Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

RECHARGEABLE BATTERIES 36.5
36.4 PERFORMANCE
36.4.1 First Cycle Discharge
The rechargeable batteries are manufactured and shipped in a charged state, as are the pri-
mary cells. Because of their good shelf life, they can retain most of this capacity (depending
on storage prior to use) and do not need to be recharged before first use. The discharge
characteristics of the rechargeable zinc/alkaline/manganese dioxide batteries are similar to
those of the primary batteries. However, due to the zinc-limited design of the rechargeable
cell, its terminal voltage on a medium- or high-load discharge drops rapidly once 0.8 V is
reached. On a low-load regime, a slope to about 0.6 to 0.7 V is sometimes noticeable before
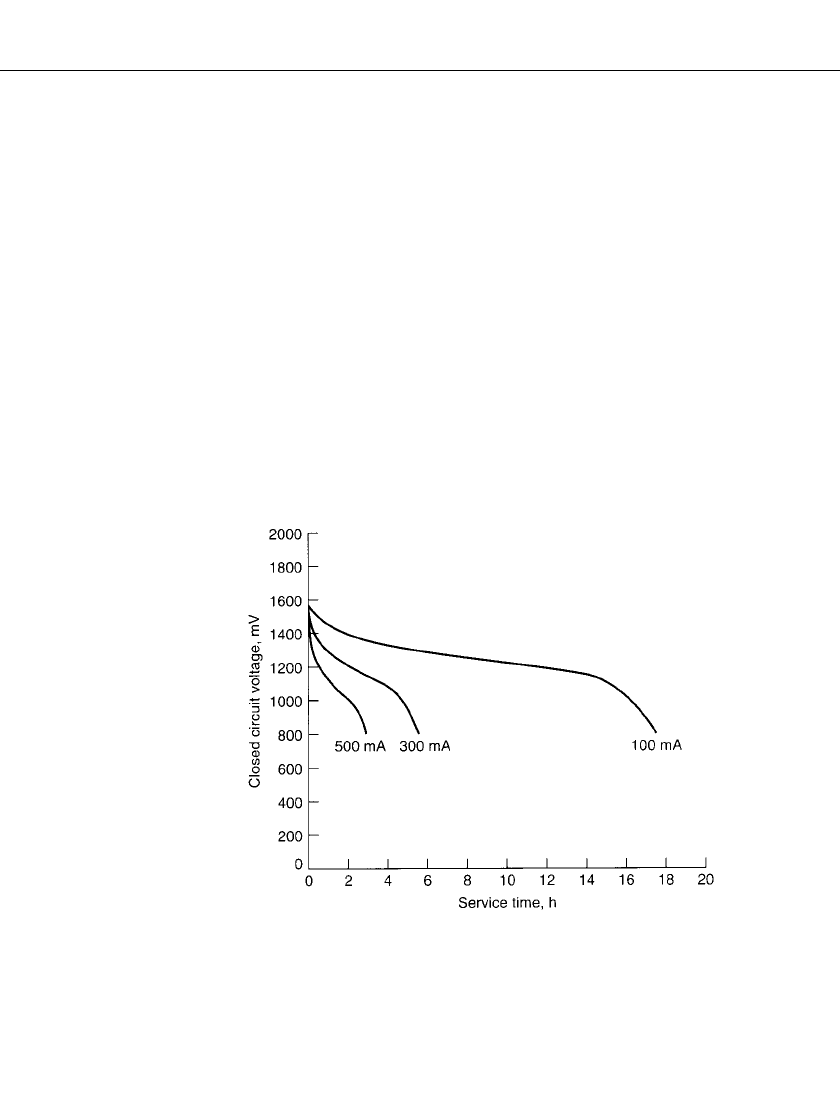
the voltage drops practically to zero. Figure 36.4 shows the discharge curves of fresh AA-
size rechargeable batteries on the first cycle of discharge at several constant-current discharge
loads. Figure 36.5 shows similar discharge curves for discharges at constant-resistance loads.
FIGURE 36.4 First-cycle discharge characteristics of rechargeable
zinc / alkaline / manganese dioxide AA-size batteries discharged contin-
uously at different constant-current loads at 22⬚C. (Courtesy of Battery
Technologies, Inc.)
