Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


BASIC CONCEPTS 1.5
1.2.1 Primary Cells or Batteries
These batteries are not capable of being easily or effectively recharged electrically and,
hence, are discharged once and discarded. Many primary cells in which the electrolyte is
contained by an absorbent or separator material (there is no free or liquid electrolyte) are
termed ‘‘dry cells.’’
The primary battery is a convenient, usually inexpensive, lightweight source of packaged
power for portable electronic and electric devices, lighting, photographic equipment, toys,
memory backup, and a host of other applications, giving freedom from utility power. The
general advantages of primary batteries are good shelf life, high energy density at low to
moderate discharge rates, little, if any, maintenance, and ease of use. Although large high-
capacity primary batteries are used in military applications, signaling, standby power, and
so on, the vast majority of primary batteries are the familiar single cell cylindrical and flat
button batteries or multicell batteries using these component cells.
1.2.2 Secondary or Rechargeable Cells or Batteries
These batteries can be recharged electrically, after discharge, to their original condition by
passing current through them in the opposite direction to that of the discharge current. They
are storage devices for electric energy and are known also as ‘‘storage batteries’’ or ‘‘accu-
mulators.’’
The applications of secondary batteries fall into two main categories:
1. Those applications in which the secondary battery is used as an energy-storage device,
generally being electrically connected to and charged by a prime energy source and
delivering its energy to the load on demand. Examples are automotive and aircraft sys-
tems, emergency no-fail and standby (UPS) power sources, hybrid electric vehicles and
stationary energy storage (SES) systems for electric utility load leveling.
2. Those applications in which the secondary battery is used or discharged essentially as a
primary battery, but recharged after use rather than being discarded. Secondary batteries
are used in this manner as, for example, in portable consumer electronics, power tools,
electric vehicles, etc., for cost savings (as they can be recharged rather than replaced),
and in applications requiring power drains beyond the capability of primary batteries.
Secondary batteries are characterized (in addition to their ability to be recharged) by high
power density, high discharge rate, flat discharge curves, and good low-temperature perform-
ance. Their energy densities are generally lower than those of primary batteries. Their charge
retention also is poorer than that of most primary batteries, although the capacity of the
secondary battery that is lost on standing can be restored by recharging.
Some batteries, known as ‘‘mechanically rechargeable types,’’ are ‘‘recharged’’ by replace-
ment of the discharged or depleted electrode, usually the metal anode, with a fresh one.
Some of the metal /air batteries (Chap. 38) are representative of this type of battery.
1.2.3 Reserve Batteries
In these primary types, a key component is separated from the rest of the battery prior to
activation. In this condition, chemical deterioration or self-discharge is essentially eliminated,
and the battery is capable of long-term storage. Usually the electrolyte is the component that
is isolated. In other systems, such as the thermal battery, the battery is inactive until it is
heated, melting a solid electrolyte, which then becomes conductive.

1.6 CHAPTER ONE
The reserve battery design is used to meet extremely long or environmentally severe
storage requirements that cannot be met with an ‘‘active’’ battery designed for the same
performance characteristics. These batteries are used, for example, to deliver high power for
relatively short periods of time, in missiles, torpedoes, and other weapon systems.
1.2.4 Fuel Cells
Fuel cells, like batteries, are electrochemical galvanic cells that convert chemical energy
directly into electrical energy and are not subject to the Carnot cycle limitations of heat
engines. Fuel cells are similar to batteries except that the active materials are not an integral
part of the device (as in a battery), but are fed into the fuel cell from an external source
when power is desired. The fuel cell differs from a battery in that it has the capability of
producing electrical energy as long as the active materials are fed to the electrodes (assuming
the electrodes do not fail). The battery will cease to produce electrical energy when the
limiting reactant stored within the battery is consumed.
The electrode materials of the fuel cell are inert in that they are not consumed during the
cell reaction, but have catalytic properties which enhance the electroreduction or electro-
oxidation of the reactants (the active materials).
The anode active materials used in fuel cells are generally gaseous or liquid (compared
with the metal anodes generally used in most batteries) and are fed into the anode side of
the fuel cell. As these materials are more like the conventional fuels used in heat engines,
the term ‘‘fuel cell’’ has become popular to describe these devices. Oxygen or air is the
predominant oxidant and is fed into the cathode side of the fuel cell.
Fuel cells have been of interest for over 150 years as a potentially more efficient and less
polluting means for converting hydrogen and carbonaceous or fossil fuels to electricity com-
pared to conventional engines. A well known application of the fuel cell has been the use
of the hydrogen /oxygen fuel cell, using cryogenic fuels, in space vehicles for over 40 years.
