Litton C.W., . Reynolds D.C., Collins T.C. Zinc Oxide Materials for Electronic and Optoelectronic Device Applications
Подождите немного. Документ загружается.


considerable skepticism, sparked an ongoing debate about doping and fueled interest in the
material as well. Intensive research efforts were initiated aimed at a number of electronic
and optoe lectronic device applications such as blue/UV light emitters and detectors,
energy efficient white lighting, transparent thin film transi stors (TTFTs) as well as
transparent conductors for large area displays to provide a more cost effective alternative
to tin-doped indium oxide (ITO). LEDs and laser diodes (LD s) aim to take advantage of
the large band gap (E
g
3.35 eV at room temperature) and large free exciton binding
energy ( 60 meV)
[34]
in ZnO, and have been demonstrated
[35,36]
to produce blue/near-UV
emission. Combining such an efficient UV emitter with appropriate phosphors for solid-
state white lighting could save as much as $130B yr
1
in the USA by the year 2025
according to some estimates.
[37,38]
For electronic applications, ZnO TTFTs opera ting at
frequencies in the GHz range have recently been demonst rated.
[39]
However, to take full
advantage of the promise of these devices, it is necessary to understand the bulk electrical
characteristics of ZnO as well as the influence of impurities, defects and surface layer
effects on transport properties. In this chapter, these fundamental aspects of the material
are reviewed and some of the major challenges that remain to be solved are discussed. One
of the most common and powerful probes of the electrical properties in semiconductors is
the temperature-dependent Hall effect since it provides a wealth of information about
charge transport in these materials including details about carrier scattering and about the
donor and acceptor levels which control n- and p-type dop ing. We will begin with a brief
review of the basic theory of the Hall effect for bulk conduction in a single band.
Extensions of these concepts will then be presented which allow for quantitative analysis
of conduction by surfaces as well as mixed conduction effects which can play an important
role in the performance of p-type samples.
3.2 Hall-Effect Analysis
3.2.1 Single-Band Conduction
Carrier transport can be accurately described by the Boltzmann transport equation which
can be generalized to include photo-excited carriers:
[40]
@f
@t
þ r
.
r
r
f þ k
.
r
k
f
@f
@t
coll
þGR ¼ 0 ð3:1Þ
Here f is the occupation distribution function in phase space, G is the generation rate and R
is the recombination rate of photo-excited carriers. Using the relaxation time (t)
approximation (RTA) to the Boltzmann transport equation, the current, conductivity, and
Hall coefficient for n-type material are given by:
[41]
j
x
¼
ne
2
t
hi
m*
E
x
¼nem
c
E
x
ð3:2Þ
R
H
¼
E
y
j
x
B
z
¼
1
ne
t
2
t
hi
2
¼
r
ne
ð3:3Þ
62 Electrical Transport Properties in Zinc Oxide

m
H
¼ R
H
s ¼
1
ne
Gt
2
H
GtH
2
!
ne
2
GtH
m
*
!
