Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

SOLID-ELECTROLYTE BATTERIES 15.9
15.2.5 Storage
Long-term storage tests show that there is no loss of capacity after storage periods up to 1
year at 20, 45, and 60
⬚C (Fig. 15.7). The battery can withstand storage to 200⬚C, but for
best performance, such high temperatures should be avoided. Temperatures above 200
⬚C
may cause bulging and failure of the seal. The long shelf life results from the chemical
compatibility of the cell components, the absence of chemical reaction between the electrodes
and the electrolyte, and the low electronic conductivity of the solid electrolyte which mini-
mizes self-discharge. Longer-term tests show that there is no measurable loss of capacity
after storage periods of 4 year at 20
⬚C and up to 1 year at temperatures as high as 100⬚C.
On the basis of these tests it is projected that the shelf life of these batteries is at least 15–
20 years under normal storage conditions.
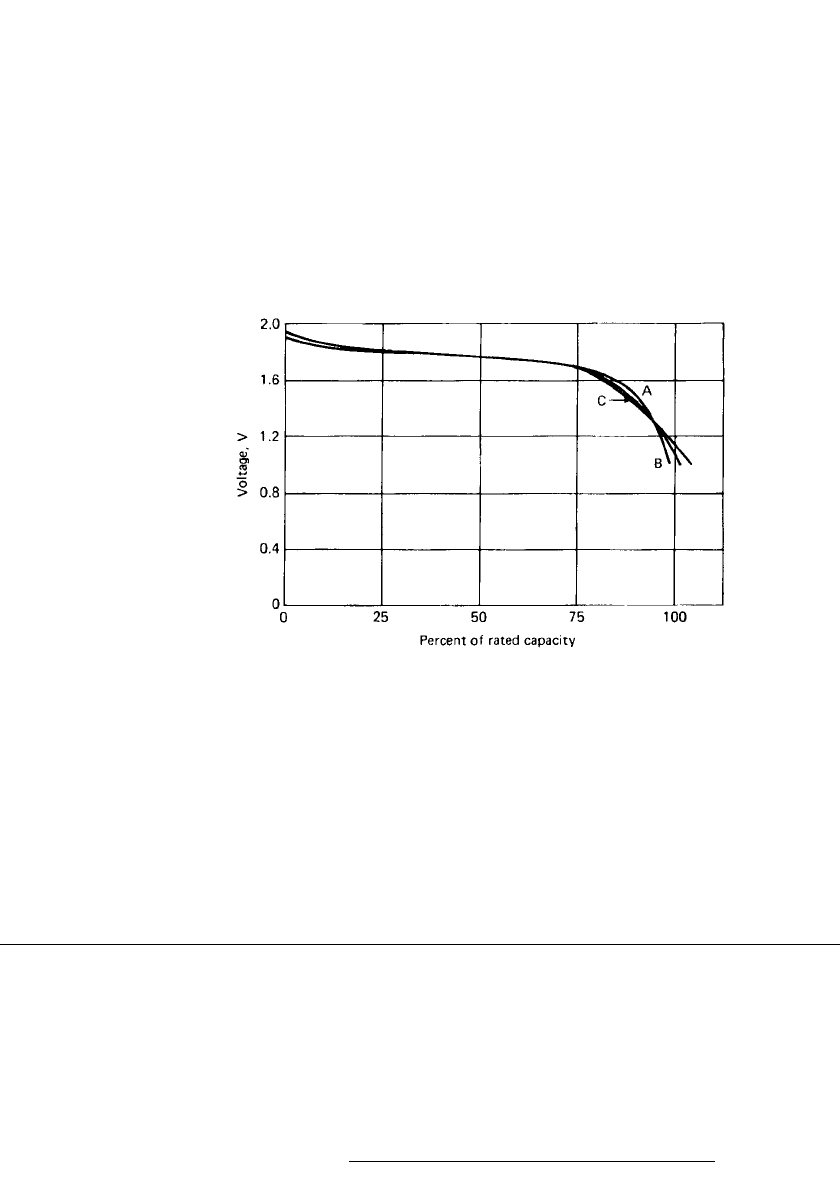
12
FIGURE 15.7 Storage tests of solid-state battery. Discharge after (a)no
storage: (b) 1 year at 45⬚C: (c) 1 year at 60⬚.(Courtesy of Duracell, Inc.)
15.2.6 Handling
Solid-state batteries are designed primarily for low power and long service life. These bat-
teries can withstand short circuit and voltage reversal, although these conditions should be
avoided. No explosion due to pressure buildup or chemical reaction is known to occur under
recommended operating temperatures. Prolonged short-circuiting will result in a separation
between the electrode and the electrolyte, making the cell inoperative.
