Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

16
Microbially Influenced Corrosion
Dominique Thierry
Swedish Corrosion Institute, Stockholm, Sweden
Wolfgang Sand
Universität Hamburg, Hamburg, Germany
INTRODUCTION
Microbially influenced corrosion (MIC) is, by definition, corrosion associated
with the action of microorganisms present in the system. Microbially influenced
corrosion is therefore an interdisciplinary subject that embraces the fields of
materials science, chemistry, microbiology, and biochemistry.
The first report of MIC on metals was published in 1891 by Garrett [43],
who found that the corrosion of covered cables could be attributed to biogenic
ammonia, nitrite, and nitrate. Almost two decades later, it was shown by Gaines
[42] that sulfate-reducing, sulfur-oxidizing, and iron bacteria were responsible for
part of the corrosion of iron in soils. These observations were associated with
aerobic conditions. In 1934, Von Wolzogen Kühr and Van der Vlugt [130] presented
the first evidence that microorganisms play a direct role in the corrosion of metals
under anaerobic conditions. They postulated that sulfate-reducing bacteria were
able to pick up adsorbed hydrogen from the metal surface. From 1934 up to now
there has been an increasing number of reports of MIC on metallic and nonmetallic
constructions and in various environments.
The cost of MIC is very significant. Several estimates have been made in the
United States and in the United Kingdom. For instance, it has been reported by
Iverson [58] that the annual cost of MIC of buried pipes in the United States was
$500 million to $2000 million. In the United Kingdom, it was postulated that at
least 50% of the corrosion occurring on buried metals was of microbial origin [8].
The main purpose of this chapter is to provide an overview of present
knowledge of the mechanisms of microbially influenced corrosion on common con-
structional materials. This review includes both metallic and nonmetallic materials.
The reason for including nonmetallic materials is that biodegradation occurs on
these materials and that they are widely used for construction purposes. Because
detailed knowledge in the fields of microbiology and biochemistry is necessary in
order to understand the mechanisms of microbially influenced corrosion, considerable
space has been devoted to a general description of microorganisms and to a
563
Copyright © 2002 Marcel Dekker, Inc.
description of natural cycles of matter in which microorganisms play an important
role. A later section briefly presents the most commonly used countermeasures for
avoiding or minimizing the influence of microorganisms on the degradation of
materials.
MICROORGANISMS
The term microorganism covers a vast diversity of life forms. Bacteria, blue-green
cyanobacteria, algae, lichens, and fungi together with protozoa are all classed as
microorganisms. The main characteristic is the size of the individual organism. As a
rule, it ranges between 0.5 and 10 μm, although organisms several meters in size are
known (e.g., brown algae). Because of their small size, microorganisms have a large,
catalytically active surface. A volume of 1cm
3
can contain 10
12
bacterial cells having
a surface of approximately 1m
2
(cell size 1 to 1 μm, length to width). Through this
enormous surface area, agents may be excreted into the surrounding medium, caus-
ing detrimental effects on materials. The vast majority of microorganisms cannot be
detected with the naked eye. Techniques such as light and/or electron microscopy
(transmission and/or scanning) are required to magnify single cells so that they
become visible [6]. The detection may be enhanced using dyes, which bind to cells
and make them colored or fluorescent. Specific detection can be achieved using the
polymerase chain reaction (PCR) technique with specific gene probes and/or
immunolabeled antibodies binding to selected microorganisms. Thus, microorgan-
isms with special characteristics may be detected in a mixed population.
Other indirect techniques measure the effect of the metabolism of
microorganisms on the environment, such as acidification; alkalization; oxygen
consumption; degradation of substrates, production of insoluble, dissolved, and/or
gaseous intermediates and/or end products of catabolism; and heat production
[107]. An indirect technique for visualization of “single” cells of microorganisms
is transfer of a cell to a solidified nutrient solution and subsequent incubation at a
given temperature, which enables the cell to grow and to multiply. After several
multiplication cycles, a colony (consisting now of at least 10
7
daughter cells)
becomes visible to the naked eye. The solidifying agent may be agar-agar, derived
from algae, a polysaccharide forming a gel.