¼
e
m
*
Gt
2
H
GtH
ð3:4Þ
where
t
n
ðEÞ
hi
¼
Ð
1
0
t
n
ðE ÞE
3=2
e
E=
kT
dE
Ð
1
0
E
3=2
e
E=
kT
dE
ð3:5Þ
for nondegenerate electrons (Boltzmann statistics) where electric and magnetic fields are
applied in the x- and z-directions, respectively. Equations (3.1)–(3.5) can also be used to
describe nondegenerate hole transport by making the substitutions n !p and e !-e. The
relaxation time arises from carrier scattering by a variety of mechanisms:
t
1
¼ t
1
ii
þt
1
ac
þt
1
po
þt
1
pe
þt
1
dis
ð3:6Þ
where the most common scattering sources include ionized impurities (t
ii
), acoustic
phonons (t
ac
), polar-optical phonons (t
po
), the piezoel ectric potential (t
pe
) and charged
dislocation defects (t
dis
), each with its own scattering rate (inverse relaxation time) which
simply add to produce the total scattering rate as shown. Standard approximat ions for these
scattering mechanisms have been developed
[40–46]
and provide the connection between
experimental Hall-effect measurements and intrinsi c materials parameters of interest as
well as donor, acceptor, and dislocation concentrations. For completeness, expressions for
these scattering modes are listed below (constants and materials-related parameters are
listed in Table 3.1):
t
ii
ðEÞ¼
2
9=2
p«
2
o
ðm
*
Þ
1=2
E
3=2
e
4
ð2N
A
þnÞ½lnð1 þyÞy=ð1 þyÞ
ð3:7Þ
Table 3.1 Physical constants and ZnO specific materials
parameters for Hall-effect analysis
h 1.055 10
34
Js
e 1.602 10
19
C
k
B
1.380 10
23
JK
1
m
0.3
m
e
¼2.73 10
31
kg
«
o
(300 K) 8.12
« ¼7.19 10
11
Fm
1
«
1
(300 K) 3.72
« ¼3.29 10
11
Fm
1
T
po
837 K
E
1
15 eV
r
d
5.675 10
3
kg m
3
s 6.006 10
3
ms
1
P
pe
0.21
c
latt
5.2069 10
10
m
Hall-Effect Analysis 63

for scattering from ionized impurities in nondegenerate, n-type material where «
0
is the
static dielectric constant, y ¼8«
0
m
k
B
TE/(h
2
e
2
n), k
B
is the Boltzmann constant, and h is
Planck’s constant divided by 2p. This is the well-known Brooks–Herring formula which is
valid
[46]
when ka
o
/2 1, where a
o
is the Bohr radius. Equation (3.7) can be applied to
p-type samples by replacing (2N
A
þn) with (2N
D
þp). The relaxation time for acoustic
mode deformation potential scattering is:
t
ac
ðE Þ¼
ph
4
r
d
s
2
E
1=2
2
1=2
e
2
E
2
1
ðm
*
Þ
3=2
k
B
T
ð3:8Þ
where r
d
is the density, s is the speed of sound in the material and E
1
is the deformation
potential. Since scattering from polar optical phonons is inelastic, we can only get an
approximate expression for the relaxation time:
[41,47]
t
po
ðEÞ¼
2
3=2
ph
2
ðe
T
po
=T
1Þ½0:5446E
1=2
þ0:5888ðk
B
T
po
Þ
1=2
0:1683ðk
B
T
po
Þ
1=2
E
e
2
k
B
T
po
ðm
*
Þ
1=2
ð«
1
1
«
1
o
Þ
ð3:9Þ
where T
po
is the Debye temperature and «
1
is the high-frequency dielectric constant.
Polar-optical mode scattering can be significant at room temperature and above, but this
scattering weakens consider ably in ZnO for T G 150 K and the RTA analysis provides
quite accurate results. The relaxation time for acoustic mode piezoelectric-potential
scattering can be written:
t
pe
ðE Þ¼
2
3=2
ph
2
«
o
E
1=2
e
2
P
2
ðm
*
Þ
1=2
k
B
T
ð3:10Þ
where P ¼(h
pz
2
/rs
2
«
0
)
1/2
is the coefficient of piezoelectric coupling. Finally,
t
dis
ðE Þ¼
h
3
«
2
o
c
2
latt
1 þ
8m
*
l
2
«
h
2
3=2
N
dis
m
*
e
4
l
4
ð3:11Þ
for scattering from dislocations, where l ¼(«
0
k
B
T/e
2
n)
1/2
is the screening parameter.
Since dislocation densities of 10
10
cm
2
and higher are quite common for ZnO samples
grown on non-native substrates, this mechanism can be very important and may reduce the
room temperature Hall mobility to values m
H
G100 cm
2
V
1
s
1
for epitaxial samples.