Use of the fuel cell in terrestrial applications has been developing slowly, but recent advances
has revitalized interest in air-breathing systems for a variety of applications, including utility
power, load leveling, dispersed or on-site electric generators and electric vehicles.
Fuel cell technology can be classified into two categories
1. Direct systems where fuels, such as hydrogen, methanol and hydrazine, can react directly
in the fuel cell
2. Indirect systems in which the fuel, such as natural gas or other fossil fuel, is first converted
by reforming to a hydrogen-rich gas which is then fed into the fuel cell
Fuel cell systems can take a number of configurations depending on the combinations of
fuel and oxidant, the type of electrolyte, the temperature of operation, and the application,
etc.
More recently, fuel cell technology has moved towards portable applications, historically
the domain of batteries, with power levels from less than 1 to about 100 watts, blurring the
distinction between batteries and fuel cells. Metal/ air batteries (see Chap. 38), particularly
those in which the metal is periodically replaced, can be considered a ‘‘fuel cell’’ with the
metal being the fuel. Similarly, small fuel cells, now under development, which are ‘‘refu-
eled’’ by replacing an ampule of fuel can be considered a ‘‘battery.’’
Fuel cells were not included in the 2nd Edition of this Handbook as the technical scope
and applications at that time differed from that of the battery. Now that small to medium
size fuel cells may become competitive with batteries for portable electronic and other ap-
plications, these portable devices are covered in Chap. 42. Information on the larger fuel
cells for electric vehicles, utility power, etc can be obtained from the references listed in
Appendix F ‘‘Bibliography.’’
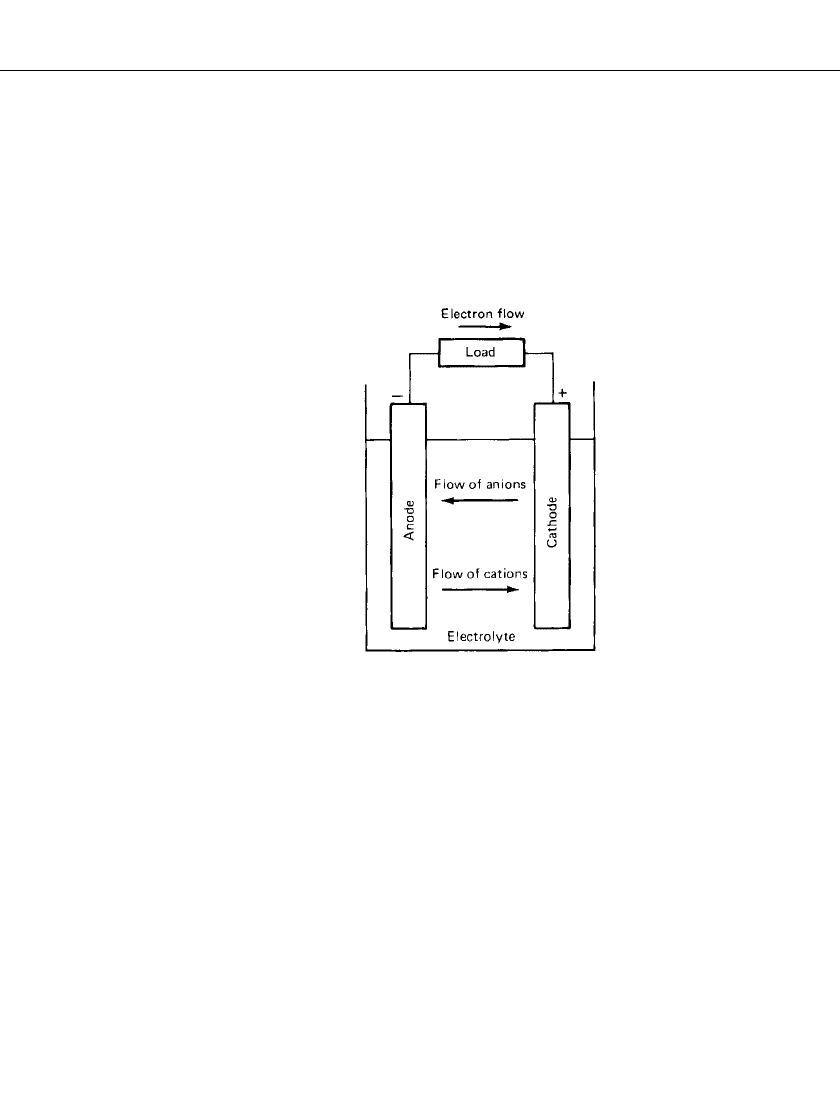
BASIC CONCEPTS 1.7
1.3 OPERATION OF A CELL
1.3.1 Discharge
The operation of a cell during discharge is also shown schematically in Fig. 1.1. When the
cell is connected to an external load, electrons flow from the anode, which is oxidized,
through the external load to the cathode, where the electrons are accepted and the cathode
material is reduced. The electric circuit is completed in the electrolyte by the flow of anions
(negative ions) and cations (positive ions) to the anode and cathode, respectively.