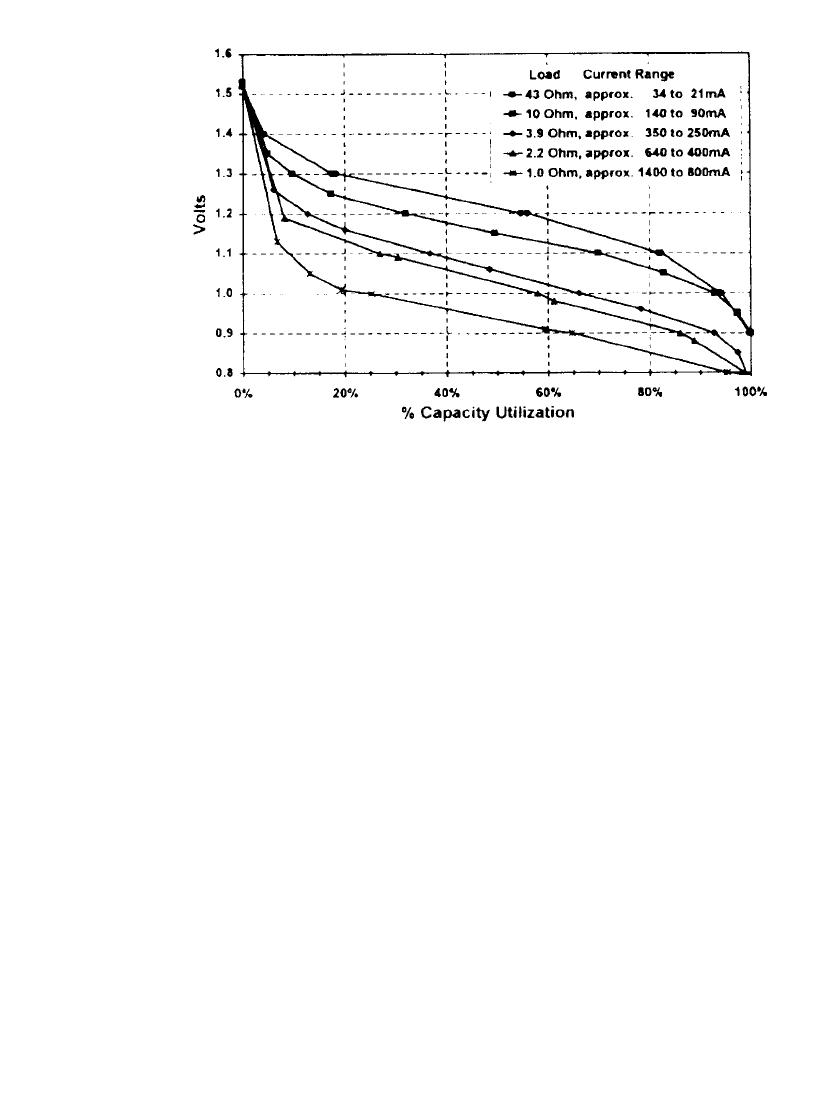
36.6 CHAPTER THIRTY-SIX
FIGURE 36.5 First-cycle discharge characteristics of rechargeable zinc / alkaline / man-
ganese dioxide AA-size batteries discharged continuously at different constant-resistance
loads at 22⬚C: (Courtesy of Battery Technologies, Inc.) (From Ref. 12a.)
36.4.2 Cycling
The rechargeable battery has its highest capacity on the first cycle, and that value at 20⬚C
is about 70 to 80% of the capacity of the primary cell. On subsequent charge-discharge
cycles, if the cells are completely discharged before being recharged, 20 discharge cycles
can be obtained until the capacity drops to about 50% of the initial capacity. The shape of
the discharge curve changes slightly during cycling, but the voltage level drops with cycling,
as shown in Fig. 36.6. Additional deep discharge cycles can be obtained, but at reduced
capacity, if the cycling is continued.
The number of useful cycles and the cycle capacity increases when the batteries are only
partially discharged and recharged after use. Figure 36.7a shows the increased cycle life
obtained on a intermittent discharge of a AA-size battery on a 10-ohm load, applied daily
for 4h (about a 25% depth of discharge). The cells are charged overnight on a constant
voltage charger set at about 1.65 V. Although the same drop in voltage level with cycling
as shown in Fig. 36.6 is present, more than 200 cycles can be obtained with the terminal
voltage about 0.9 volts.