We can use these analytic expressions for the dominant carrier scattering mechanisms to
fit experimental Hall mobility data as a function of temperature, m
H
(T). Of particular
interest is the scattering from ionized impurities, which usually dominates at low
temperatures. Using Equation (3.7), we can determine the concentration of acceptors,
N
A
, in n-type material or the donor concentration, N
D
, for p-type material. In practice, it is
necessary to simultaneously fit both m
H
(T) and n
H
(T) since the carrier concentration
appears in Equation (3.7). The fit of n
H
(T) provides shallow donor concentrations and
donor energies through the charge-balance equation (CBE):
64 Electrical Transport Properties in Zinc Oxide
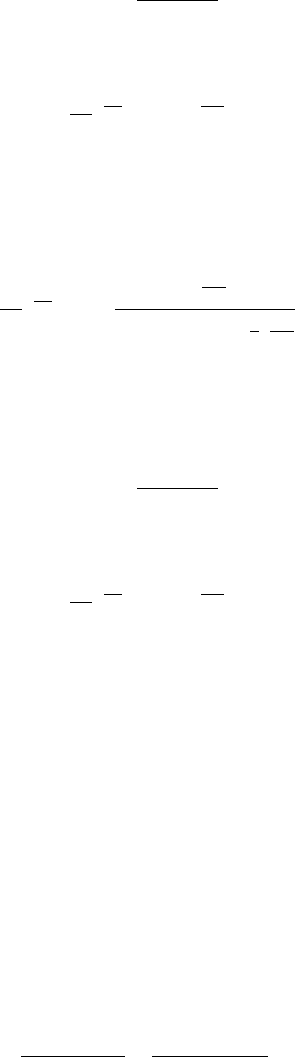
n þN
A
¼
X
i
N
D
i
1 þn=f
D
i
ð3:12Þ
where the sum extends over separate donor levels, i, and
f
D
i
¼
g
0
i
g
1
i
e
a
D
i
k
B
N
0
C
T
3=2
e
E
D0
i
k
B
T
ð3:13Þ
For each donor, g
0
/g
1
is a degeneracy factor, N
0
C
¼2(2pm
n
k
B
)
3/2
/h
3
is the effective densi ty
of states at 1 K, E
D
i
is the energy of the ith donor and E
D
i
¼E
D0i
a
Di
T. For effective-mass-
like donor levels, the analysis can be generalized to account for hydrogenic-type excited
states:
f
D
i
¼
g
0
i
g
1
i
e
a
D
i
k
B
N
0
C
T
3=2
e
E
D0
i
k
B
T
1 þ
P
m
2
j
2
e
1
1
j
2
E
D0
i
k
B
T
ð3:14Þ
where it is assumed that g
j
/g
0
¼j
2
(similar to the H atom) and a
D
i
is assumed to be the same
for each excited state as in the ground sta te (i. e. independent of j).
Similar expressions can be derived for p-type samples, where the CBE becomes:
p þN
D
¼
X
i
N
A
i
1 þn=f
A
i
ð3:15Þ
with
f
A
i
¼
g
1
i
g
0
i
e
a
A
i
k
B
N
0
V
T
3=2
e
E
A0
i
k
B
T
ð3:16Þ
where N
0
V
¼2(2pm
p
k
B
)
3/2
/h
3
and E
A
i
¼E
A0
i
a
A
i
T. Note that the degeneracy factor,
g
1
/g
0
, is inverted from the analogous term in Equation (3.13) because the degeneracies
refer to electrons rather than holes.
[41]
3.2.2 Two-Band Mixed Conduc tion
One important special case of two-layer conduction in ZnO involves a thin conducting
n-type layer on p-type bulk material. In this case, for transport by electrons in the
conduction band and holes in the valence band, the current, conductivity, and Hall
coefficient take the form:
[41]
j
x
¼ j
px
þj
nx
ð3:17Þ
s ¼ s
p
þs
n
¼ eðpm
p
þnm
n
Þð3:18Þ
R
H
¼
R
p
s
2
p
þR
n
s
2
n
ðs
p
þs
n
Þ
2
¼
pm
2
p
nm
2
n
eðpm
p
þnm
n
Þ
2
ð3:19Þ
Hall-Effect Analysis 65

The sign of R
H
is used to assign the sample ‘type’ from a given Hall-effect measure-
ment; a sample will be classified as n-type if pm
2
p
Gnm
2
n
or as p-type when pm
2
p
Hnm
2
n
.
