Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

Many microorganisms, bacteria and fungi, are able to use this reduction reaction.
Even the ferrous iron–oxidizing bacteria may be able to perform this reaction in
the event of oxygen shortage. The reactions are not restricted to mesophilic bacteria.
Thermophilic bacteria are known to participate in these reactions (oxidatively as
well as reductively).
As mentioned, sulfuric acid is produced concomitantly with the metal sulfide
oxidation. Sulfuric acid may react with metallic iron, Fe
0
, to give ferrous sulfate
and hydrogen. Both compounds may be oxidized biologically. Furthermore, ferric
iron may react with metallic iron to form ferrous iron, Fe
0
+ 2Fe
3+
→ 3Fe
2+
, which
may then be oxidized to ferric iron. Another reaction with iron results from the
sulfur cycle and demonstrates the intimate connection between both cycles.
Reduction of sulfate under anaerobic conditions, e.g., by SRB, results in the
formation of hydrogen sulfide. This may react with ferrous iron to form a precipitate
of ferrous sulfide and, if sulfide is in excess, finally pyrites (FeS
2
).
In summary, it becomes obvious that most reactions of materials with the
environment are influenced by microorganisms and are often even controlled by
them [36]. Thus, in the case of microbially influenced corrosion, the partici-
pation of microorganisms needs to be anticipated [54,95]. Furthermore, cases
are known in which excreted metabolic products, e.g., EPS free from microbial
cells, cause corrosion.
MECHANISMS OF BIODETERIORATION
Despite the vast diversity of microorganisms participating in various natural
cycles, the biological mechanisms contributing to or causing biodeterioration may be
summarized in a few main categories [48]. It needs to be pointed out, however,
that one microorganism may exert multiple detrimental effects. In addition,
under natural conditions pure cultures do not exist. Thus, mixed cultures called
biocoenoses are active by usually creating growth conditions for the corrosion-
causing microorganisms. In most cases microorganisms cause an attack resulting
from a chemical compound produced and excreted by metabolism. Thus, the basic
action will be a chemical reaction.
Excretion of Acid
Specialized bacteria are able to produce and excrete strong mineral acids. Usually
under aerobic conditions, thiobacilli oxidize inorganic sulfur compounds and sulfur
to sulfuric acid. The energy of this oxidation is coupled via special enzymes to
growth. Besides sulfur and sulfur compounds, the bacteria need only carbon dioxide
(cell mass). The genus Thiobacillus consists of several species that are able to
grow at moderately alkaline down to strongly acidic pH values. The species able
to grow and proliferate on alkaline materials are pioneers for the species growing
only under acidic conditions. After the buffer substance of, e.g., concrete (lime)
has been exhausted, the pH value in the surface water declines and the acidophilic
species start to proliferate. This finally causes severe biogenic sulfuric acid corrosion
[6,7,80,89,96].
Microbially Influenced Corrosion 573
Copyright © 2002 Marcel Dekker, Inc.
The second group to be mentioned here is the nitrifying bacteria excreting nitric
acid. The nitrifying bacteria consist of two groups. The ammonia oxidizers convert
ammonia to nitrite, and the nitrite oxidizers subsequently form nitrate. Like sulfuric
acid, nitric acid can react with alkaline materials forming highly soluble salts
(in contrast, sulfates are much less soluble). Biogenic deterioration results
[7,64,66,76]. Nitrifiers are also lithoautotrophs. The energy source is a nitrogen
compound; cell carbon is derived from CO
2
.
The third important acid is produced by all life forms. Carbon dioxide is
excreted as the end product of metabolism. It reacts with water to carbonic acid,
which may dissolve, e.g., to carbonates forming soluble bicarbonates. In this way
the binding material of concrete, lime, may be dissolved.