The cycle life, when the battery is discharged to other depth of discharge, is shown in
Fig. 36.7b. This figure shows the results of repeated discharge of the rechargeable AA-size
battery to approximately 12%, 18% and 25% depth-of-discharge, followed by recharge. The
cycle life increases with reducing depth-of-discharge and the voltage drop increases with
lowering the depth-of-discharge.
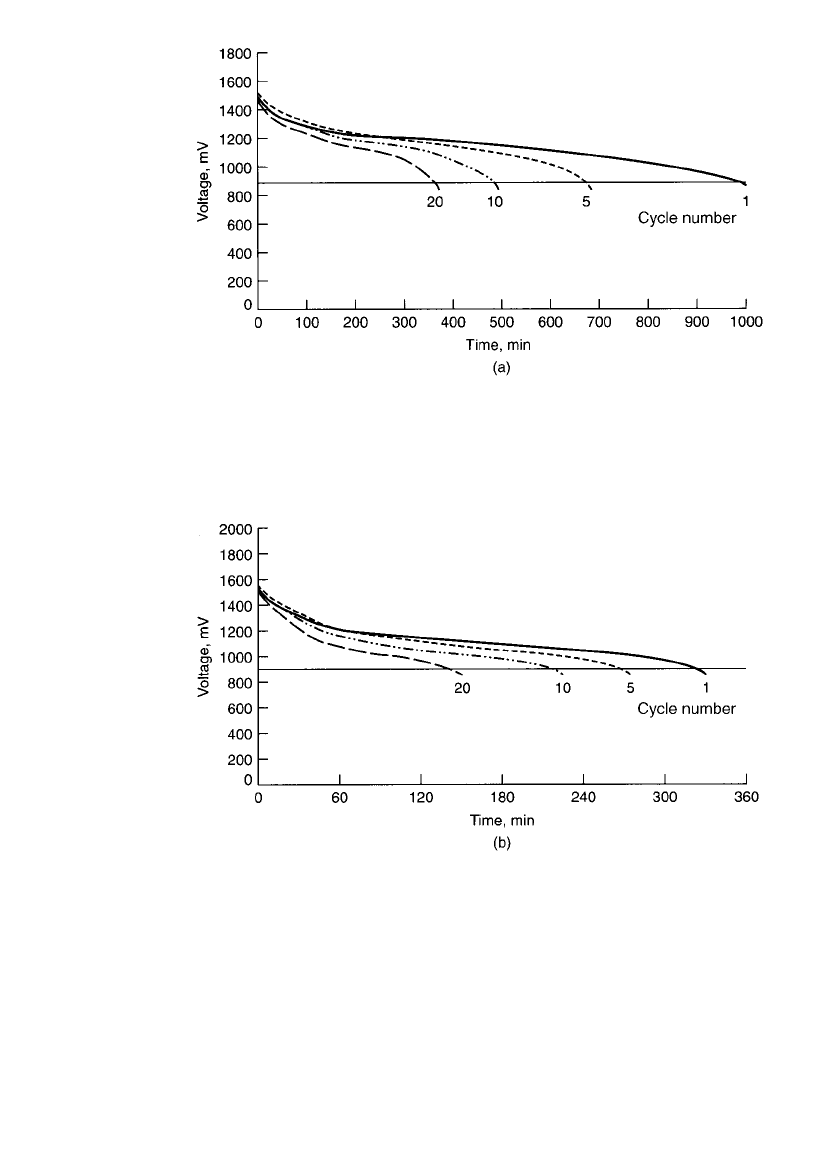
RECHARGEABLE BATTERIES 36.7
FIGURE 36.6 Continuous-discharge characteristics of rechargeable zinc / alkaline /
manganese dioxide AA-size batteries after cycling at 20⬚C. (a) 10-⍀ cycling. (b)4-⍀
cycling. (Courtesy of Battery Technologies, Inc.)