A powerful, recently developed tool is the PCR technique. The technique
allows theoretically the detection of a single cell in 10
7
or more cells. A probe
containing a counterpart of that piece traps a piece of genetic information, unique
for a microorganism (a genetic fingerprint). Afterward, the double-stranded piece
is multiplied by means of an enzyme, taq polymerase. After several multiplication
cycles, the genetic information is sufficiently amplified to become detectable, e.g.,
by autoradiography (because of the use of radioactive components). However, so
far only a few absolutely specific probes for detection exist.
Microorganisms exhibit a vast diversity with regard to their metabolism.
Nevertheless, this diversity may be described by six main terms. Microorganisms
obtaining their metabolic energy by use of light are called phototrophs. In constrast,
those gaining energy through chemical reactions are known as chemotrophs. The
source of reducing power is used for a further subdivision. In the case of inorganic
hydrogen donators, the microorganisms are called lithotrophs, whereas those using
564 Thierry and Sand
Copyright © 2002 Marcel Dekker, Inc.
organic compounds are called organotrophs. Finally, the source of cell carbon is
important. If CO
2
is used, the organisms are called autotrophs. If carbon is derived
from organic molecules, they are designated heterotrophs. By combing these six
terms, bacteria can easily be described with regard to their nutritional requirements.
For instance, if energy is derived from inorganic hydrogen donators and
biomass is derived from organic molecules, they are called mixotrophs (= chemo-
lithoorganotroph). Escherichia coli, for example, a bacterium occuring in the gut
of animals, is a chemoorganoheterotroph because it grows by chemical oxidation
of organic compounds such as glucose in the medium and derives cell carbon by
assimilating part of the glucose. Another example is the chemolithotrophic bacterium
Acidithiobacillus thiooxidans, which grows by the chemical oxidation of inorganic
sulfur compounds using the energy of the oxidation for metabolism and deriving the
cell carbon from CO
2
of the air.
Most microorganisms may be classified as chemoorganoheterotrophs.
Exceptions are photosynthetic microorganisms (mainly a few bacterial genera),
cyanobacteria, algae, and in part lichens because of the algal or cyanobacterial
symbiont. Exclusively restricted to bacteria are chemolithotrophic life forms.
An important feature of microbial life is the possibility to degrade any naturally
occuring compound. This is called microbial omnipotence. Exceptions to this rule are
a few manmade compounds such as highly polymerized materials (resins, plastics,
etc.) and halogenated compounds. These are called xenobiotica because they are
strange to the living world and living organisms have had too little time to adopt to
these compounds and develop degradative enzymes. Eventually, genetic engineering
of microorganisms may help in the development of degradatively active ones.
Besides energy and carbon sources, microorganisms need nitrogen,
phosphorus, and trace elements. Nitrogen compounds may be inorganic ammonium
and/or nitrate (sometimes nitrite, too) as well as organically bound nitrogen (amino
acids, nucleotides, etc). Some microorganisms (bacteria and cyanobacteria) are able
to fix nitrogen from atmospheric nitrogen with the help of an enzyme called
nitrogenase. The end product is ammonia, which is incorporated in cell constituents.
Phosphorous is usually taken up as inorganic phosphate or as (organically
bound) phosphorylated compounds such as phosphorus-containing sugars and lipids.
Phosphate in the form of adenosine triphosphate (ATP) serves as the main energy
storage compound. Whenever a reaction takes place generating metabolically useful
energy, ATP is produced to conserve at least a part of it.
Trace elements are needed for many metabolic purposes. They make up only a
negligible amount of the total cell weight, but they support vital functions. Iron as
Fe
2+
or Fe
3+
is necessary for the electron transport system. It functions as an
oxidizable/reducible central atom in cytochromes or in nonheme iron-sulfur proteins.
Magnesium plays this role in the chlorophyll molecule. Cobalt functions in the
transfer of methyl groups from/to organic or inorganic molecules (vitamin B
12
,
cobalamine, is involved in the methylation of heavy metals such as Hg). Copper is an
integral part of a cytochrome (aa
3
), which at the terminal end of the electron
transport system mediates the reduction of oxygen to water (cytochrome oxidase).
Further examples exist for other metals.
Microbial growth is influenced and sometimes restricted by several chemical
and physical factors. Life generally cannot exist without water. Hence, water is
Microbially Influenced Corrosion 565
Copyright © 2002 Marcel Dekker, Inc.