The fourth group of microorganisms consists of those which during their
metabolism excrete organic acids such as oxalic, citric, malic, lactic, or acetic
acid, amino acids, uronic acids, etc. [25,34]. Usually, these acids are excreted
because of an unbalanced metabolic state. However, they may thus be taken up
again by the cells in later growth phases or be metabolized by other microorganisms
present in the biotope. This means that organic acids are usually available only
temporarily. Nevertheless, their presence may have caused transformations in the
crystal lattice. Organic acids may be excreted by almost all bacteria, cyanobacteria,
algae, lichens, and fungi. Sometimes the excretion is coupled to the uptake of
limiting cations into the cell.
Chelatization
Besides acid attack on materials, organic acids are chelating cations. Because of the
stability of the complexes, metals may be dissolved from a crystal lattice resulting in
a weakening of the structure. The action is sometimes deliberate because it allows the
cells to replenish limiting cation concentrations; e.g., pathogenic bacteria possess
special iron chelators to replenish growth-limiting ferric iron at the expense of the
host.
Organic Solvents
Many microorganisms are able to metabolize organic substances under anaerobic
conditions. If an electron acceptor such as nitrate, Fe
3+
, Mn
4+
, or sulfate is not
available, fermentation results [73]. This means that hydrogen (= redox equivalent)
is transferred from one to another organic compound. By this transfer, growth may
be possible because of a substrate phosphorylation. The result of a fermentation
process is thus usually another organic compound, sometimes carbon dioxide. The
organic compounds are in many cases organic acids, as mentioned before, or
organic solvents such as ethanol, propanol, or butanol. These solvents may react
with materials of natural and/or synthetic origin, causing swelling, partial or total
dissolution, etc., which finally results in deterioration.
Other Metabolic Compounds
An important compound for MIC is hydrogen sulfide. It is produced under anaerobic
conditions by the action of sulfate-reducing bacteria from sulfate, sulfite, and
sometimes sulfur [88]. It has also been shown that thiosulfate can by used by SRB
574 Thierry and Sand
Copyright © 2002 Marcel Dekker, Inc.
[75]. As mentioned before, the SRB use the not fully reduced sulfur compounds
as electron acceptors in anaerobic respiration.
Hydrogen sulfide may act in several ways. Either it is reoxidized to sulfuric acid
under aerobic conditions [101] or in the presence of compounds such as nitrate or it
may react with cations to form metal sulfides that precipitate [50]. Further, H
2
S may
be oxidized anaerobically in the light by photosynthetic bacteria (mostly to sulfur,
sometimes to sulfate). In the case of materials containing acid-reactive substances,
the biogenic acid will become harmful. In the case of metals the presence of H
2
S may
cause MIC. Another source of H
2
S is not restricted to anaerobiosis. Amino acids
containing sulfur are degraded under aerobic conditions, giving rise to H
2
S (cysteine
and cystine).
Another important compound is ammonia. It may be generated as a result of
amino acid or urea degradation by microorganisms. Furthermore, ammonia (or
mainly ammonium salts such as sulfates or chlorides) is a major part of airborne
gases (or dust particles). By dry or wet deposition ammonia/ammonium salts
reach the surface of materials and, in the case of porous materials, enter the pore
system and become biologically available. Nitrifying bacteria as already described
may degrade ammonia/ammonium compounds. However, chemical reactions with
materials may also be possible.
Nitrogen oxides,N
2
O, NO, NO
2
, resulting from biological activity (nitrogen
cycle—oxidation of ammonia or nitrite reduction to N
2
) as well as from processes
such as the burning of fuels (heat and power generation, traffic), are a group of
compounds that may react with materials. NO
2
is a water-soluble gas dissociating
upon dissolution into nitric and nitrous acid. These acids may attack susceptible
materials. NO is less reactive but may be oxidized to NO
2
by light. N
2
O is not
known to interfere with materials.
Biofilm
Microorganisms tend to adhere to surfaces [48,56,106,141]. It is believed that
more than 90% of all microorganisms grow in this way [14]. Concomitantly,
exopolymeric substances are excreted. This may result in a slimy superficial layer
on materials and/or, in the case of porous materials, in total clogging of the free pore
volume [60]. The exopolymers are usually hydrated and may contain ionic groups
favoring water inclusion. Thus, one result may be an increase in water content in the
case of porous materials (a consequence is freeze–thaw attack; see the following).