15.3 THE LITHIUM/ IODINE BATTERY
15.3.1 General Characteristics
Lithium/ iodine batteries are based on the reaction
1
Li ⫹ ⁄
2
I → LiI
2
The specific reactions for the cell using poly-2-vinylpyridine (P2VP) in the cathode are
Anode 2Li → 2Li
⫹
⫹ 2e
Cathode 2Li
⫹
⫹ 2e ⫹ P2VP 䡠 nI
2
→ P2VP 䡠 (n ⫺ 1)I
2
⫹ 2LiI
Overall 2Li ⫹ P2VP 䡠 nI
2
→ P2VP 䡠 (n ⫺ 1)I
2
⫹ 2LiI

15.10 CHAPTER FIFTEEN
Lithium and iodine are consumed, and their reaction product, LiI, precipitates in the region
between the two reactants. The LiI not only is the discharge product, but also serves as the
cell separator and electrolyte. The theoretical energy density is 1.9 Wh/cm
3
(see Table 15.2).
Practical values approaching 1 Wh /cm
3
can be obtained at discharge rates of 1 to 2
A/
cm
2
. Commercially available lithium/iodine batteries have a solid anode of lithium and a
polyphase cathode which is largely iodine. The iodine is made conductive by the addition
of an organic material. Pyridine-containing polymers are most often used for this purpose,
the additive in all present commercial batteries being P2VP. At ambient temperatures the
iodine/ P2VP mixtures are two-phase in undischarged batteries, liquid plus excess solid io-
dine.
23
The iodine content of the cathode decreases during discharge of the battery, and the
remaining cathode material becomes hard as the battery nears depletion. As discharge pro-
ceeds, the layer of lithium iodide becomes thicker. The resistance of the battery also increases
because of the growing amount of discharge product.
The volume change accompanying the cell discharge is negative. The theoretical value
for this volume change is
⫺15% for complete discharge of a balanced mixture of pure iodine
and lithium.
23
It is somewhat less when the chemical cathode is not pure iodine. For example,
a volume change of
⫺12% is expected if the cathode is 91% iodine by weight.
24
The volume
change may be accommodated by the formation of a porous discharge product or of mac-
roscopic voids in the cell.
Cells are formed by contacting the iodine-containing cathode directly with the lithium
anode. The chemical reaction between these two materials immediately forms a thin layer
of lithium iodide between anode and cathode. This layer serves to separate the two elec-
troactive materials electronically and prevents failure due to internal short-circuiting of the
anode and cathode. This makes them especially suitable for applications requiring very high
reliability.
Features of this system include low self-discharge, high reliability, and no gassing during
discharge. Shelf life is 10 years or longer, and the cells can take a considerable amount of
abuse without any catastrophic effects. Batteries of this type have found commercial appli-
cations powering various low-power devices such as cardiac pacemakers, solid-state mem-
ories, and digital watches. Power sources for portable monitoring and recording instruments
and the like are also possible applications.
All the currently available Li /I
2
batteries have a nominal capacity of 15 Ah or less, and
most have deliverable capacities under 5 Ah. All the Li /I
2
batteries intended for medical
applications are designed to be cathode-limited.
15.3.2 Cell Construction
Several generic types of Li /I
2
cells have been produced, three of which were designed for
medical applications such as cardiac pacemakers. Figure 15.8 shows the first type, which
was phased out in the early 1980s. This unit had a case-neutral design and consisted of a
stainless-steel housing with a plastic insulator that lined the inside of the case. A lithium
envelope (the anode) fitted inside the plastic and contained the I
2
(P2VP) depolarizer. The
cathode current collector was located in the center of the cell. Current collector leads from
both the anode and the cathode went through hermetic feed-throughs in the case. This cell
was formed by pouring molten iodine depolarizer into the lithium envelope. After the cathode
material solidified, the top of the lithium envelope was closed, the plastic cup added, and
the final assembly completed. The construction used in this cell eliminates any contact be-
tween the case and the iodine depolarizer.
