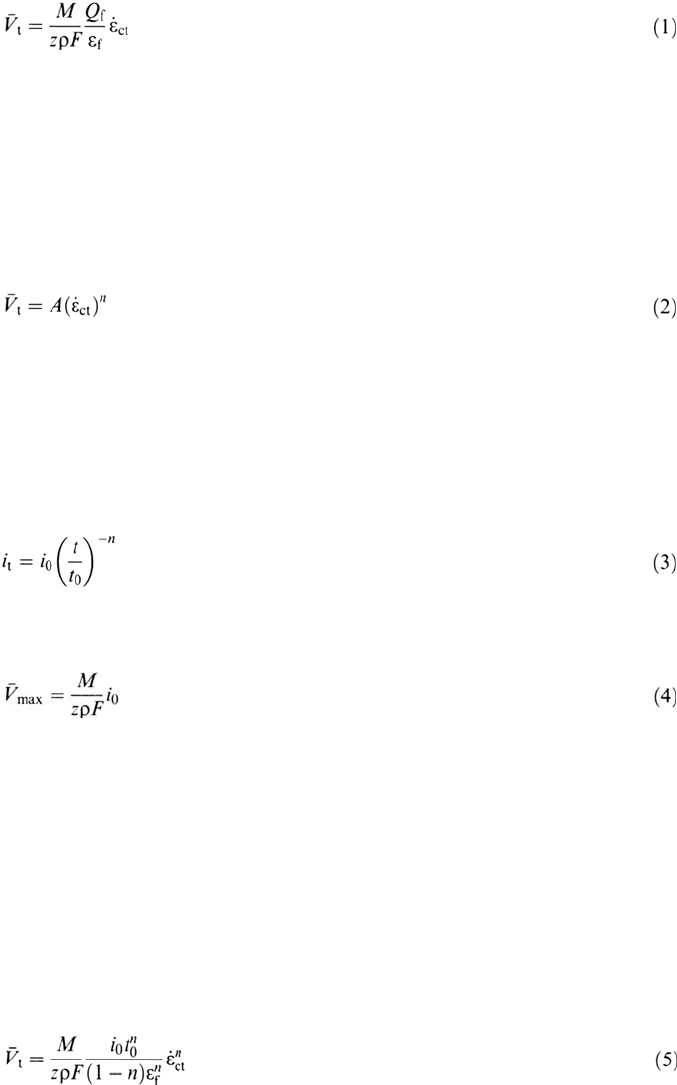
controlled crack propagation rate,
–
V
t
, can be related to the oxidation charge density
passed between film rupture events, Q
f
, and the strain rate at the crack tip,
⋅
ε
ct
:
Corrosion in Nuclear Systems 611
where M, ρ = atomic weight and density of the crack tip metal
F = Faraday’s constant
z = number of electrons involved in the overall oxidation of an atom of
metal
Because the oxidation charge density on a bare surface varies with time at a
rate that is dependent on the material and environment compositions that control
the passivation rate at the crack tip, Eq. (1) can be adequately represented (and
reformulated) in terms of a power law relationship:
where A and n are constants that depend on the material and environment
compositions at the crack tip. These constants are related to the oxidation reaction
rates, or current densities, in the specific crack tip material-environment system
[45]. The current transients generally have an initially high bare surface dissolution
current density, i
0
for a short time, t
0
; thereafter, oxide formation or precipitation
leads to a decay in the oxidation current density which often follows a general
power law relationship:
If a bare surface condition is maintained at the crack tip (i.e., ε
f
/
⋅
ε
ct
< t
0
), then
integration of Eq. (3) leads to a predicted maximum crack propagation rate:
This relationship is the quantitative basis for the early empirical observations
discussed at the beginning of this section relating the oxidation current density on
a straining surface and the crack propagation rate. However, these early correlations
were obtained primarily for alloys in concentrated environments (boiling MgCl
2
,
9 M NaOH solutions, etc.) under dynamic straining conditions. By comparison, in
relatively dilute environments characteristic of LWRs it is expected that (a) the
passivation rate will be high, and thus “n” [in Eq. (3)] will be high and t
0
will be
short, and (b) under constant load or displacement conditions, the periodicity of
oxide rupture, ε
f
/
⋅
ε
ct
, will be much greater than t
0
. Under these circumstances a
bare surface will not be maintained at the crack tip, and the crack propagation rate
will be given by the integration of Eq. (3).
This is an expanded version of Eq. (2) and relates the parameters A and n to the specific
oxidation rates [e.g., Eq. (3)] and the fracture strain of the oxide at the crack tip.
Copyright © 2002 Marcel Dekker, Inc.
Under constant load or displacement conditions, the crack tip strain rate can
be related, as discussed later, to the creep processes at a moving crack tip [1,45].