Furthermore, the biofilm may act as insulation, reducing heat transfer. In the
case of heating systems, the efficiency may be considerably reduced [40]. Another
effect is the reduction of the cruising speed of ships due to increased friction caused
by the surfacial biofilm [5]. Finally, biofilm development may cause technical
processes to become susceptible to trouble. In paper machines, the inclusion of
exopolymers in the pulp may cause the paper web to break in the paper machine. The
coating of cans with resins may also be affected. Cans for food are coated on the
inside with a resin to protect the metal from corrosion by foodborne acids. If the metal
is covered at a few spots with biofilm (microcolonies), the coating process will not
be totally successful. Pores will remain in the resin at these spots, rendering the metal
accessible to acids from the food. Corrosion from the inside combined with
deterioration and spoilage of the contents will result.
Microbially Influenced Corrosion 575
Copyright © 2002 Marcel Dekker, Inc.
Salt Stress
The biogenic reactions that have been mentioned result in the generation and,
generally, accumulation (except in an aquatic environment) of salts as reaction
products. Because salts are hydrophilic they are usually hydrated, resulting in an
increase in the water content of a porous material. This may increase the
susceptibility to physical attack by freezing and thawing because of the volume
change in water/ice crystals.
Furthermore, upon desiccation, salt crystals may develop, causing the surfacial
removal of layers of material, e.g., deterioration destroying wall paintings on natural
stone. The third detrimental effect of salts results from the formation of large crystals
causing a swelling attack. A well-known example is the formation of ettringite
from gypsum crystals destroying concrete and bricks.
Exoenzymes and Emulsifying Agents
Besides exopolymers of a lipopolysaccharidic nature, microorganisms excrete
lipoproteins and proteins. The latter include exoenzymes for the degradation of high-
molecular-weight compounds such as cellulose. Cellulases degrade the insoluble
cellulose fibrils to soluble molecules such as cellobiose (dimer) and glucose,
which may be taken up by the cells for metabolism. Similar exoenzymes exist for
other materials, e.g., esters, amines, and waxes. Other molecules, although not as
large as cellulose, may also be insoluble and, thus, only poorly degradable.
In some cases microorganisms excrete emulsifying agents to increase the
solubility of hydrophobic substances [112]. In the case of elemental sulfur, which
is highly insoluble due to its hydrophobic nature, the excretion of such emulsifying
agents causes an increase in dispersibility from 5 to 20,000 μg/L [114]. This holds
for other hydrophobic materials as well. Water-insoluble hydrocarbons become water
soluble because of microbial production of emulsifying agents and consequently
become degradable.
These microbial deterioration mechanisms represent the main categories of
action. Several mechanisms are usually active jointly, and because these mechanisms
may also influence other types of physical or chemical attack, the microbial share
of the total attack can rarely be determined. Much too often, studies of deterioration
mechanisms suffer from inadequate microbiological analysis (if it is done at all). If
corrosion occurs in the range where life is possible, a microbial contribution needs
to be taken into account and, hence, a microbiologist should be consulted [54,84].
MICROBIAL CORROSION OF CONSTRUCTIONAL MATERIALS
Metallic Materials
Metallic materials are an important group of construction materials. Microbially
influenced corrosion may occur for these materials for many industrial applications,
as listed in Table 1 [27]. Microbially induced corrosion of metallic materials
does not involve any new form of corrosion. Thus it is necessary to discuss the
electrochemical nature of corrosion briefly before continuing with the mechanisms
of MIC for different construction metals.
576 Thierry and Sand
Copyright © 2002 Marcel Dekker, Inc.

The corrosion of metals in the presence of water is of an electrochemical
nature. This includes corrosion in water-containing solutions, atmospheric corro-
sion due to the presence of a humidity film at the metal surface, and corrosion in
soils due to the humidity of the soil. A detailed discussion of electrochemically
induced corrosion is outside the scope of this chapter. For more details, a large
number of good comprehensive reviews may be consulted (for instance, Ref. 63).