FIGURE 1.1 Electrochemical op-
eration of a cell (discharge).
The discharge reaction can be written, assuming a metal as the anode material and a
cathode material such as chlorine (Cl
2
), as follows:
Negative electrode: anodic reaction (oxidation, loss of electrons)
2
⫹
Zn → Zn ⫹ 2e
Positive electrode: cathodic reaction (reduction, gain of electrons)
⫺
Cl ⫹ 2e → 2Cl
2
Overall reaction (discharge):
2
⫹⫺
Zn ⫹ Cl → Zn ⫹ 2Cl (ZnCl )
22
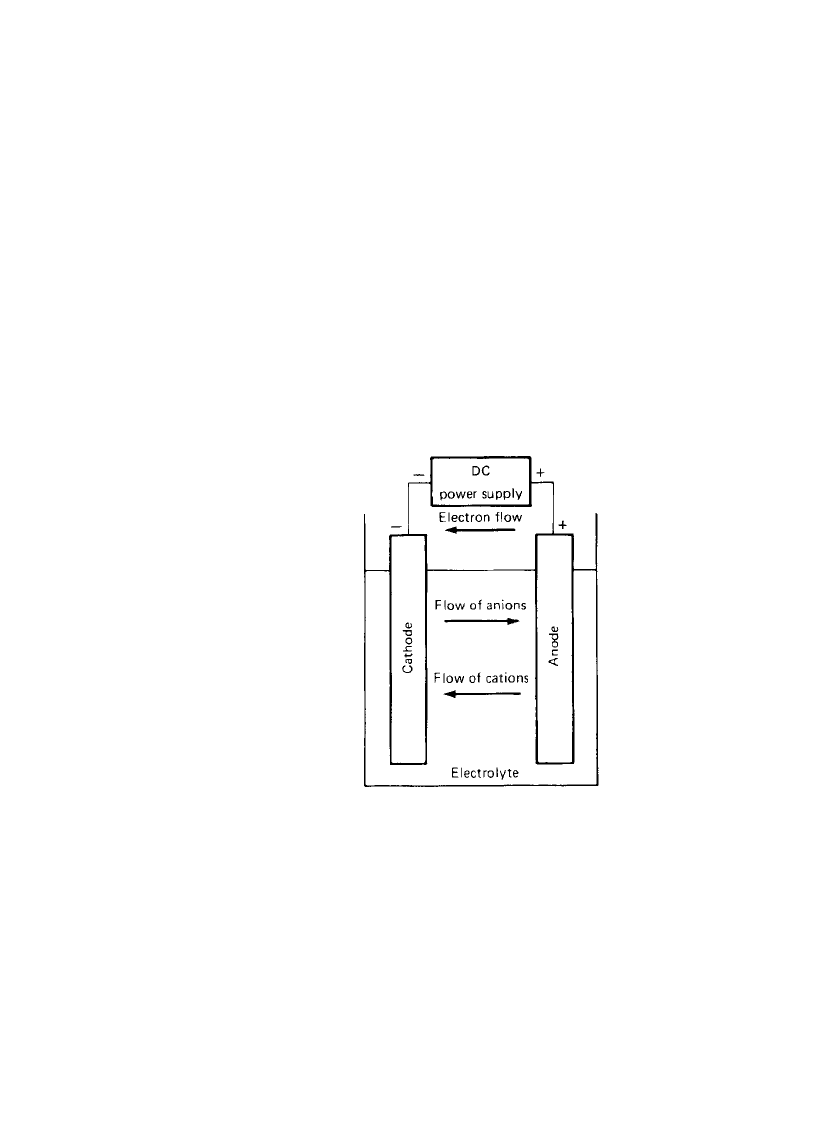
1.8 CHAPTER ONE
1.3.2 Charge
During the recharge of a rechargeable or storage cell, the current flow is reversed and oxi-
dation takes place at the positive electrode and reduction at the negative electrode, as shown
in Fig. 1.2. As the anode is, by definition, the electrode at which oxidation occurs and the
cathode the one where reduction takes place, the positive electrode is now the anode and
the negative the cathode.