a prerequisite for microbial life and growth. Microorganisms differ considerably
with regard to the amount of water needed. In particular, fungi are able to
live under extremely dry conditions. Three types of water surrounding a solid
material need to be distinguished: hygroscopic, pellicular, and gravitational
water. Hygroscopic water, a film 3 × 10
2
μm thick, directly surrounds the solid
particle. It is not available for life and does not freeze or move. Pellicular water
and gravitational water are biologically available and, thus, may be used by
microorganisms. The biologically available water is usually measured as the water
activity a
w
of a sample:
566 Thierry and Sand
Most bacteria are restricted to a
w
values of more than 0.90 (equivalent to an
osmotic pressure of –150 bar). Exceptions are bacteria living in salterns or
salinas—halobacteria—that exhibit a
w
values of 0.75 (equivalent to an osmotic
pressure of –400 bar). The lowest known value for a (micro) organism has been
found for the fungus Xeromyces bisporus [115]. The organism is able to grow at
an a
w
value of 0.61, equivalent to –681 bar. For comparison, egg powder has an
a
w
value of 0.40 (–1260 bar) and biscuits of 0.30 (–1660 bar). Lichens, because of
the symbiosis of a photosynthetic partner (alga or cyanobacterium) with a fungus,
may resemble fungi in their need for available water. All other microorganisms
(and also higher organisms) are very sensitive to water stress. They barely tolerate
water shortage. The tolerated a
w
value is 0.99 (–15 bar). Physical factors may
contribute to water shortage. In porous systems such as soil or sedimentary stone
(sandstone), water activity is already reduced because of the capillary bonding in
pores of low diameter (below 10 μm).
Another important factor is the hydrogen ion concentration. Microorganisms
may be distinguished by their ability to grow under acidic, neutral, or alkaline
conditions. Hence, they are called acidophiles, neutrophiles, or alkaliphiles. The
bacterium A. thiooxidans has been detected in samples exhibiting a negative pH
value, whereas in soda lakes life has been detected at pH values of 12 and above.
Fungi are able to grow over a large range of pH values. Species of Penicillium
have been found at pH 2 and up to pH 12. Most microorganisms, however, thrive
in the neutral pH range from 6 to 8.
The redox potential is another factor determining microbial growth. If, under
standard conditions, hydrogen is assumed to have a redox potential (E
h
) of –420 mV
and oxygen to have an E
h
value of +820 mV, this gives the range in which metabolism
can take place. The basic process of life is the reaction of hydrogen with oxygen to form
water. Depending on oxidizing or reducing conditions, different types of metabolism
are found. Under oxidizing conditions with E
h
values of +500 mV and above, oxygen
is usually used as terminal electron acceptor. E
h
values of +400 mV favor the reduction
of nitrate if oxygen is not available. If neither oxygen nor nitrate is available, Mn
4+
may serve as an electron acceptor, being reduced to soluble Mn
2+
species. At an E
h
value of –180 mV, ferric iron starts to be used as terminal electron acceptor. At an even
lower redox potential sulfate is reduced to sulfide (–220 mV). The lowest potential
(–250 mV) is necessary for the reduction of carbon dioxide to methane.
Copyright © 2002 Marcel Dekker, Inc.
Closely linked to the redox potential is the available oxygen. Microbial life is
possible under well-aerated as well as under totally oxygen-free conditions. The
differences are used to characterize the oxygen needs of microorganisms. Aerobes are
organisms living with the amount of oxygen contained in the air. They may tolerate
shortage of up to 5 or 10% of the regular partial pressure of oxygen. Facultative
aerobes are able to live with the oxygen partial pressure even under conditions of
exhaustion. In this case, they convert their metabolism to the use of chemically
bound oxygen (nitrate) or to fermentation. Anaerobes are organisms that perform
their metabolism without any free oxygen. They are able to use only bound oxygen
(sulfate, carbon dioxide) or to ferment organic compounds [73]. In the latter group
there are a few species that are called strict anaerobes because oxygen is highly
toxic to these organisms. The process of utilization of chemically bound oxygen
is called anaerobic respiration. It is also used for the reduction of inorganic metal
ion species such as Mn
4+
or Fe
3+
.