Hence only some basics will be given here insofar as they may help in understanding
MIC of metals.
The anodic reaction involves the oxidation of the metal into metal ions,
whereas the cathodic reaction involves the reduction of some component in the
corrosive environment:
Microbially Influenced Corrosion 577
Table 1 Industrial Applications for Which Microbial Corrosion Has Been Reported
Source: Ref. 27.
Cathodic reactions may be summarized as follows:
Copyright © 2002 Marcel Dekker, Inc.
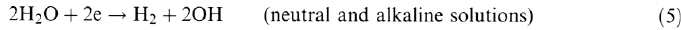
The rates of the anodic and cathodic reactions must be balanced in order to preserve
electroneutrality.
The nature of the cathodic reaction depends on the pH of the solution and
on the presence of dissolved oxygen or other oxidizers such as dissolved CO
2
.
For instance, in the pH range between 4 and 10, the diffusion of dissolved
oxygen to the metal surface controls the rate of corrosion of iron. In this pH range
and in the absence of oxygen, very low corrosion rates should therefore be
expected.
The term “uniform corrosion” implies that the anodic and cathodic sites are
virtually inseparable, whereas “localized corrosion” implies that macroscopic
anodic and cathodic sites are physically separable.
As already stated, MIC of metallic materials does not involve any new form
of corrosion. The mechanisms involved in the biodeterioration of materials have
already been given in detail. The main ways in which microorganisms may
enhance the rate of corrosion of metals and/or the susceptibility to localized corrosion
in an aqueous environment are given below in relation to known corrosion
mechanisms (for each case some examples are also given):
Formation of concentration cells at the metal surface and in particular oxygen
concentration cells. This effect may occur when a biofilm or bacterial growth
develops heterogeneously on the metal surface. Concentration cells are
also associated with the tubercles formed by iron-oxidizing bacteria, such
as Gallionella. Certain bacteria may also trap heavy metals such as copper
and cadmium within their extracellular polymeric substance, resulting in the
formation of ionic concentration cells.
Modification of corrosion inhibitors. To this group belong microorganisms that
may destroy corrosion inhibitors such as bacteria that transform nitrite
(corrosion inhibitor for iron and mild steel) to nitrate, or nitrate (corrosion
inhibitor for aluminum and aluminum alloys) to nitrite and ammonia and N
2
.
Production of corrosive metabolites. Bacteria may produce different metabolites,
such as inorganic acids (e.g., T.thiooxidans), organic acids (almost all
bacteria, algae, and fungi), sulfide (sulfate-reducing bacteria), and
ammonia, that are corrosive to metallic materials.
Destruction of protective layers. Various microorganisms may attack organic
coatings, and this may lead to corrosion of the underlying metal.
Stimulation of electrochemical reactions. An example of this type of action is
the evolution of cathodic hydrogen from microbially produced hydrogen
sulfide.
Hydrogen embrittlement. Microorganisms may influence hydrogen embrittlement
on metals by acting as a source of hydrogen or/and through the production of
hydrogen sulfide.
In summary, all known cases of microbial corrosion of metals can be attributed
to known corrosion mechanisms. In the following sections, the mechanisms of
microbially induced corrosion for different metals and alloys are discussed.
578 Thierry and Sand
Copyright © 2002 Marcel Dekker, Inc.