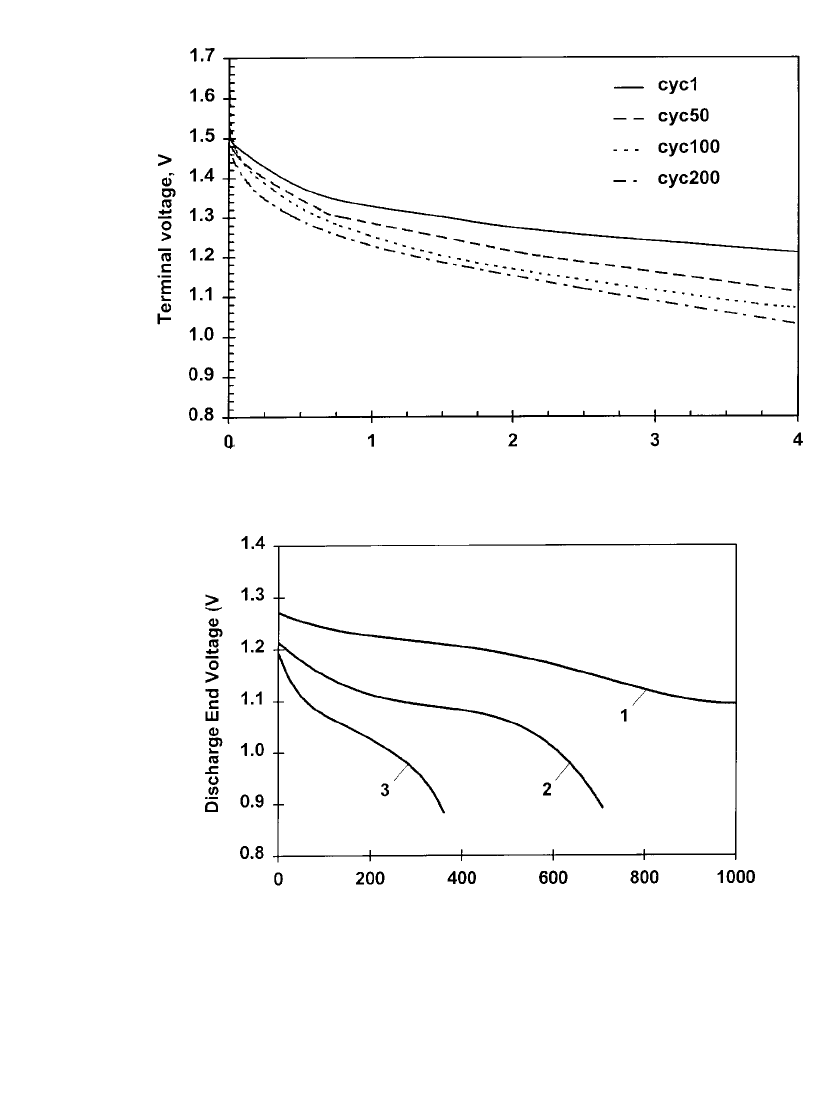
36.8 CHAPTER THIRTY-SIX
Time, hours
(a)
Number of Cycles
(b)
FIGURE 36.7 (a) Discharge characteristics of rechargeable zinc /alkaline /man-
ganese dioxide AA-size batteries after cycling at 20⬚C. Cells discharged 4h per day
at 10 ⍀; 1.65 V constant voltage charging overnight. (b) The cycle life of AA-size
batteries on a 10 ⍀ load. Curve 1 discharged for 200 mAh, then recharged: curve 2
discharged for 300 mA then recharged: curve 3 Discharged for 400 mAh, then re-
charged. (Courtesy of Battery Technologies, Inc.)
RECHARGEABLE BATTERIES 36.9
36.4.3 Performance of Different Sizes of Batteries
The rechargeable alkaline-manganese dioxide batteries are available in other sizes. Figures
36.8 and 36.9 show the performance of C- and D-size batteries and Fig. 36.10 that of AAA-
size.
Note that the AA-size and AAA-size batteries, with their thinner positive electrodes give
relatively better performance than the larger diameter C-size and D-size batteries. The effi-
ciency of utilization of the manganese dioxide and the deep cycling performance of the
rechargeable battery are related to the thickness of this electrode. This is further illustrated
in Fig. 36.11, which shows the utilization of the manganese dioxide and the ampere-hour
capacity delivered from the batteries as a function of load current. The thinner batteries
deliver a higher percentage of their capacity than the larger diameter batteries when dis-
charged at the higher discharge currents.