Last but not least, the temperature needs to be discussed. Microbial life is
possible between –5°C and +114°C. Again, the temperature needs of microorganisms
are used for their classification.
Psychrophiles are organisms living in the range of –5°C up to 20°C.
Psychotrophs live between 5°C and 30°C. Mesophiles thrive in temperatures
between 20°C and 45°C. At higher temperatures, moderate thermophiles find their
habitat (40–55°C). The next group are the thermophiles, living in the range from
55°C to 85°C. The extreme thermophiles grow at temperatures up to about 120°C.
Above that, living organisms have not so far been detected. Most organisms live
in the mesophilic range corresponding to the usual temperature on the surface of
the earth. Interestingly, only a special group of bacteria is able to grow at
elevated temperatures (above 70°C). They are called archaebacteria and are
distinguished from eubacteria by many chemical and physiological traits. Many of
them seem to represent ancestors who are believed to have been active in the early
history of this planet (high temperature, reducing atmosphere). Organisms are
usually not restricted to grow at a certain temperature. Adaptation within a range
of 20 to 30°C above or below their optimized growth temperature is possible. It is
achieved by a modification of the fatty acid composition in the cytoplasmic
membrane. At higher temperatures, unsaturated and/or branched fatty acids
prevail, whereas at lower temperatures saturated, unbranched ones are found.
In summary, microbial life is influenced by many parameters. Adaptation to
a change is possible [13,26]. This allows microorganisms to be present wherever
a substrate is available for metabolism. A variation in the parameters previously
mentioned may be used to control microbial growth. However, care must be taken
to ensure that merely adaptation of the microorganisms present and not a change
in the composition of the microbial biocoenosis takes place.
Microorganisms grow predominantly attached to surfaces. It is estimated that
more than 90% of all microorganisms live sessile on the surface of a solid material
(called the substratum, in contrast to the nutrient, which is called the substrate).
Attachment of microorganisms is mediated by a complex series of events by
excretion of linking exopolymers resulting in firmly substratum-bound cells.
The exopolymers consisting of lipopolysaccharides, lipoproteins, and
sometimes nucleic acids [14] constitute the outer membrane of the cells and, because
Microbially Influenced Corrosion 567
Copyright © 2002 Marcel Dekker, Inc.
they are strain specific, may be used for identification purposes. Furthermore they
may contain metal cations, some of which are known to be necessary for substrate
degradation [47]. If several cells are growing together on a substratum, the
exopolymers fill the voids. This is called a biofilm. If a biofilm grows and increases
in thickness, it becomes stratified or patchy. The oxygen supply of cells buried
deeply in a biofilm may become limiting. Thus, anaerobic zones or layers develop
[70]. This may result in the appearance of closely associated zones with aerobic
and anaerobic types of metabolism. Localized biofilm (microcolonies) may have
serious detrimental effects on materials [55]. Because of the oxygen consumption
by a microcolony or a biofilm, localized aeration elements exist, causing in the
case of metals an enhanced dissolution by stimulation or electrochemical effects.
Another effect may be reduced heat transfer in heat exchange systems [40].
Furthermore, biofilms protect the organisms living there because of reduced
penetrability of poisons such as heavy metals, biocides, and natural enemies such
as viruses (phages) and grazing protozoa [20,41]. In addition, a biofilm protects
against dryness. The microorganisms growing in a biofilm have, thus, created a
microenvironment of their own favorable for their survival and growth. Another
aspect is that in dilute solutions, due to ionic interactions, (organic) molecules tend to
adsorb to surfaces and thus becoming increasingly available for attached organisms.
MICROBIALLY INFLUENCED GEOCHEMICAL CYCLES
Most elements on earth are subject to cycling. During this process the oxidation
status may be changed from fully oxidized to totally reduced. All cycles are
influenced by microorganisms, and some are controlled by them [36]. Four examples,
the carbon cycle, the nitrogen cycle, the sulfur cycle, and the iron cycle, are selected
here to illustrate the importance of microorganisms.