Notice that when pm
2
p
¼ nm
2
n
the Hall coefficient R
H
¼0. This feature results in a
singularity in the apparent carrier concentration [Equation (3.3)] when a simple sing le-
band analysis is used and a change of sign for R
H
across this transition. Likewise, since the
Hall mobility is derived from the Hall coefficient and conductivity [Equation (3.4)], it will
go to zero when pm
2
p
¼ nm
2
n
. These behaviors provide clear signatures of the presence of
mixed conduction effects.
3.2.3 Conducting Surface Layers
As was pointed out in the introduction, ZnO surfaces are highly reactive and surface
conducting or depletion layers can dramatically change the electrical characteristics of the
material. For such a case, where the carrier concentration and mobility are depth
dependent, the analysis provided above can be generalized:
s
sq
¼
ð
d
0
sðzÞdz ¼ e
ð
d
0
nðzÞmðzÞdz ð3:20Þ
R
Hsq
s
2
sq
¼
ð
d
0
nðzÞm
2
ðzÞdz ð3:21Þ
where s
sq
and R
H
sq
denote sheet (areal) quantities (cm
2
) rather than volume quantities
(cm
3
). For the simple case of a two-layer system, with a thin surface conducting layer on a
thick, bulk sample, the effective mobility and carrie r concentration can be determined
explicitly:
m ¼
n
b
m
2
b
þn
s
m
2
s
n
b
m
b
þn
s
m
s
ð3:22Þ
n ¼
ðn
b
m
b
þn
s
m
s
Þ
2
n
b
m
2
b
þn
s
m
s
2
ð3:23Þ
The impact of such conductive surface layers on Hall-effect measurements can be
substantial, as seen in Figures 3.1 and 3.2. Recent work
[47–49]
has extended this analysis
to provide more quantitative information about the characteristics of the surface. Examples
of surface effects will be discussed in more detail later.
3.3 Donor States and n-type Doping
As-grown, unintentionally doped ZnO generally exhibits n-type conduction, with
typical electron concentrations on the order of n 10
15
–10
17
cm
3
.
[50]
For such samples,
66 Electrical Transport Properties in Zinc Oxide

temperature-dependent Hall -effect measurements usually show one or more shallow
donors with binding energies in the range of E
d
30–75 meV.
[51–53]
Surprisingly, a
definitive assignment for the source of these background carriers has not been clearly
established and this question continues to be actively studied and debated.
[54]
Measured
values of the room temperature electron mobility are typically m
e
200 cm
2
V
1
s
1[55]
for
high quality samples and peak values m
peak
H2000 cm
2
V
1
s
1
have been observed at low
temperature. Material prepared by vapor-phase-growth typically exhibits the highest
mobility although melt-grown material can sometimes have peak values that are almost
as high. Hydrothermal ZnO shows lower values of mobility, sometimes much lower, partly
because of much higher background acceptor impurity concentrations.
10
11
10
12
10
13
10
14
0 10 20 30 40
combined bulk and
surface electrons
surface electrons
HYD #1
bulk electrons
10
3
/T (K
-1
)
n
meas
(cm
-3
)
Figure 3.1 Representative temperature-dependent Hall-effect data and two-layer model fits
showing the impact of a surface conducting layer on carrier concentration
0
200
400
600
0 100 200 300
combined bulk and
surface electrons
surface electrons
bulk electrons
Sample HYD #1
T (K)
µ
meas
(cm
2
V
–1
s
–1
)
Figure 3.2 Representative temperature-dependent Hall-effect data and two-layer model fits
showing the impact of a surface conducting layer on carrier mobility
Donor States and n-type Doping 67
3.3.1 Native Point Defects – Donors
Basic bonding chemistry tells us that native point defects, such as oxygen vacancies (V
O
)
or zinc interstitials (Zn
i
), should be donors, and for many years it was widely believed that
these defects were responsible for the intrinsic n-type behavior observed
[6,56]
in uninten-
tionally doped material, since ZnO growth is often performed under Zn-rich conditions.