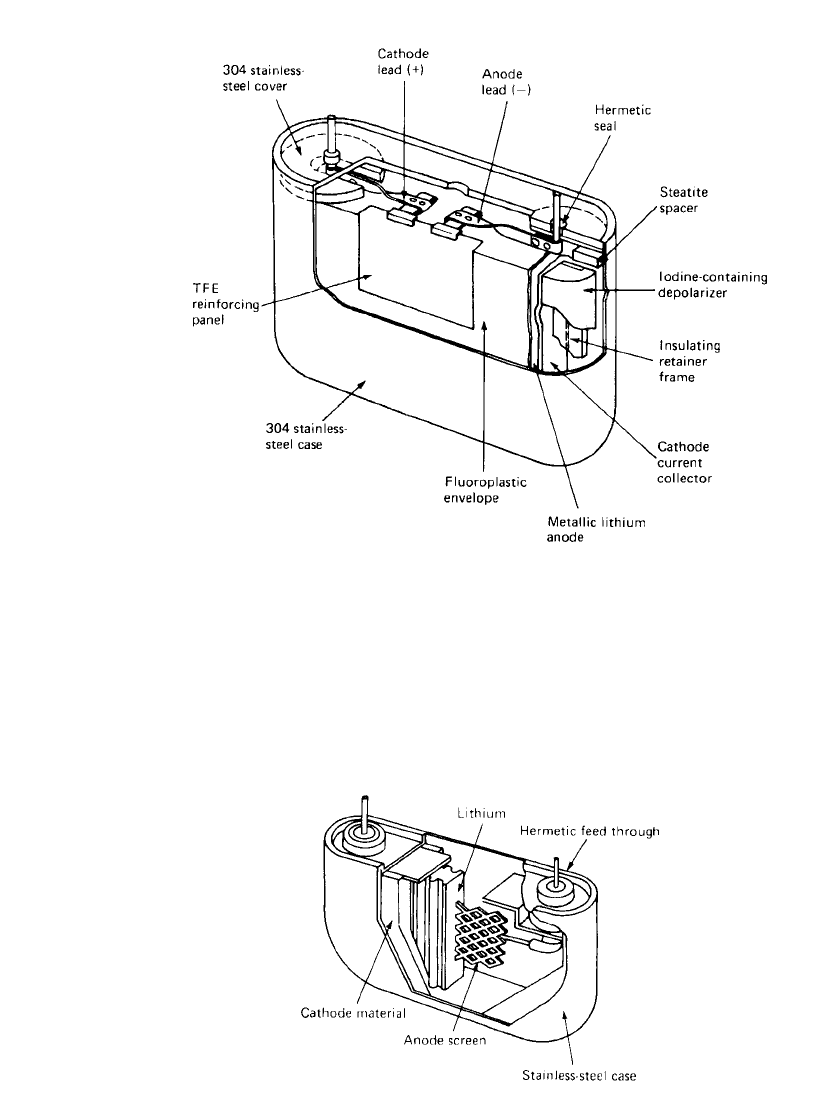
SOLID-ELECTROLYTE BATTERIES 15.11
FIGURE 15.8 Model 802/ 35 Li / I
2
cell. (Courtesy of Catalyst Research Corp.)
FIGURE 15.9 Cutaway view of typical can-positive
Li/I
2
cell. (Courtesy of Wilson Greatbatch, Ltd.)
A second construction uses a case-positive design of similar size. This cell is the type
used today for most medical applications. A cutaway view is shown in Fig. 15.9. This cell
is manufactured in a slightly different manner from that in Fig. 15.8. It contains a central
lithium anode and uses the stainless-steel case of the cell as the positive-current collector.
Most models are completely assembled with their header welded to the can before the cath-
ode is added to the cell. Hot depolarizer is poured into the cell can through a small fill port,
which is later welded shut. The anode current collector is brought out via a glass-to-metal
feed-through.

15.12 CHAPTER FIFTEEN
FIGURE 15.10 Exploded view of case-positive
Li/I
2
cell. (Courtesy of Catalyst Research Corp.)
Manufacturers of these case-positive designs also precoat their anode assembly with a
layer of pure P2VP prior to assembly. This coating is designed to protect the anode from
the environment before assembly, but it also alters the electrical discharge behavior (see Sec.
15.3.4).
Another case-positive cell type has been used for medical applications. This unit is very
similar to the other case-positive designs, but the cathode is not poured into the battery can.
The iodine and P2VP are pelletized and then pressed onto the central anode assembly. After
the pressing operation, the entire unit is slipped into a nickel can. An exploded view of this
cell is shown in Fig. 15.10.
Case-neutral designs were developed to prevent corrosion of the exterior case and to
minimize leakage to the feed-through by the iodine depolarizer. However, 5 years of real-
time data have shown that no significant corrosion of stainless steel in contact with the
cathode depolarizer or its vapor takes place in sealed can-positive cells. Tests show that
corrosion occurs during the first few months after assembly and is limited to a 50-
m layer.
Even at 60
⬚C, corrosion of stainless steel by the iodine depolarizer has not proved to be a
problem in the dry environment of the cell.