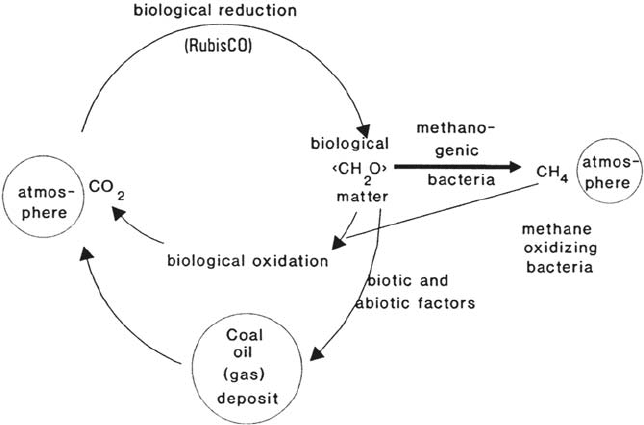
Carbon Cycle
Carbon occurs in the biosphere either reduced (methane, fatty acid, carbohydrate)
or oxidized (alcohol, aldehyde, carbonic acid, carbon dioxide). The valence state
0 is found only in coal (a compound of biological origin).
(Micro)organisms control the carbon cycle (see Fig. 1). By fixing CO
2
from the
air, cell carbon is generated. Primary producers such as green plants, algae,
cyanobacteria, and photosynthetic bacteria are responsible for this reaction. The
enzyme mainly involved in this process is called ribulose-1,5-bisphosphate-
carboxylase/oxygenase (RubisCO). It is the most abundant protein on earth. The
resulting cell carbon is degraded by organisms, the end product being carbon dioxide,
which may be fixed again. Another possibility is the incomplete degradation
occurring in sediments, etc. This may give rise to accumulations of organic matter
as observed in shallow marine areas, swamps, and marshes. Presumably, deposits
of carbon such as oil, gas, and coal were formed in this way [2]. Another pathway
is the fixation of CO
2
followed by its use in structural elements of organisms such
as valves and bones. In this way, inorganic deposits such as limestone and shales
are formed.
568 Thierry and Sand
Copyright © 2002 Marcel Dekker, Inc.
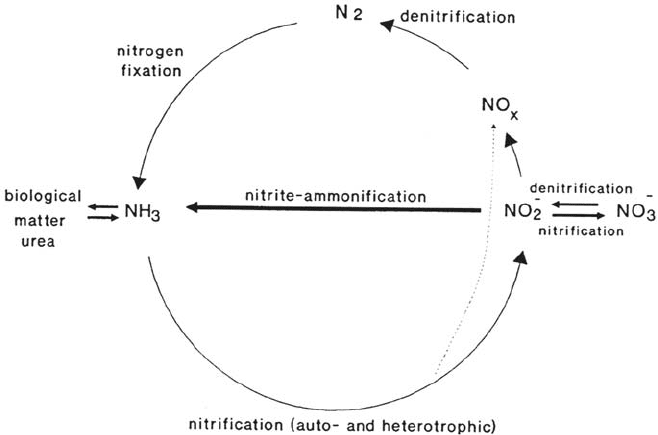
Nitrogen Cycle
Nitrogen occurs in the biosphere either reduced (to ammonium) or oxidized
(nitrite, nitrate), together with intermediates such as hydroxylamine (NH
2
OH),
nitrogen (N
2
), and nitrogen oxides (N
2
O, NO, NO
2
). In addition, organically
bound nitrogen is found in many compounds such as proteins, nucleic acids,
amines, and urea. Microorganisms mediate all and control most of the reactions in
this cycle (see Fig. 2). The cycle starts with an enzymatic reaction unique to
bacteria and cyanobacteria. Atmospheric dinitrogen, N
2
, is reduced and fixed by
the action of the enzyme nitrogenase. The product NH
3
is incorporated in cell
constituents, the amount of ammonia that becomes available for the biosphere
being comparable to the amount produced in technical ammonia production.
When cell constituents are degraded, ammonia is liberated and may be used for
the synthesis of other nitrogenous compounds. Because nitrogen is usually a limiting
factor for life, organisms take care not to lose it. However, upon the death of cells,
ammonia may become available. Usually, a process called nitrification starts.