In the example of the Zn /Cl
2
cell, the reaction on charge can be written as follows:
Negative electrode: cathodic reaction (reduction, gain of electrons)
2
⫹
Zn ⫹ 2e → Zn
Positive electrode: anodic reaction (oxidation, loss of electrons)
⫺
2Cl → Cl ⫹ 2e
2
Overall reaction (charge):
2
⫹⫺
Zn ⫹ 2Cl → Zn ⫹ Cl
2
FIGURE 1.2 Electrochemical op-
eration of a cell (charge).
1.3.3 Specific Example: Nickel-Cadmium Cell
The processes that produce electricity in a cell are chemical reactions which either release
or consume electrons as the electrode reaction proceeds to completion. This can be illustrated
with the specific example of the reactions of the nickel-cadmium cell. At the anode (negative
electrode), the discharge reaction is the oxidation of cadmium metal to cadmium hydroxide
with the release of two electrons,
⫺
Cd ⫹ 2OH → Cd(OH) ⫹ 2e
2
At the cathode, nickel oxide (or more accurately nickel oxyhydroxide) is reduced to nickel
hydroxide with the acceptance of an electron,
⫺
NiOOH ⫹ HO⫹ e → OH ⫹ Ni(OH)
22

BASIC CONCEPTS 1.9
When these two ‘‘half-cell’’ reactions occur (by connection of the electrodes to an external
discharge circuit), the overall cell reaction converts cadmium to cadmium hydroxide at the
anode and nickel oxyhydroxide to nickel hydroxide at the cathode,
Cd
⫹ 2NiOOH ⫹ 2H O → Cd(OH) ⫹ 2Ni(OH)
222
This is the discharge process. If this were a primary non-rechargeable cell, at the end of
discharge, it would be exhausted and discarded. The nickel-cadmium battery system is, how-
ever, a secondary (rechargeable) system, and on recharge the reactions are reversed. At the
negative electrode the reaction is:
⫺
Cd(OH) ⫹ 2e → Cd ⫹ 2OH
2
At the positive electrode the reaction is:
⫺
Ni(OH) ⫹ OH → NiOOH ⫹ HO⫹ e
22
After recharge, the secondary battery reverts to its original chemical state and is ready for
further discharge. These are the fundamental principles involved in the charge–discharge
mechanisms of a typical secondary battery.
1.3.4 Fuel Cell
A typical fuel cell reaction is illustrated by the hydrogen/ oxygen fuel cell. In this device,
hydrogen is oxidized at the anode, electrocatalyzed by platinum or platinum alloys, while at
the cathode oxygen is reduced, again with platinum or platinum alloys as electrocatalysts.
The simplified anodic reaction is
⫹
2H → 4H ⫹ 4e
2
while the cathodic reaction is
⫹
O ⫹ 4H ⫹ 4e → 2H O
22
The overall reaction is the oxidation of hydrogen by oxygen, with water as the reaction
product.
2H
⫹ O → 2H O
22 2
1.4 THEORETICAL CELL VOLTAGE, CAPACITY, AND ENERGY
The theoretical voltage and capacity of a cell are a function of the anode and cathode
materials. (See Chap. 2 for detailed electrochemical theory.)
1.4.1 Free Energy
Whenever a reaction occurs, there is a decrease in the free energy of the system, which is
expressed as
00
⌬G ⫽⫺nFE
where F
⫽ constant known as Faraday (⬇96,500 C or 26.8 Ah)
n
⫽ number of electrons involved in stoichiometric reaction
E
0
⫽ standard potential, V

1.10 CHAPTER ONE
1.4.2 Theoretical Voltage
The standard potential of the cell is determined by the type of active materials contained in
the cell. It can be calculated from free-energy data or obtained experimentally. A listing of
electrode potentials (reduction potentials) under standard conditions is given in Table 1.1. A
more complete list is presented in Appendix B.
The standard potential of a cell can be calculated from the standard electrode potentials
as follows (the oxidation potential is the negative value of the reduction potential):
Anode (oxidation potential)
⫹ cathode (reduction potential) ⫽ standard cell potential.
For example, in the reaction Zn
⫹ Cl
2
→ ZnCl
2
, the standard cell potential is:
2
⫹
Zn → Zn ⫹ 2e ⫺(⫺0.76 V)
⫺
Cl → 2Cl ⫺ 2e 1.36 V
2
E⬚ ⫽ 2.12 V
The cell voltage is also dependent on other factors, including concentration and temper-
ature, as expressed by the Nernst equation (covered in detail in Chap. 2).