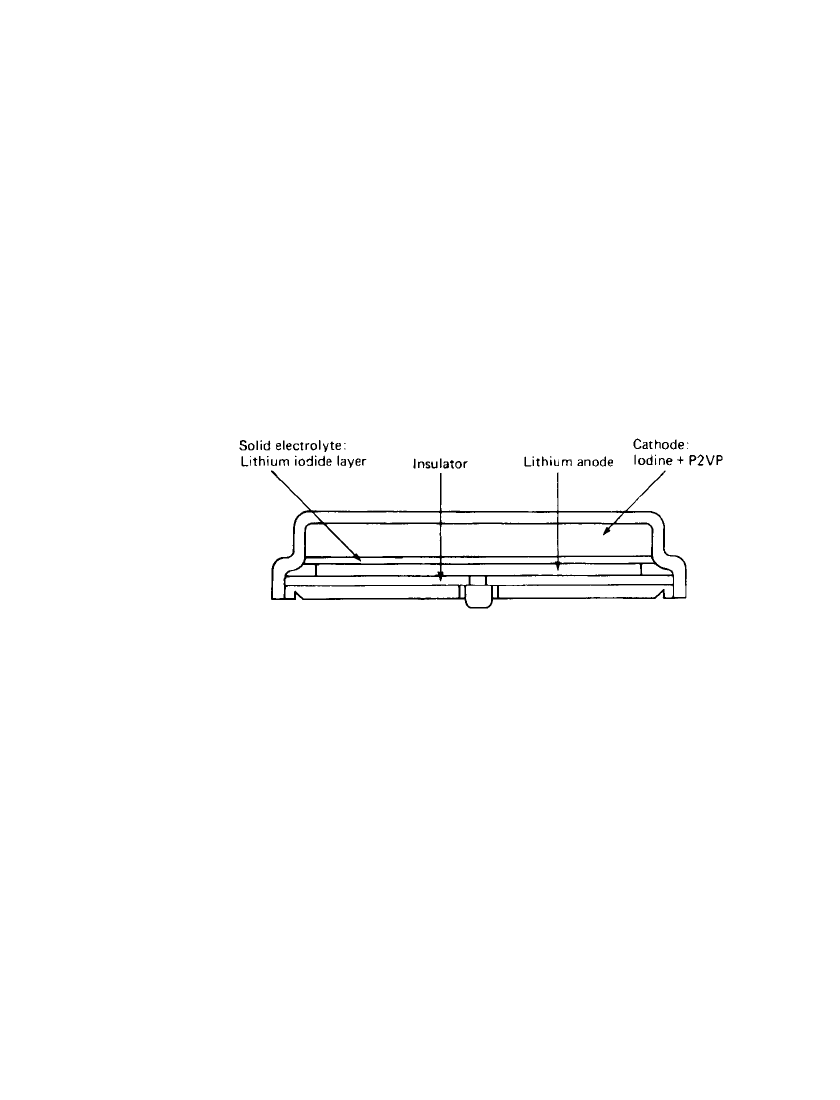
SOLID-ELECTROLYTE BATTERIES 15.13
Li/I
2
medical batteries are produced in a variety of sizes and shapes to meet specific
applications. Their profiles range from rectangular to semicircular, or a combination. All of
them are made quite thin and have flat sides because their primary application is in cardiac
pacemakers. Cell thickness is typically 5 to 10 mm. The area of the lithium anodes ranges
between 10 and 20 cm
2
in current batteries. Some cells use a ribbed anode (see Fig. 15.9)
to increase the amount of active anode surface area in the battery.
The nonmedical batteries are made in more conventional button and cylindrical config-
urations. The hermetically sealed button-cell batteries are intended for powering digital
watches and serving as backup power for computer memories. These batteries are made by
pressing iodine cathode and lithium anode layers into a stainless-steel cup. The cup is the
positive-current collector. A glass-to-metal feed-through brings the negative terminal to the
exterior. Figure 15.11 shows a view of this cell type. The cylindrical (D-cell diameter) Li /
I
2
battery is welded hermetically, like the other batteries described. It is case-positive; the
negative connection is a button on the end of the cell. It is designed to withstand substantial
shock, vibration, and abuse without venting, swelling, leaking, or exploding. These button-
type batteries are no longer commercially available.
FIGURE 15.11 Button-type Li/ I
2
battery. (Courtesy of Catalyst Re-
search Corp.)
Manufacturing of all Li/ I
2
batteries is done in a dry environment (typically less than 1%
RH). In addition, all these batteries are sealed hermetically to prevent exchange of any
material with the environment. Good sealing is required in order to maintain the desired
electrical characteristics.
Connection to the medical-grade batteries is made by soldering or spot welding. The
case-positive varieties usually have a pin or wire welded to the case to facilitate making the
positive connection (see Figs. 15.9 and 15.10).
Manufacturers keep detailed records of the construction and manufacture of each battery
intended for medical applications and each unit is individually serialized. This procedure
allows the systematic tracing of the history and behavior of every battery, should the need
arise.
15.3.3 Commercially Available Batteries
Table 15.6 summarizes the manufacturer’s specifications for some typical pacemaker batter-
ies. Batteries for medical use are relatively expensive because of the low manufacturing
volume and demands for high reliability.