Nitrifying bacteria oxidize ammonia via nitrites to nitrate. The nitrifying bacteria
consist of two groups: the ammonia oxidizers, which are responsible for the
oxidation of ammonia to nitrite, and the nitrite oxidizers, which are responsible for
converting nitrite to nitrate. Hence, a cation is converted to an anion plus protons
(acidification). The process has positive and negative consequences. Whereas
ammonia may be adsorbed to clay particles and, thus, may become unavailable for
plant growth, nitrate is a mobile ion, which can be washed out of soil by rainfall
and enter into ground water. Because of this, nitrification is responsible for
nitrogen loss from fertilizers and ground water pollution by nitrate. In the case of
a large supply of organic matter, the availability of oxygen is often limiting for
Microbially Influenced Corrosion 569
Figure 1 Carbon cycle.
Copyright © 2002 Marcel Dekker, Inc.
degradation. Under these circumstances, a reductive process called denitrification
or nitrate (nitrite) respiration gains importance.
Nitrate and other oxidized nitrogen compounds may serve as electron acceptors
and be reduced to nitrite, NO, N
2
O, and finally N
2
[4]. The oxygen is released as
water. Thus, denitrifiers can get rid of hydrogen resulting from the oxidation of
organic matter. By this mechanism a considerable amount of organic matter may
be mineralized under anaerobic conditions. Growth yields with chemically bound
oxygen are in the range of atmospheric oxygen usage (less than 10%). The reduction
does not always proceed until dinitrogen is released. Often, NO or N
2
O are the end
products. These may either be reoxidized or be further reduced by microorganisms.
In any case, the nitrogen cycle is closed by these microbiologically catalyzed
reactions. It needs to be added that there is an enormous input of ammonia mainly
from two processes: the degradation of manure from livestock breeding and loss
of fertilizers from farmland. Compared with industrial processes such as the use
of ammonia for flue gas desulfurization, these two processes dominate the ammonia
emission. Nitrogen oxides are becoming increasingly important because of their
influence on the global climate. In contrast to the emissions of dust particles and
sulfur dioxide, the emission of nitrogen oxides is still increasing because of
increasing use of combustion processes, e.g., for automobiles and heating systems.
Microbiology will adapt to the increased supply of these compounds, which furthers
the growth of ammonia-oxidizing and NO-oxidizing microorganisms.
Sulfur Cycle
Sulfur occurs in the biosphere in many compounds. It is essential for the formation
of the sulfurylated amino acids methionine and cysteine/cystine. Other highly
570 Thierry and Sand
Figure 2 Nitrogen cycle.
Copyright © 2002 Marcel Dekker, Inc.
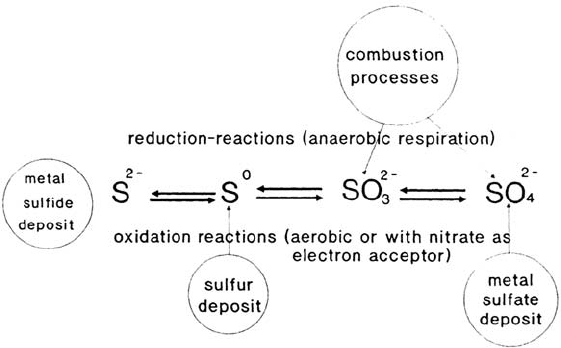
important compounds are those containing reactive thiol groups, such as coenzyme
A or iron-sulfur redox centers involved in electron transfer reactions. The
commercially most important sources are deposits of metal sulfides (production of
valuable metals by mining) and sulfur (source of sulfur for sulfuric acid production).