1.4.3 Theoretical Capacity (Coulombic)
The theoretical capacity of a cell is determined by the amount of active materials in the cell.
It is expressed as the total quantity of electricity involved in the electrochemical reaction
and is defined in terms of coulombs or ampere-hours. The ‘‘ampere-hour capacity’’ of a
battery is directly associated with the quantity of electricity obtained from the active mate-
rials. Theoretically 1 gram-equivalent weight of material will deliver 96,487 C or 26.8 Ah.
(A gram-equivalent weight is the atomic or molecular weight of the active material in grams
divided by the number of electrons involved in the reaction.)
The electrochemical equivalence of typical materials is listed in Table 1.1 and Appen-
dix C.
The theoretical capacity of an electrochemical cell, based only on the active materials
participating in the electrochemical reaction, is calculated from the equivalent weight of the
reactants. Hence, the theoretical capacity of the Zn/Cl
2
cell is 0.394 Ah /g, that is,
—
Zn ⫹ Cl → ZnCl
22
(0.82 Ah/ g) (0.76 Ah/ g)
1.22 g/ Ah
⫹ 1.32 g /Ah ⫽ 2.54 g /Ah or 0.394 Ah /g
Similarly, the ampere-hour capacity on a volume basis can be calculated using the ap-
propriate data for ampere-hours per cubic centimeter as listed in Table 1.1.
The theoretical voltages and capacities of a number of the major electrochemical systems
are given in Table 1.2. These theoretical values are based on the active anode and cathode
materials only. Water, electrolyte, or any other materials that may be involved in the cell
reaction are not included in the calculation.

BASIC CONCEPTS 1.11
TABLE 1.1 Characteristics of Electrode Materials*
Material
Atomic or
molecular
weight, g
Standard
reduction
potential
at 25
⬚C, V
Valence
change
Melting
point, ⬚C
Density,
g/cm
3
Electrochemical equivalents
Ah/g g/Ah Ah/cm
3
‡
Anode materials
H
2
Li
Na
Mg
Al
Ca
Fe
Zn
Cd
Pb
(Li)C
6
(1)
MH
(2)
CH
3
OH
2.01
6.94
23.0
24.3
26.9
40.1
55.8
65.4
112.4
207.2
72.06
116.2
32.04
0
⫺0.83†
⫺3.01
⫺2.71
⫺2.38
⫺2.69†
⫺1.66
⫺2.84
⫺2.35†
⫺0.44
⫺0.88†
⫺0.76
⫺1.25†
⫺0.40
⫺0.81†
⫺0.13
⬃⫺2.8
⫺0.83†
—
2
1
1
2
3
2
2
2
2
2
1
2
6
—
180
98
650
659
851
1528
419
321
327
—
—
—
—
0.54
0.97
1.74
2.69
1.54
7.85
7.14
8.65
11.34
2.25
—
—
26.59
3.86
1.16
2.20
2.98
1.34
0.96
0.82
0.48
0.26
0.37
0.45
5.02
0.037
0.259
0.858
0.454
0.335
0.748
1.04
1.22
2.10
3.87
2.68
2.21
0.20
2.06
1.14
3.8
8.1
2.06
7.5
5.8
4.1
2.9
0.84
—
—
Cathode materials
O
2
Cl
2
SO
2
MnO
2
NiOOH
CuCl
FeS
2
AgO
Br
2
HgO
Ag
2
O
PbO
2
Li
x
CoO
2
(3)
I
2
32.0
71.0
64.0
86.9
91.7
99.0
119.9
123.8
159.8
216.6
231.7
239.2
98
253.8
1.23
0.40†
1.36
—
1.28‡
0.49†
0.14
—
0.57†
1.07
0.10†
0.35†
1.69
⬃2.7
0.54
4
2
1
1
1
1
4
2
2
2
2
2
0.5
2
—
—
—
—
—
—
—
—
—
—
—
—
—
—
—
—
—
5.0
7.4
3.5
—
7.4
—
11.1
7.1
9.4
—
4.94
3.35
0.756
0.419
0.308
0.292
0.270
0.89
0.432
0.335
0.247
0.231
0.224
0.137
0.211
0.30
1.32
2.38
3.24
3.42
3.69
1.12
2.31
2.98
4.05
4.33
4.45
7.29
4.73
1.54
2.16
0.95
4.35
3.20
2.74
1.64
2.11
—
1.04
* See also Appendixes B and C.
† Basic electrolyte: all others, aqueous acid electrolyte.
‡ Based on density values shown.
(1) Calculations based only on weight of carbon.