This explanation continues to be cited frequently in the current literature even though there
is no direct evidence to support the assignment of these defects as the dominant
donors.
[16,57,58]
While variations in stoichiometry do appear to play an important role
in determining the surface conduc tivity of ZnO,
[7]
this native defect model was chal-
lenged
[59]
for bulk tra nsport by theoretical calculations that suggested that V
O
and Zn
i
do
not have shallow levels, but are in fact deep donors, and have high formation energies in n-
type material.
[54,60–67]
However, it was quickly realized that Zn
i
is actually a shallow
donor, but the formation energy is still high. Electron paramagnetic resonance (EPR) and
positron annihilation spectroscopy (PAS) measurements
[68–74]
have reported that the V
O
donor level is deep, and that it is not observed in as-grown material. Electron irradiation
experiments, which have been used to produce point defects in ZnO,
[71–77]
show that V
O
is
stable up to 400
C.
[78]
These irradiation experiments have also demonstrated
[75,76]
that
Zn
i
is actually a shallow donor (E
d
E
c
30 meV), but for n-type growth conditions, the
Zn
i
concentration should still be very low because of its high formation energy. In addition,
the Zn
i
defect diffuses rapidly at 500
C
[79–81]
so it is unlikely to be stable at room
temperature.
[74]
Ion bombardment experiments indicate point defects are unstable above
300
C.
[82,83]
There has been some debate whether the Zn anti-site defect (Zn occupying an O
sublattice site, Zn
O
) is a shallow or deep donor
[59–61]
but recent work
[62]
suggests that it
induces a lattice distortion resulting in a shallow level. However, Zn
O
has an even higher
formation energy than either V
O
or Zn
i
defects, so it is not expected to be present in any
significant quantity, especially in n-type material. No evidence of electrical or optical
activity for the Zn
O
level has been observed experimentally.
Recent theo retical analyses
[60–62]
conclude that isolated native defects are not respon-
sible for the high residual electron concentration found in unintentionally doped material.
There is, however, some experimental evidence that complexes
[84]
involving Zn
i
and
acceptors such as N or Li substituting for O (N
O
,Li
O
) may exist as shallow donors in n-
type material and have low enough formation energies to be produced in significant
concentrations. Density functional calculations indicate that such defect complexes can
form through Coulombic interactions
[85]
or as a result of quantum mechanical hybridiza-
tion effects.
[86]
It has even been suggested recently that an attracti ve interaction between
V
O
and Zn
i
may exist and produce a donor defect complex (V
O
-Zn
i
) with a low formation
energy.
[86]
A complex between a deep V
O
donor and an unidentified shallow donor has
been observed using optically detected EPR.
[78]
For intentionally doped material, the formation energies of V
O
,Zn
i
, and Zn
O
defects are
large enough under n-type growth conditions that even the shallow levels should have low
enough concentrations that they will not influence the electrical transport properties.
However, in p-type material, since the Fermi level (E
F
) is close to the valence band
maximum, these defects will have lower formation energies, especially under Zn-rich
growth conditions, and may limit p-type doping through self-compensation.
68 Electrical Transport Properties in Zinc Oxide
3.3.2 Substitutional Donors
The group III elements, Al, Ga, and In, are all well-known donors
[6,87–91]
when
substituting in the Zn sublattice, and recent work
[92–94]
indicates that B is also a donor,
as expected. One or more of these elements is almost always present in ZnO, as seen by
photoluminescence (PL) measurements,
[53,95,96]
and they are the most common dopants
used to produce n-type material, with electron concentrations in the mid-10
20
cm
3
range
[87–91]
achieved easily. The donor levels
[53]
for Al (E
d
E
c
52 meV), Ga (E
d
E
c
55 meV), and In (E
d
E
c
63 meV) are all in the range of values observed in undoped
material, so residual concentrations of one or more of these impurities are likely to
contribute to the background carriers responsible for the bulk n-type conductivity in these
films.
[88]
There have also been a few reports of n-type doping with the group VII elements, F or
Cl, replacing O.