15.14 CHAPTER FIFTEEN
TABLE 15.6 WGL Specifications for Lithium/ Iodine Pacemaker Batteries
Type
Manufacturer’s
rated capacity,
Ah Wt., g Vol., cm
3
Size, mm
(length
⫻
width ⫻
height)
Energy
density
Wh/ cm
3
Specific
energy
Wh/g
9107
9114
9331
8711
8831
9412
9085
9438
9105
9236
8426
9074
8708
8431
8402
8843
8207
8950
8077
8206
8041
9111
0.43
0.56
0.82
0.86
0.89
0.92
0.98
0.98
0.99
1.00
1.07
1.15
1.21
1.28
1.29
1.36
1.55
1.58
1.78
1.98
2.10
2.36
5.3
7.0
9.1
9.5
10.7
10.2
10.9
10.7
10.6
11.0
11.6
12.9
13.4
13.8
14.4
13.6
17.2
15.9
18.9
20.4
22.0
22.8
1.40
1.82
2.34
2.52
2.75
2.20
2.93
2.93
2.95
2.85
3.02
3.28
3.52
3.67
3.72
3.73
4.70
4.45
5.11
5.54
5.81
6.24
30
⫻ 4.2 ⫻ 13
28
⫻ 6 ⫻ 14
32
⫻ 5 ⫻ 19
27
⫻ 5 ⫻ 22
43
⫻ 5 ⫻ 16
32
⫻ 5 ⫻ 21
27
⫻ 5 ⫻ 25
27
⫻ 5 ⫻ 25
27
⫻ 6 ⫻ 21
24
⫻ 6 ⫻ 25
33
⫻ 5 ⫻ 23
43
⫻ 5 ⫻ 18
47
⫻ 5 ⫻ 22
27
⫻ 5 ⫻ 31
45
⫻ 5 ⫻ 22
32
⫻ 6 ⫻ 23
27
⫻ 7.8 ⫻ 25
27
⫻ 6 ⫻ 31
45
⫻ 7 ⫻ 22
27
⫻ 7.8 ⫻ 31
33
⫻ 8.6 ⫻ 26
33
⫻ 7 ⫻ 31
0.81
0.82
0.93
0.90
0.86
1.11
0.89
0.89
0.89
0.93
0.94
0.93
0.91
0.92
0.92
0.97
0.87
0.94
0.92
0.95
0.96
1.00
0.22
0.21
0.24
0.24
0.22
0.24
0.24
0.24
0.25
0.24
0.24
0.24
0.24
0.25
0.24
0.27
0.24
0.26
0.25
0.26
0.25
0.27
Courtesy Wilson Greatbatch Ltd.
15.3.4 Discharge Performance Characteristics
The open-circuit voltage of a Li /I
2
battery is very near 2.80 V. This value is maintained
through the useful life of the battery. The resistance of the lithium iodide discharge product
controls the load voltage for most of the discharge. The morphology of the lithium iodide
discharge product, in turn, depends on the way the battery is constructed. Only at the end
of the discharge cycle is the resistance of the iodine depolarizer significant in the total internal
resistance of the battery. Because the battery resistance is quite high and increases throughout
life, the discharge curves are not flat, even at moderate current drains. Two characteristic
types of discharge curves are observed.
Batteries made by putting the depolarizer against bare lithium anode metal discharge by
growing a nearly planar layer of lithium iodide. This layer becomes thicker in proportion to
the amount of discharge; hence the load voltage during this first phase of the discharge
decreases linearly with the amount of charge removed from the battery, until all the available
iodine is consumed. At that time the voltage drops at a steeper rate, indicative of the in-
creasingly significant resistance of the iodine-deficient depolarizer. In the final, third phase
the open-circuit as well as the load voltage drops as iodine is no longer available at unit
thermodynamic activity. The cells for medical use are made cathode-limited so their end-of-
life voltage change is much less abrupt than if they were lithium-limited. The normalized
slope (Volts per Ampere-hour) for the straight-line portion of the discharge curves is ap-
proximated by:
⌬E 3600Mi i
⫽ 䡠 ⫽ K
22
⌬QF
dA A

SOLID-ELECTROLYTE BATTERIES 15.15
where ⌬ E/⌬Q ⫽ slope of discharge curve, V /Ah
M
⫽ molecular weight of LiI
d
⫽ density of LiI
⫽ lithium ion conductivity of LiI
F
⫽ Faraday’s constant
i
⫽ discharge current
A
⫽ area of anode, cm
2
Literature values of the Li
⫹
conductivity in LiI range from 0.2 to 0.8 ⫻ 10 ⍀ 䡠 cm at
⫺
6
⫺
1
⫺
1
37⬚C.
16–18
K then lies between 1.5 and 6.0 ⫻ 10
6
. Experimental values of K measured from
discharge data have been found to be between 1.0 and 1.5
⫻ 10
6
. At high discharge rates,
fracturing of the LiI layer apparently occurs, and this equation holds only over small portions
of the discharge curve.