(2) Based on 1.7% H
2
storage by weight.
(3) Based on x
⫽ 0.5; higher valves may be obtained in practice.
1.12
TABLE 1.2 Voltage, Capacity and Specific Energy of Major Battery Systems—Theoretical and Practical Values
Battery type Anode Cathode Reaction mechanism
Theoretical values†
V g/Ah Ah/kg
Specific
energy
Wh / kg
Practical battery‡
Nominal
voltage
V
Specific
energy
Wh/kg
Energy
density
Wh / L
Primary batteries
Leclanche´ Zn MnO
2
Zn ⫹ 2MnO
2
→ ZnO Mn
2
O
3
1.6 4.46 224 358 1.5 85
(4)
165
(4)
Magnesium Mg MnO
2
Mg ⫹ 2MnO
2
⫹ H
2
O → Mn
2
O
3
⫹ Mg(OH)
2
2.8 3.69 271 759 1.7 100
(4)
195
(4)
Alkaline MnO
2
Zn MnO
2
Zn ⫹ 2MnO
2
→ ZnO ⫹ Mn
2
O
3
1.5 4.46 224 358 1.5 145
(4)
400
(4)
Mercury Zn HgO Zn ⫹ HgO → ZnO ⫹ Hg 1.34 5.27 190 255 1.35 100
(6)
470
(6)
Mercad Cd HgO Cd ⫹ HgO ⫹ H
2
O → Cd(OH)
2
⫹ Hg 0.91 6.15 163 148 0.9 55
(6)
230
(6)
Silver oxide Zn Ag
2
OZn⫹ Ag
2
O ⫹ H
2
O → Zn(OH)
2
⫹ 2Ag 1.6 5.55 180 288 1.6 135
(6)
525
(6)
Zinc / O
2
Zn O
2
Zn ⫹
1
⁄
2
O
2
→ ZnO 1.65 1.52 658 1085 — — —
Zinc / air Zn Ambient air Zn
⫹ (
1
⁄2O
2
) → ZnO 1.65 1.22 820 1353 1.5 370
(6)
1300
(6)
Li / SOCl
2
Li SOCl
2
4Li ⫹ 2SOCl
2
→ 4LiCl ⫹ S ⫹ SO
2
3.65 3.25 403 1471 3.6 590
(4)
1100
(4)
Li/SO
2
Li SO
2
2Li ⫹ 2SO
2
→ Li
2
S
2
O
4
3.1 2.64 379 1175 3.0 260
(5)
415
(5)
LiMnO
2
Li MnO
2
Li ⫹ Mn
IV
O
2
→ Mn
IV
O
2
(Li
⫹
) 3.5 3.50 286 1001 3.0 230
(5)
535
(5)
Li / FeS
2
Li FeS
2
4Li ⫹ FeS
2
→ 2Li
2
S ⫹ Fe 1.8 1.38 726 1307 1.5 260
(5)
500
(5)
Li / (CF)
n
Li (CF)
n
nLi ⫹ (CF)
n
→ nLiF ⫹ nC 3.1 1.42 706 2189 3.0 250
(5)
635
(5)
Li/I
2
(3)
Li I
2
(P2VP) Li ⫹
1
⁄2I
2
→ LiI 2.8 4.99 200 560 2.8 245 900
Reserve batteries
Cuprous chloride Mg CuCl Mg ⫹ Cu
2
Cl
2
→ MgCl
2
⫹ 2Cu 1.6 4.14 241 386 1.3 60
(7)
80
(7)
Zinc / silver oxide Zn AgO Zn ⫹ AgO ⫹ H
2
O → Zn(OH)
2
⫹ Hg 1.81 3.53 283 512 1.5 30
(8)
75
(8)
Thermal Li FeS
2
See Section 21.3.1 2.1–1.6 1.38 726 1307 2.1–1.6 40
(9)
100
(9)

1.13
Secondary batteries
Lead-acid Pb PbO
2
Pb ⫹ PbO
2
⫹ 2H
2
SO
4
→ 2PbSO
4
⫹ 2H
2
O 2.1 8.32 120 252 2.0 35 70
(10)
Edison Fe Ni oxide Fe ⫹ 2NiOOH ⫹ 2H
2
O → 2Ni(OH)
2
⫹ Fe(OH)
2
1.4 4.46 224 314 1.2 30 55
(10)
Nickel-cadmium Cd Ni oxide Cd ⫹ 2NiOOH ⫹ 2H
2
O → 2Ni(OH)
2
⫹ Cd(OH)
2
1.35 5.52 181 244 1.2 35 100
(5)
Nickel-zinc Zn Ni oxide Zn ⫹ 2NiOOH ⫹ 2H
2
O → 2Ni(OH)
2