[97–103]
High electron concentrations have been achieved using the alkali
halides, with measured mobilities comparable to those seen for doping with group III
metals. However, F and Cl are not commonly seen as residual impurities in unintentionally
doped material, and they are unlikely to be utilized for intentional doping given the safety
and environmental issues associated with them. The ease of doping ZnO with group III
metals makes them preferable.
A number of transition metals have also been investigated as potential dopants, such as
Fe, Cr, Co, Ni, Mn, Mo and V.
[6,83,104–113]
However, these elements do not provide shallow
levels and their impact on the electrical properties of ZnO is quite limited. The main
interest in these impurities is for potential magnetic/spintronic applications.
[113,114]
Hydrogen has also been suggested as a promising shallow donor candidate;
[115–119]
given the potentially important role played by H in the electronic properties of ZnO, it will
be discussed separately, and in more detail in the next section.
3.4 Hydrogen
Subjecting ZnO to annealing in a H ambient or exposure to H plasma has long been known
to increase its conductivity.
[6,120–122]
Hydrogen diffuses easily in ZnO, with early
measurements indicating an activation energy for diffusion, E
a
¼0.91 eV,
[6,120,121]
although more recent studies have determined a much lower value, E
a
¼0.17 eV,
[123]
and indicate that all of the H can be removed from a sample by annealing at T H 500
C.
[123–130]
In most semiconductors, H is an amphoteric impurity,
[131]
incorporating as an
H
acceptor in n-type material and as an H
þ
donor in p-type material. However, this is not
the case in ZnO, where calculations using densi ty functional theory show interstitial H
incorporates only in the H
þ
donor configuration for all Fermi level positions
[115]
because
of the strong O-H bond. Experiments using EPR and muon spin rotation confirmed this
doping behavior and determined a donor energy level of E
d
E
c
30 meV.
[116,118]
Similar
values for the H donor level, E
d
E
c
25–70 meV,
[76,118,119,125,127,132–134]
are determined
by electrical and optical measurements. However, infrared (IR) spectroscopy measure-
ments looking at O-H and O-D (deuterium) vibrations suggest that H occupies the anti-
bonding configuration but this molecular complex is unstable at room temperature.
[135,136]
More recent theoretical work suggests that H can form complexes with Zn vacancies
Hydrogen 69
(V
Zn
)
[137]
or substitute in the O sublattice by forming multi-center bonds,
[117,128]
although
there is some disagreement about the latter.
[138]
Of course, H
can also serve to passivate
acceptors in ZnO, as has been observed in several reports.
[27,76,84,133,135,139–149]
Passiv-
ation of acceptors by H can significantly impact the electrical properties if the acceptor
concentration is large,
[142,150]
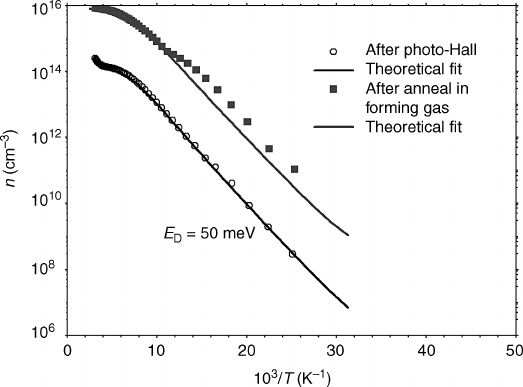
as shown in Figure 3.3 for a hydrothermal ZnO sample. If a
sample with a high concentration of acceptors is exposed to H at sufficient temperature for
diffusion to occur, passivation of these acceptors will increase the Fermi level by
repopulating existing donors that were ionized as a result of compensation, as can be
seen in Table 3.2.
3.5 Acceptor States and p-type Doping
The development of reproducible and stable p-type doping in ZnO has proven to be a
very difficult problem.