The resistance of these batteries behaves consistently with the load voltage curves. It
builds up linearly with discharge until the cells are depleted of iodine. At discharge rates
much above 10
A/cm
2
a nonohmic component of the polarization begins to become in-
creasingly important.
25
The nonohmic component appears to originate near the discharge
product-cathode interface. The effects of this polarization may last a long time after high-
rate discharge. Thus extrapolation of performance between rates is not simple.
A different discharge curve is obtained from lithium /iodine batteries which are con-
structed with a coating of pure P2VP applied to the anode before the iodine depolarizer is
added to the battery. This coating alters the discharge behavior of these cells. The discharge
product no longer grows in a planar fashion but rather in columnar-type groupings. It has
been shown that a liquid breakdown reaction product of the P2VP anode coating has been
observed in special experimental cells constructed on microscope slides.
26
This liquid is
thought to be a liquid electrolyte which wets the lithium iodide discharge product and forms
a lower resistance path in parallel to the lithium iodide.
Plots of discharge voltage versus state of discharge for these batteries are not linear. The
battery resistance increases exponentially with the state of discharge. This dependence ex-
tends from the beginning of life throughout the region of discharge where the cathode is
two-phase, consisting of crystalline iodine plus a conductive liquid, which is a product of a
reaction between iodine and P2VP. During most of the discharge, the resistance is dominated
by the accumulating discharge product and the charge-transfer reaction. Once the crystalline
iodine is depleted, the resistivity of the cathode rises rapidly and soon dominates the resis-
tance of the battery. The crossover from electrolyte to cathode domination of battery resis-
tance gives the discharge curves of Li/ I
2
batteries their characteristic shape.
Detailed studies of Li/ I
2
batteries have shown that under conditions of mild discharge
(
ⱖ5
A/cm
2
) the voltage loss below 2.8 V results from five physical processes: (1) a drop
in open-circuit potential as the discharge proceeds into the single-phase region of cathode
composition, (2) an exponential increase in electrolyte resistance throughout the discharge,
(3) bulk ohmic cathode resistance, which decreases slightly as the discharge proceeds in the
two-phase region of cathode composition, but which rises sharply after the crystalline iodine
is depleted, (4) a charge-transfer resistance at the case-to-cathode interface, and (5) concen-
tration polarization in the cathode. All of these processes have been characterized quantita-
tively in a general predictive steady-state discharge model of Li /I
2
battery discharge.
27
Figure 15.12 shows a typical plot of load voltage versus state of discharge for both coated-
and uncoated-anode groups of otherwise identical batteries at 37
⬚C. The voltage data are
shown between the initial voltage of 2.8 V and the cutoff voltage of 2.0 V. All data were
obtained at a current density of 6.7
A/cm
2
. The voltage of the uncoated batteries decreases
linearly until iodine depletion starts to occur. At this time the slope of the curve changes.
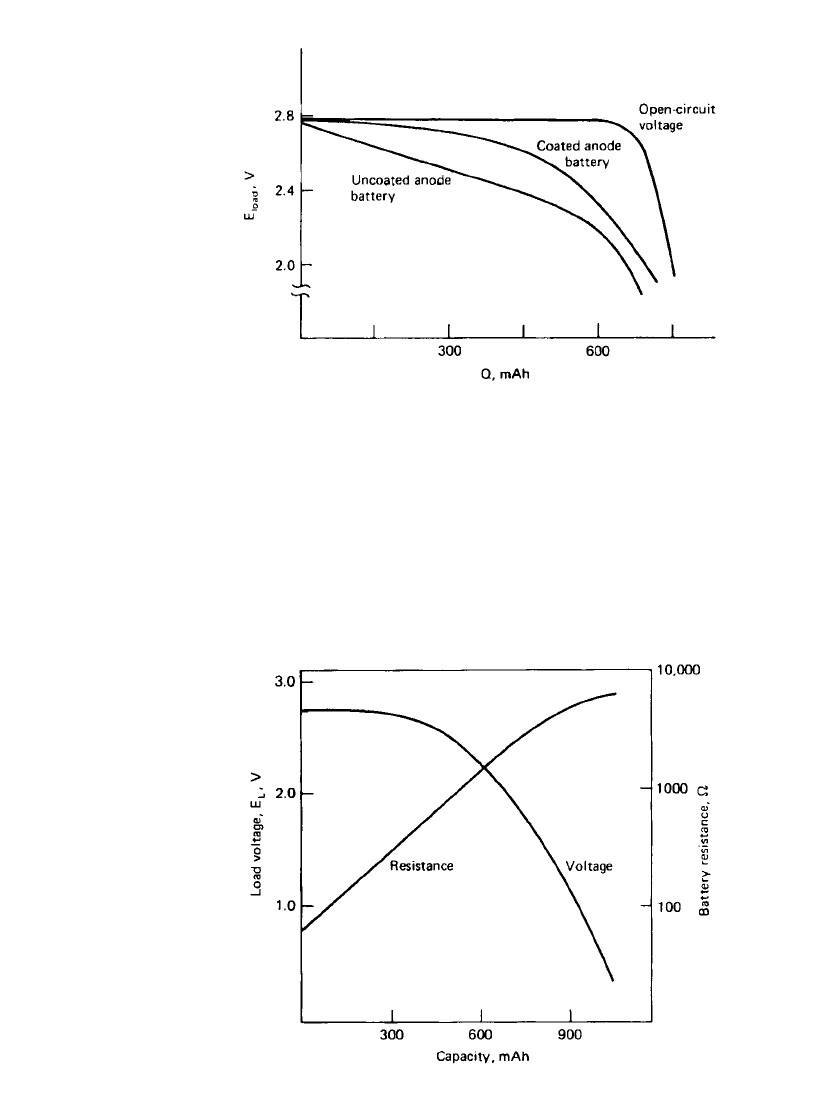
15.16 CHAPTER FIFTEEN
FIGURE 15.12 Load voltage vs. discharge state for uncoated and
P2VP-coated anode Li/ I
2
batteries discharged at 6.7
A/cm
2
.37⬚C.