⫹ Zn(OH)
2
1.73 4.64 215 372 1.6 60 120
Nickel-hydrogen H
2
Ni oxide H
2
⫹ 2NiOOH → 2Ni(OH)
2
1.5 3.46 289 434 1.2 55 60
Nickel-metal hydride MH
(1)
Ni oxide MH ⫹ NiOOH → M ⫹ Ni(OH)
2
1.35 5.63 178 240 1.2 75 240
(5)
Silver-zinc Zn AgO Zn ⫹ AgO ⫹ H
2
O → Zn(OH)
2
⫹ Ag 1.85 3.53 283 524 1.5 105 180
(10)
Silver-cadmium Cd AgO Cd ⫹ AgO ⫹ H
2
O → Cd(OH)
2
⫹ Ag 1.4 4.41 227 318 1.1 70 120
(10)
Zinc / chlorine Zn Cl
2
Zn ⫹ Cl
2
→ ZnCl
2
2.12 2.54 394 835 — — —
Zinc / bromine Zn Br
2
Zn ⫹ Br
2
→ ZnBr
2
1.85 4.17 309 572 1.6 70 60
Lithium-ion Li
x
C
6
Li
(i–x)
CoO
2
Li
x
C
6
⫹ Li
(i–x)
CoO
2
→ LiCoO
2
⫹ C
6
4.1 9.98 100 410 4.1 150 400
(5)
Lithium / manganese dioxide Li MnO
2
Li ⫹ Mn
IV
O
2
→ Mn
IV
O
2
(Li
⫹
) 3.5 3.50 286 1001 3.0 120 265
Lithium / iron disulfide
(2)
Li(Al) FeS
2
2Li(Al) ⫹ FeS
2
→ Li
2
FeS
2
⫹ 2Al 1.73 3.50 285 493 1.7 180
(11)
350
(11)
Lithium / iron monosulfide
(2)
Li(Al) FeS 2Li(Al) ⫹ FeS → Li
2
S ⫹ Fe ⫹ 2Al 1.33 2.90 345 459 1.3 130
(11)
220
(11)
Sodium / sulfur
(2)
Na S 2Na ⫹ 3S → Na
2
S
3
2.1 2.65 377 792 2.0 170
(11)
345
(11)
Sodium / nickel chloride
(2)
Na NiCl
2
2Na ⫹ NiCl
2
→ 2NaCl ⫹ Ni 2.58 3.28 305 787 2.6 115
(11)
190
(11)
Fuel cells
H
2
/O
2
H
2
/ air
H
2
H
2
O
2
Ambient air
H
2
⫹
1
⁄
2
O
2
→ H
2
O
H
2
⫹ (
1
⁄
2
O
2
) → H
2
O
1.23
1.23
0.336
0.037
2975
26587
3660
32702
Methanol / O
2
CH
3
OH O
2
CH
3
OH ⫹
3
⁄2O
2
→ CO
2
⫹ 2H
2
O 1.24 0.50 2000 2480 — — —
Methanol / air CH
3
OH Ambient air CH
3
OH ⫹ (
3
⁄2O
2
) → CO
2
⫹ 2H
2
O 1.24 0.20 5020 6225 — — —
† Based on active anode and cathode materials only, including O
2
but not air (electrolyte not included).
* These values are for single cell batteries based on identified design and at discharge rates optimized for energy
density, using midpoint voltage. More specific values are given in chapters on each battery system.
(1) MH
⫽ metal hydride, data based on 1.7% hydrogen storage (by weight).
(2) High temperature batteries.
(3) Solid electrolyte battery (Li/I
2
(P2VP)).
(4) Cylindrical bobbin-type batteries.
(5) Cylindrical spiral-wound batteries.
(6) Button type batteries.
(7) Water-activated.
(8) Automatically activated 2- to 10-min rate.
(9) With lithium anodes.
(10) Prismatic batteries.
(11) Value based on cell performance, see appropriate chapter for details.