Metal sulfides can be attacked and degraded microbiologically by the action of
highly specialized bacteria, which oxidize the metal sulfide to a metal sulfate
[105]. This is accompanied by sulfuric acid production, which keeps the metal
ions solubilized. The metals may be recovered by processes such as sedimentation,
solvent extraction, or ion exchange and further purified for practical use. The
oxidation of the sulfide moiety takes place in several steps including sulfur,
polythionate, and sulfite (see Fig. 3). The energy liberated by this oxidation is
recovered by ATP production. The microorganisms active in this process tolerate
high heavy metal concentrations (up to several grams per liter) and low pH values
(pH 1.5 and below). They may grow at temperatures from 4°C up to 90°C. Once
sulfate has been produced, a process similar to denitrification may take place. If
sufficient organic matter is available and anaerobic conditions prevail, sulfate may
act as an electron acceptor for reduction equivalents, being reduced via sulfite and
sulfur to sulfide [31]. The process is called sulfate reduction (sulfate respiration)
and the bacteria are referred to as sulfate reducers or sulfate-reducing bacteria
(SRB). This is a physiologically diverse group of microorganisms including
photosynthetically active bacteria. Besides mesophilic eubacteria, very ancient
life forms belong to this group. It contains archaebacteria, which are able to live
at 110°C by sulfate reduction, conditions probably resembling early stages of life
on this planet. If sulfide accumulates, two different pathways are selected depending
on the oxygen availability. Under aerobic conditions, oxidation to sulfate occurs as
described before. Under anaerobic conditions in the light another type of oxidation
takes place. Photosynthetic microorganisms oxidize sulfide to sulfur and, at least
some of them, to sulfate using the electrons liberated to fill up their photosynthetic
system. Without light it has been discovered that sulfide oxidation coupled to
nitrate reduction can take place. A small part of the sulfide is used for the
Microbially Influenced Corrosion 571
Figure 3 Sulfur cycle.
Copyright © 2002 Marcel Dekker, Inc.
production of sulfur-containing biomolecules. Thus, biology may oxidize and
reduce sulfur and its compounds by many reactions. Deposits of sulfur compounds
resulting from this activity may include elemental sulfur, metal sulfides, and sulfates
like barites. The biological origin can be detected by measurement of the
32
S/
34
S
ratio. The enzymes involved in sulfur metabolism often prefer to handle the lighter
isotope. In consequence, a biological deposit will be enriched with
32
S compared
with sulfur from meteorites which has a strictly defined ratio of 22.22/1.
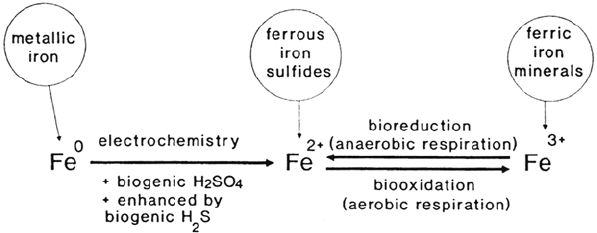
Iron Cycle
Iron is an essential element for life. Although it belongs to the trace elements, a
shortage endangers many vital functions. Generally, it exists as Fe
2+
or Fe
3+
mediating redox reactions combined with electron transfer (see Fig. 4). For example,
iron is the central atom of cytochromes (heme type) and is part of redox proteins
having an iron-sulfur reaction center (nonheme). Ferrous iron may serve as energy
source for many bacteria. Well known are the acidophilic lithoautotrophic bacteria
A. ferrooxidans and Leptospirillum ferrooxidans living in sulfidic, aerobic habitats
by oxidizing ferrous to ferric iron (besides sulfide oxidation to sulfuric acid with A.
ferrooxidans). The energy of the reaction is used for metabolic purposes. Reaction
products are usually ferric iron, sulfuric acid, and to a certain extent intermediary
sulfur. Although these bacteria thrive best at a pH range of 1 to 3.5 [18], where
ferrous iron is only slowly autoxidized by oxygen, organisms such as
Metallogenium sp. (pH 3.5–6.5) and Gallionella ferruginea (pH 6–8) as well as
Leptothrix ochracea (= Sphaerotilus natans, pH 6–8) are responsible for ferrous
iron oxidation at higher pH values. As Fe
2+
becomes increasingly autoxidizable at pH
values above 3.5, these organisms must be adapted to live with low concentrations of
ferrous iron and/or a reduced oxygen partial pressure (microaerophiles). Once ferric
iron has entered the cycle, it will probably be precipitated in the form of ferric
hydroxide, Fe(OH)
3
, followed by several reactions resulting in formation of hematite,
Fe
2
O
3
. Under conditions where organic compounds are available, e.g., in anaerobic
sediments with decaying organisms, ferric iron may be used as an electron acceptor and
be reduced to ferrous iron [73]. Thus, an immobile iron form is transformed into a
mobile one. This process becomes even more important because coprecipitated
heavy metals also become solubilized, consequently endangering the environment.
572 Thierry and Sand
Figure 4 Iron cycle.
Copyright © 2002 Marcel Dekker, Inc.