(Courtesy of Medtronic, Inc.)
FIGURE 15.13 Load voltage and battery resistance vs. Q for coated-
anode Li / I
2
battery discharged at 100
A, 37⬚C. (Courtesy of Med-
tronic, Inc.)
The same batteries with P2VP-coated anodes exhibit a higher load voltage at this current
density. However, as the current density decreases, the differences between the discharge
curves for the coated- and uncoated-anode batteries become smaller. Figure 15.13 shows the
discharge voltage data for the coated-anode battery in Fig. 15.12 to 0 V with the correspond-
ing resistance data. Log R versus Q is linear, and R varies between 100 and 8000
⍀ during
discharge. Typical discharge curves for a CRC 800 series battery, as well as changes in
SOLID-ELECTROLYTE BATTERIES 15.17
battery resistance, are shown in Fig. 15.14. Catalyst Research Corporation reported that its
900 series batteries (manufactured by pressing the cathode depolarizer onto a central lithium
anode assembly) exhibited discharge behavior very similar to that of the coated-anode bat-
teries.
The button and D-size batteries made by Catalyst Research Corporation were reported to
have discharge curves like the 900 series batteries. Figures 15.15 and 15.16 show projected
discharge curves for two types of button cells. Figure 15.17 is a similar discharge plot for
the D-size battery at 25
⬚C. This cell delivered 7 Ah (0.45 Wh /cm
3
) at the 2-month (5-mA)
rate; capacities and energy densities at lower rates are expected to be larger. The projected
discharge curves for the 1- and 2-mA rates are also shown.
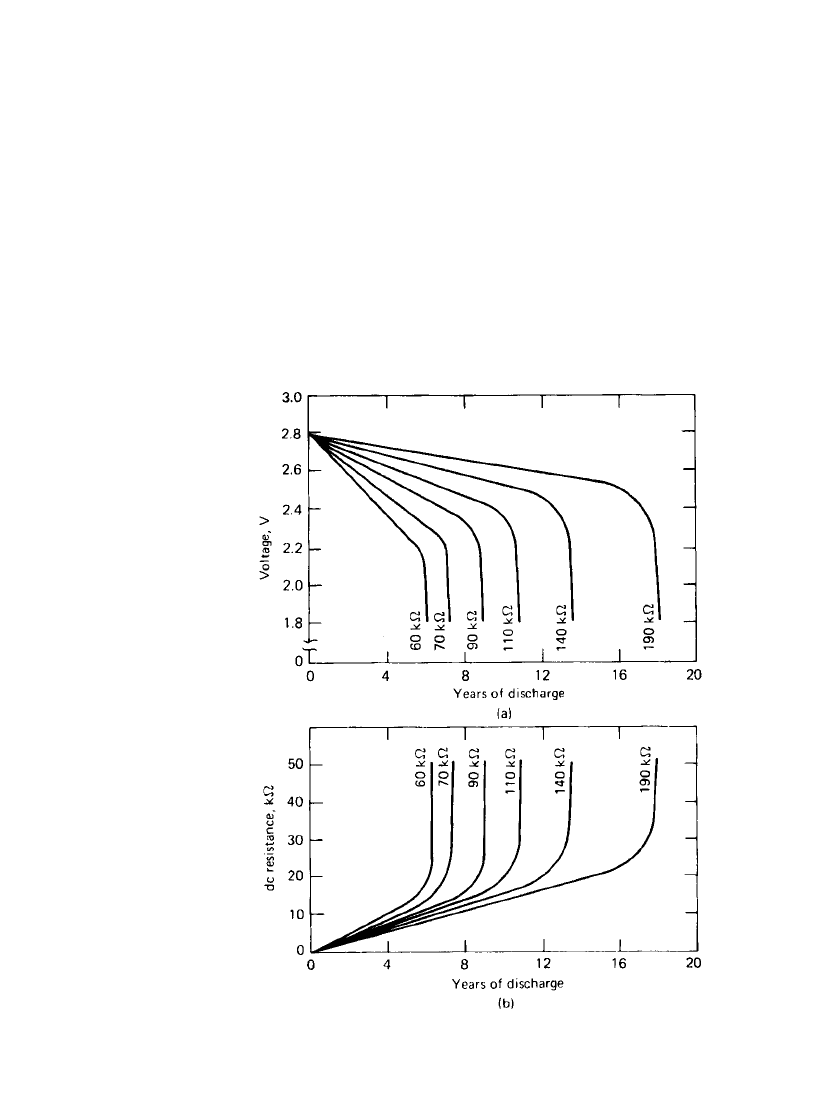
FIGURE 15.14 800 series Li / I
2
battery (a) Typical discharge
curves. (b) Changes in cell resistance during discharge. (Courtesy
of Catalyst Research Corp.)