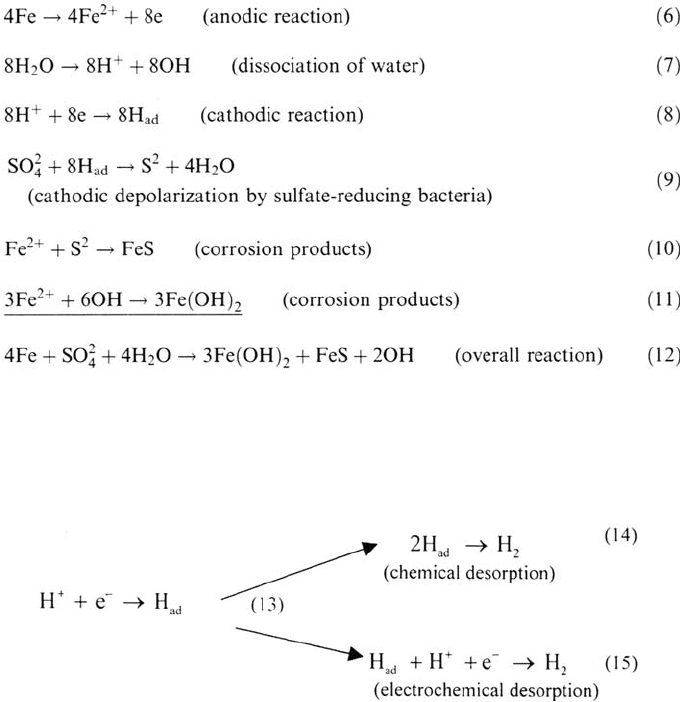
Mechanisms of Microbially Induced Corrosion of Iron and Mild Steel
Corrosion Under Anaerobic Conditions Considering the electrochemical
reactions discussed earlier, very low corrosion rates are expected for iron and steel
in near-neutral conditions and in the absence of oxygen. However, a large number
of case histories in the literature, particularly for buried pipes and marine
structures, report that the corrosion may be very severe and can be several orders
of magnitude higher than that experienced under normal aerobic conditions [47].
It is well accepted that this is due to sulfate-reducing bacteria and their ability to
produce sulfide. However, despite the large body of literature available, the exact
mechanism(s) responsible for the corrosion in such environments is still the
subject of discussion. Several good review articles present this subject in more
detail than is attempted here [50,123,124,128].
The first attempt to explain the anaerobic corrosion of iron was that by
Von Wolgozen Kühr and van der Vulgt [130]. These authors proposed as early as
1934 that sulfate-reducing bacteria were responsible of the pitting corrosion they
observed on buried cast-iron pipes through their ability to remove the hydrogen from
the metal surface via their enzyme hydrogenase for the dissimilary reduction of
sulfate according to the following reactions:
Microbially Influenced Corrosion 579
Reaction (9) represents the ability of SRB to remove the adsorbed hydrogen
from the metal surface through the enzyme hydrogenase. This reaction was referred to
by the authors as “depolarization.” This term was used only to underline that there was
an undefined change in the electrochemical response of the system studied [33]. This is
an alternative reaction path compared with the classical hydrogen evolution reaction:
Copyright © 2002 Marcel Dekker, Inc.
Reactions (14) and (15) are the rate-determining steps (i.e., a higher activation
energy is required for these reactions compared with the discharge of hydrogen
ions) for many metals and alloys, including iron in deaerated aqueous solutions.
The rate-determinating steps may, however, be different in the case of complex
corrosion layers. Sulfate-reducing bacteria, by removing adsorbed hydrogen to
form sulfide according to reaction (9), lower the activation energy for the desorption
steps. As these steps are rate determinating, this may lead to higher corrosion rates
if hydrogen evolution is thermodynamically possible (i.e., if the immunity potential
is lower than the redox potential for H
2
/H
+
).
This theory, also referred to in the literature as the classical depolarization
theory, suggests that only sulfate-reducing bacteria that are hydrogenase positive
(i.e., bacteria in which the enzyme hydrogenase is present) are responsible for the
anaerobic corrosion of iron. Even though there are data supporting this view
[9,57], it has been shown in many other works that this is not the case and that a
high corrosion rate may be obtained with hydrogenase-negative strains [10,81,92].