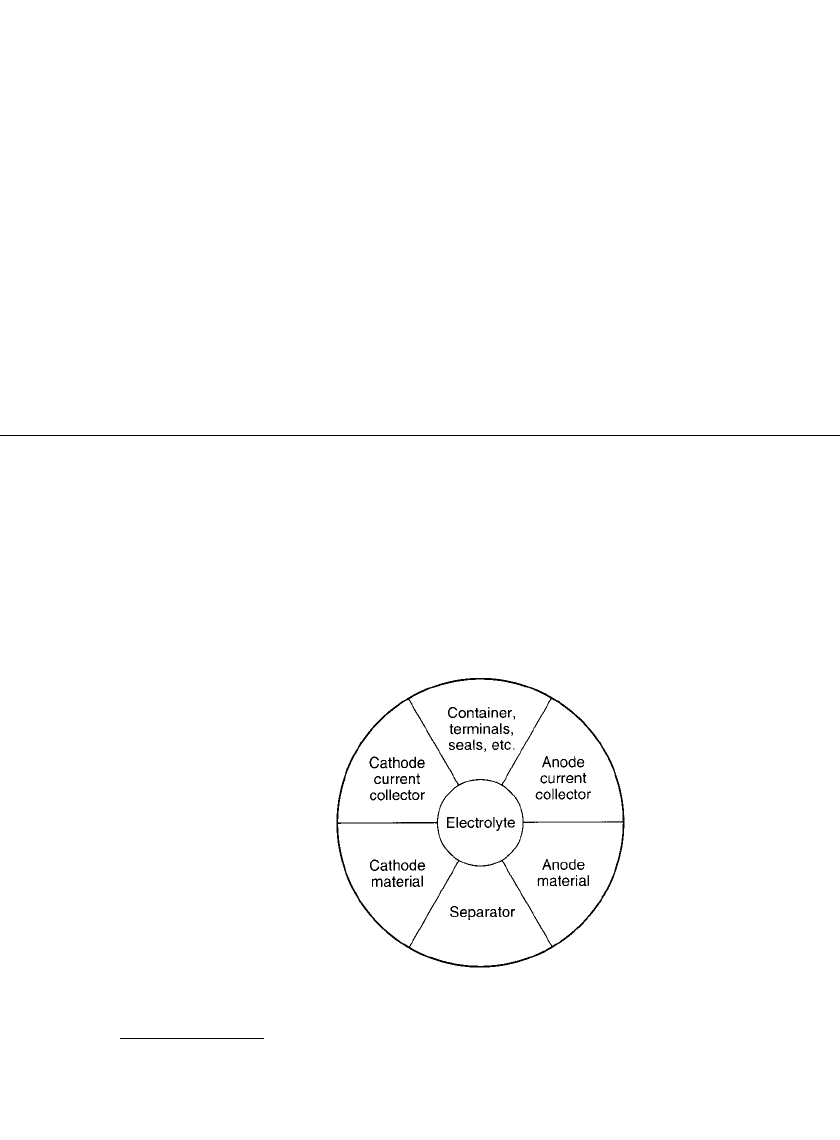
1.14 CHAPTER ONE
FIGURE 1.3 Components of a cell.
1.4.4 Theoretical Energy*
The capacity of a cell can also considered on an energy (watthour) basis by taking both the
voltage and the quantity of electricity into consideration. This theoretical energy value is the
maximum value that can be delivered by a specific electrochemical system:
Watthour (Wh)
⫽ voltage (V) ⫻ ampere-hour (Ah)
In the Zn/Cl
2
cell example, if the standard potential is taken as 2.12 V, the theoretical
watthour capacity per gram of active material (theoretical gravimetric specific energy or
theoretical gravimetric energy density) is:
Specific Energy (Watthours/gram)
⫽ 2.12 V ⫻ 0.394 Ah/g ⫽ 0.835 Wh /g or 835 Wh /kg
Table 1.2 also lists the theoretical specific energy of the various electrochemical systems.
1.5 SPECIFIC ENERGY AND ENERGY DENSITY OF PRACTICAL
BATTERIES
The theoretical electrical properties of cells and batteries are discussed in Sec. 1.4. In sum-
mary, the maximum energy that can be delivered by an electrochemical system is based on
the types of active materials that are used (this determines the voltage) and on the amount
of the active materials that are used (this determines ampere-hour capacity). In practice, only
a fraction of the theoretical energy of the battery is realized. This is due to the need for
electrolyte and nonreactive components (containers, separators, electrodes) that add to the
weight and volume of the battery, as illustrated in Fig. 1.3. Another contributing factor is
that the battery does not discharge at the theoretical voltage (thus lowering the average
*The energy output of a cell or battery is often expressed as a ratio of its weight or size.
The preferred terminology for this ratio on a weight basis, e.g. Watthours/kilogram
(Wh/ kg), is ‘‘specific energy’’; on a volume basis, e.g. Watthours/ liter (Wh /L), it is ‘‘energy
density.’’ Quite commonly, however, the term ‘‘energy density’’ is used to refer to either
ratio.