15.18 CHAPTER FIFTEEN
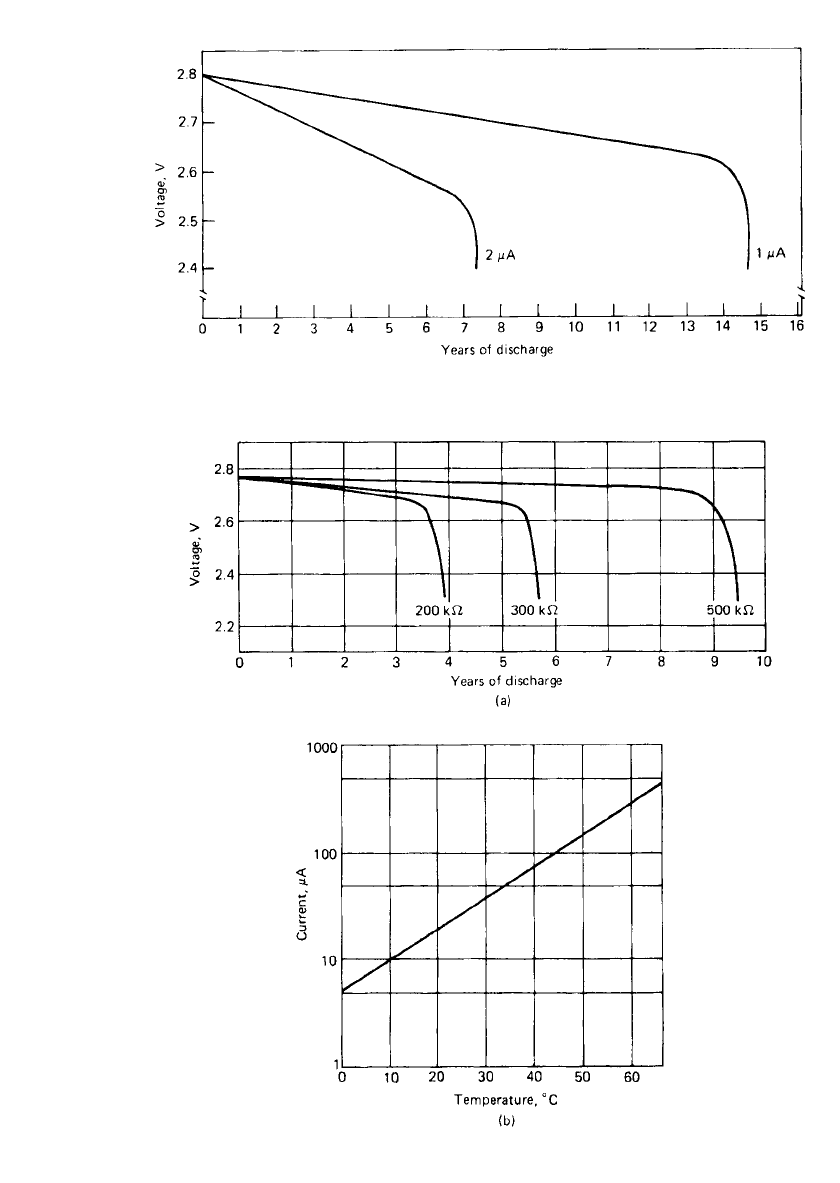
FIGURE 15.15 Projected discharge curves for S19P-20 button watch battery at 25⬚C. (Courtesy
of Catalyst Research Corp.)
FIGURE 15.16 Discharge characteristics for 2736 button-cell battery (a) Projected dis-
charge at 25⬚C. (b) Maximum continuous discharge current vs. temperature.
25
(Courtesy
of Catalyst Research Corp.)