In addition, several other important factors are not taken into account in the classical
depolarization theory: (a) the effects of sulfide, bisulfide, and hydrogen sulfide
produced from the sulfate reduction on the anodic reaction; (b) the effect of
hydrogen sulfide on the cathodic reaction; (c) the effect of elemental sulfur from
the biotic or abiotic oxidation of sulfur; (d) fluctuations in the environmental
conditions between anaerobic and aerobic conditions [47]; (e) the production of
other corrosive metabolites [128].
It is therefore now widely accepted that, even if the so-called depolarization
of the cathodic reaction by the enzyme hydrogenase occurs, it plays no more than
a secondary role in the pitting corrosion of iron in anaerobic conditions. This theory
is therefore reported here more for historical interest than as a potential mechanism
for pitting corrosion of iron in presence of SRB.
More recent theories have been proposed in which the role of the biogenic
sulfide, the formation of a galvanic cell between the metal and the iron sulfide film
formed, the role of elemental sulfur, the role of iron phosphites, and the local
acidification of anodes have been discussed. The alternative theories to the classical
depolarization theory are presented briefly in Table 2.
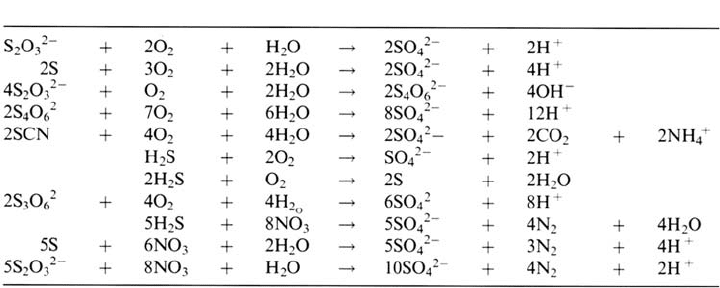
It has also been shown that the nature of the iron sulfide film plays an important
role in the initiation of pitting corrosion. Figure 5 shows the different biogenically
and chemically formed iron sulfide films [93]. When protective films such as
mackinawite and siderite are formed, the corrosion rate may be very low. However,
with changes in the environmental conditions these films may be transformed to iron
sulfides that are less protective, such as pyrrhotite, with the possible initiation of pits
as a result. The importance of the environment for the initiation of localized corrosion
by SRB as been mentioned by several authors [24,51]. Of particular importance is
the ability of SRB to regulate pH, the ionic composition, the composition of Fe
2+
,
aerobic/anaerobic cycles, and the presence of other microorganisms, as they may
lead to conditions resulting in passivation or uniform corrosion or localized
corrosion. This is probably the main reason why the severe pitting corrosion
experienced in the field is only seldom reproduced in the laboratory using growing
cultures of SRB, where uniform corrosion is generally observed. Indeed, in practice
SRB are not found in isolation but in consortia of microorganisms or biofilms
in which many physicochemical parameters such as pH and dissolved oxygen
580 Thierry and Sand
Copyright © 2002 Marcel Dekker, Inc.