[151]
For most of the twentieth century, the inability to identify
any shallow acceptors resulted in a widespread belief that it was impossible to dope ZnO
p-type. Not surprisingly, early reports of successful p-type doping
[26,27,30–33]
were largely
dismissed, but as a result of a large amo unt of work over the past decade, a general
consensus has begun to emerge that p-type material does, in fact, exist. Reports are even
beginning to appear
[152–155]
which claim to have produced stable p-type films. Consider-
able skepticism still remains
[156]
but a concerted effort is now underway to produce better
p-type material and to develop a detailed understanding of the physics of acceptor states in
ZnO. In general, films require thermal annealing treatments, often in air or an O
2
ambient,
Figure 3.3 Increase in electron density produced by forming gas anneal and best-fit two-layer
Hall-effect model. No new donor levels introduced and no change in N
D
for the two dominant
donors, but significant reduction in N
A
most likely caused by passivation with H. Reprinted with
permission from B. Claflin and D. C. Look, J. Vac. Sci. Tech. B 27, 1722 (2009). Copyright
2009, American Vacuum Society
70 Electrical Transport Properties in Zinc Oxide

to exhibit p-type characteristics.
[30,85,152,157–162]
One of the challenging aspects of
developing p-type ZnO is the low value of the hole mobility – typically around
m 1cm
2
V
1
s
1
at room temperature. Similar limitations are also seen in p-type GaN.
Such low hole mobilities make Hall-effect measurements difficult because the Hall
voltages involved are small, and other effects, such as persistent photoconductivity in
ZnO,
[163,164]
and sample homogeneity,
[165–168]
can significantly impact the results.
3.5.1 Native Point Defects – Acceptors
Theoretical calculations indicate that the Zn vacancy (V
Zn
), O interstitial (O
i
), and O anti-
site (O
Zn
) are acceptors
[59–62]
with V
Zn
having the lowest formation energy of all the
acceptor-type native point defects. For n-type material grown under O-rich conditions, V
Zn
is expected to be the dominant acceptor, and this has been confirmed experimental-
ly.
[72,169]
The V
Zn
defect has been well-characterized by PAS measurements since in n-
type material the ionized vacancy is negatively charged and traps positrons easily.
Temperature-dependen t Hall-effect measurements on unintentionally doped ZnO depos-
ited by seeded-vapor growth
[169]
show small concentrations of V
Zn
(low 10
15
cm
3
), which
account for all of the background acceptors observed in this sample. However, these
measurements do not provide any information about the V
Zn
acceptor energy level because
these defects function as com pensation centers in n-type material and thus do not require
thermal activation to be ionized. The V
Zn
acceptor concentration is determined by fitting
temperature-dependent mobility data, involving Equations (3.4–3.11), and in particular, by
analysis of ionized impurity scattering at low temperature using Equation (3.7). In p-type
material, the high formation energy of V
Zn
precludes its generation in significant
concentrations.
[62]
The O
i
defect is predicted to be unstable at the tetrahedral lattice site,
[62]
inducing a
lattice relaxation to form a split interstitial defect, O
i
(split), which is effectively an O
2
molecule embedded in the ZnO lattice.
[62]
The O
i
(split) defect is expected to be
electrically neutral for all positions of the Fermi level. However, interstitial O can also
occupy the octahedral lattice site, O
i
(oct) as a deep acceptor. The theoretical formation
energy for O
i
(oct) is very high and it is not expected to be present in any significant
concentration. Similarly, O
Zn
is predicted to have the highest formation energy of all the
native acceptor point defects and is expected to have negligible concentrations. There have
been no experimental observations of either O
i
(oct) or O
Zn
defects, even after high-energy
electron irradiation.
Table 3.2 Best-fittwo-layerHall-effectmodelparametersforhydrothermalZnOsampleindifferent
states. Donor concentrations unaffected by annealing in forming gas but acceptor concentration
decreases most likely as a result of passivation. Reprinted with permission from B. Claflin and D. C.
Look, J. Vac. Sci. Tech. B 27, 1722 (2009). Copyright 2009, American Vacuum Society
Sample N
D
(50 meV)
10
16
cm
3
N
D
(400 meV)
10
16
cm
3
N
A
10
16
cm
3
As-received 1.228 1.5 1.215
After photo-Hall 2.0 2.0 1.98
Forming gas anneal 2.0 2.0 1.2
Acceptor States and p-type Doping 71