Under monotonically or cyclically changing bulk strain conditions,
⋅
ε
ct
can be
related to an applied strain rate
⋅
ε
app
. Thus, the slip dissolution mechanism may be
applied to not only stress corrosion but also strain-induced cracking [46] and
corrosion fatigue. Under cyclic loading conditions, however, the crack is also
moving forward be irreversible cyclic plastic deformation, e.g., fatigue striation
formation. Since this mechanical crack advance is occurring independently of the
crack advance by oxidation processes, these two crack advance mechanisms
(striation formation and oxidation) are considered additive, as shown by the
dashed lines in Figure 5.
Film-Induced Cleavage Mechanism
In some incidences of transgranular cracking it has been observed [47] that the
faradaic equivalent of the oxidation change density at a strained crack tip is
insufficient to account for the observed crack advance. Moreover, the cleavage-like
crystallographic features on the fracture surfaces are hard to rationalize convincingly
in terms of a dissolution-oxidation model. Consequently, several authors [47–51]
have proposed that transgranular environmentally controlled crack propagation may
occur by a combination of oxidation and brittle fracture mechanisms. Specifically,
the crack front is envisioned to move forward by an oxidation process that is
controlled by the same rate-determining steps as those in the slip dissolution model
but, when the film rupture event occurs, the crack in the film may rapidly penetrate a
small amount, a
*
, into the underlying ductile metal matrix (Fig. 6). Thus, Eq. (1) is
modified as follows:
612 Ford and Andresen
Figure 5 Illustration of the strain rate dependence of the crack propagation rate due to the
slip dissolution model, and the additive properties of the mechanical and environmental
components of crack advance during corrosion fatigue.
Copyright © 2002 Marcel Dekker, Inc.
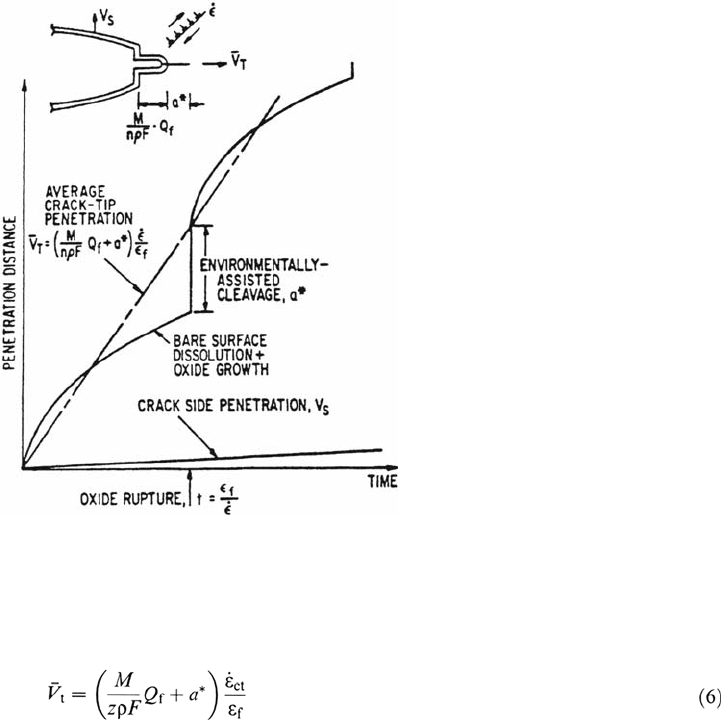
The extent of the additional film-induced cleavage component of crack
advance, a
*
, is governed [47] by the state of coherence between the surface film and
matrix, the fracture toughness of the substrate, the film thickness, and the initial
velocity of the cleavage crack emerging from the surface film. Although the surface
film has been traditionally considered to be an oxide, more recent investigations
[50,51] have shown that dealloyed surface films (e.g., copper-rich films in Cu-Zn or
nickel-rich films in Fe-Cr-Ni alloys) can also form, exhibit “passive” behavior, and
be very brittle. The extent of the cleavage propagation into the matrix is estimated
to be of the order of 1 μm and to depend on the plasticity and microstructural factors
mentioned above. Although there is evidence for this mechanism in copper-base
alloys and austenitic nickel-base alloys and stainless steels in some low-temperature
environments (i.e., <115°C), it has not been extensively tested for other alloy systems,
including those relevant to LWRs. Its attractiveness is, however, that it provides a
rational basis for explaining the interrelationships between the electrochemical
parameters and the transgranular fractographic features in e.g., the carbon and
low-alloy steel–high-temperature water systems peculiar to nuclear reactor
pressure vessels and steam generator shells and quantitatively predicting the
Corrosion in Nuclear Systems 613
Figure 6 Schematic illustration of the elements of the film induced cleavage mechanism
of crack propagation [1]. Note similarity to the slip dissolution model (Fig. 5) during initial
stages of propagation cycle.
Copyright © 2002 Marcel Dekker, Inc.