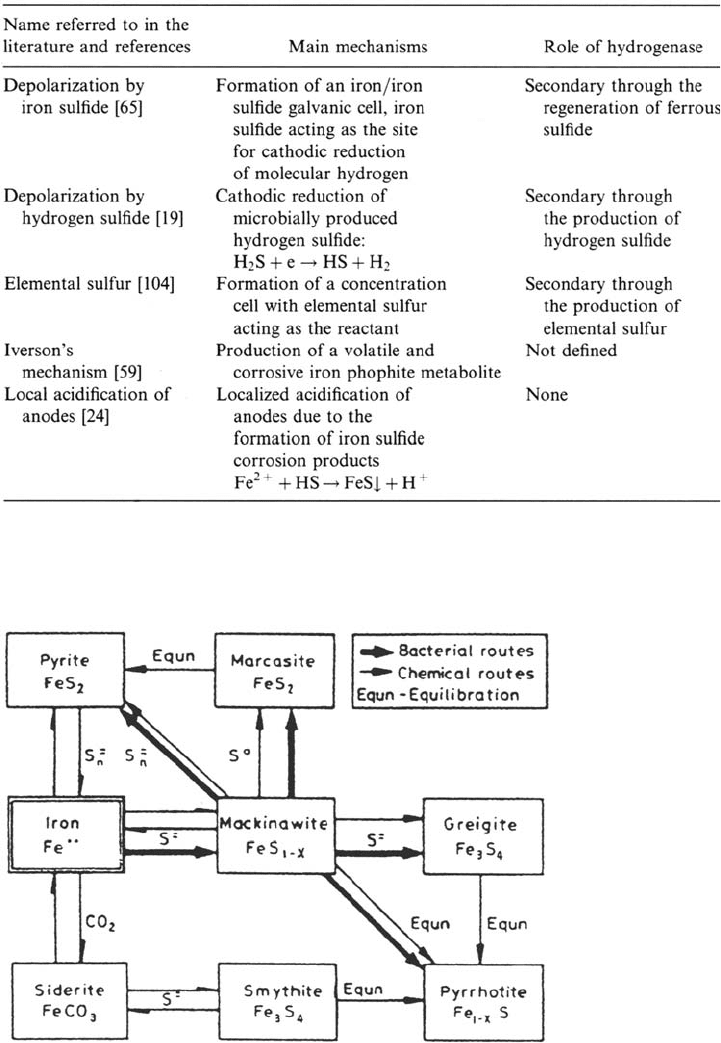
Microbially Influenced Corrosion 581
Table 2 Alternative Mechanism to the Classical Depolarization Theory
Figure 5 Chemical and biological interrelationships between iron sulfides [93].
Copyright © 2002 Marcel Dekker, Inc.
concentration change both with time and within the thickness of the biofilm. This
dynamic picture of microbial corrosion is discussed in more detail later.
Corrosion Under Aerobic Conditions Corrosion may occur under aerobic
conditions through the production of sulfuric acid by bacteria of the genus
Thiobacillus [119]. The sulfuric acid is produced through the oxidation of various
inorganic sulfur compounds, as illustrated in Table 3 [22]. Some of these bacteria
can tolerate a concentration of sulfuric acid up to 10–12%. Under these conditions,
iron and mild steel are heavily corroded.
Corrosion of iron and steel may also occur in aerobic conditions due to the
colonization of bacteria at the metal surface and the formation of an uneven patchy
biofilm. Nonuniform biofilms or bacterial colonization results in the formation of
differential aeration cells in which the anodic areas are found under respiring
colonies or in the thick part of the biofilm (zones depleted in oxygen) whereas the
cathodic areas are found in noncolonized areas or in the areas where the biofilm
is thin (regions rich in oxygen). This is schematically illustrated in Figure 6. The
iron-oxidizing bacteria such as Gallionella ferruginea, Crenothrix, and Leptothrix
are often associated in the literature with this form of corrosion, particularly in the
internal surfaces of water pipes. These bacteria are aerobes and are believed to
oxidize ferrous ions into ferric ions with the resulting formation of heavy deposits
of ferric oxide or hydroxide, also referred to as tubercles. However, even if
iron-oxidizing bacteria are the best-documented case, many other organisms may
be involved in this kind of microbially influenced corrosion.
Finally, it should be mentioned that microorganisms may cause the degradation
of corrosion inhibitors such as aliphatic amines, nitrite, and phosphate-based
corrosion inhibitors used, for instance, for steel pipes in cooling water systems. This
results in a higher demand for corrosion inhibitor and an increase in the bacterial
population, which leads to high corrosion rates, if the system is not controlled
[120,121].
Microbial Biofilms and the Interactions Between Aerobic and Anaerobic
Populations In the preceding discussion, the mechanisms of microbial corrosion
have been divided in the traditional way into anaerobic and aerobic mechanisms,
which refer to the living conditions of the microorganisms involved in the
582 Thierry and Sand
Table 3 Oxidation Reactions of Thiobacilli and Other Aerobic Sulfur-Oxidizing Bacteria
Source: Ref. 22.
Copyright © 2002 Marcel Dekker, Inc.