13–15
Time, hours
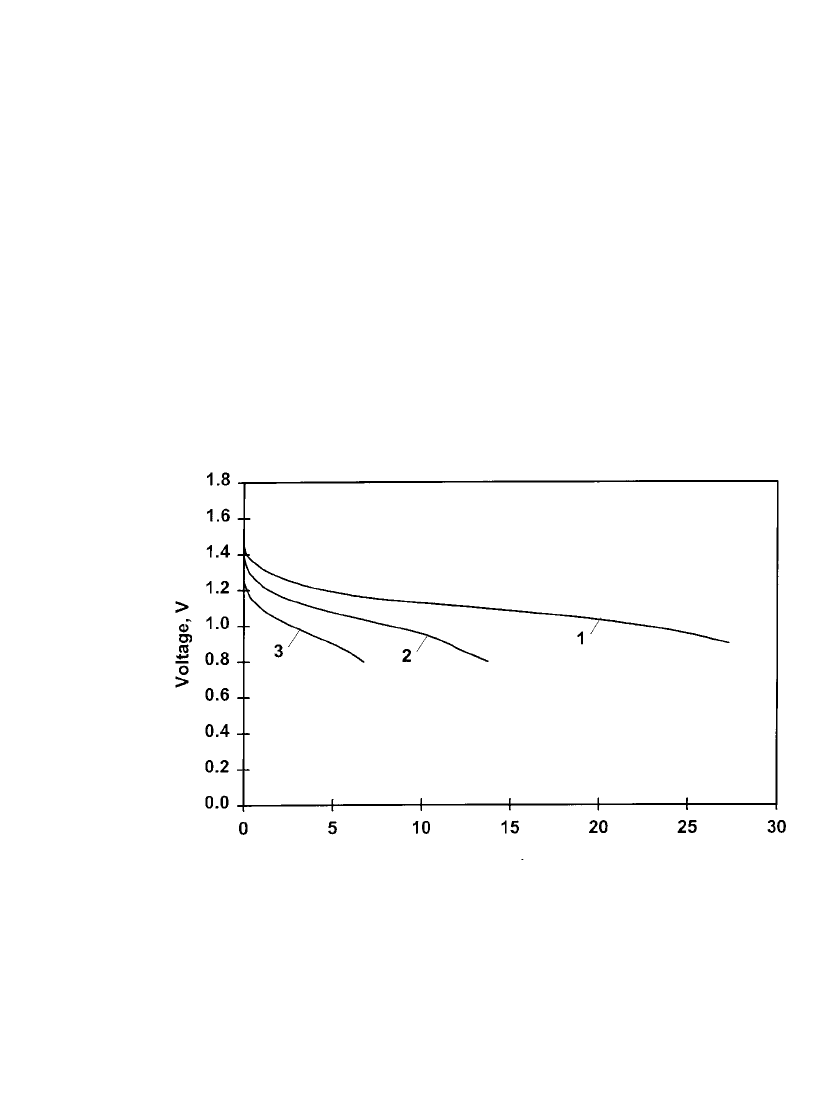
FIGURE 36.8 First cycle discharge characteristics of rechargeable zinc / alkaline / manganese
dioxide C-size batteries discharged continuously at different constant resistance loads at 20⬚C:
Curve 1 ⫺ 6.8 ⍀. 160 mA (approx.): curve 2 ⫺ 3.9 ⍀. 270 mA (approx.): curve 3 ⫺ 2.2 ⍀.
450 mA (approx.). (Courtesy of Battery Technologies Inc.)
36.10 CHAPTER THIRTY-SIX
Time, hours
FIGURE 36.9 First cycle discharge characteristics of rechargeable zinc / alkaline / manganese
dioxide D-size batteries discharged continuously at different constant resistance loads at 20⬚C.
Curve 1 ⫺ 3.9 ⍀. 280 mA (approx.): curve 2 ⫺ 2.2 ⍀. 460 mA (approx.): curve 3 ⫺ 1.0 ⍀.
1A (approx.). (Courtesy of Battery Technologies Inc.)
Time, hours
FIGURE 36.10 First cycle discharge characteristics of rechargeable zinc / alkaline / manganese
dioxide AAA-size cells discharged continuously at different constant resistance loads at 20⬚C.
Curve 1 ⫺ 10 ⍀. 110 mA (approx.): curve 2 ⫺ 5.1 ⍀. 190 mA (approx.): curve 3 ⫺ 3.9 ⍀. 260
mA (approx.). (Courtesy of Battery Technologies Inc.)
RECHARGEABLE BATTERIES 36.11
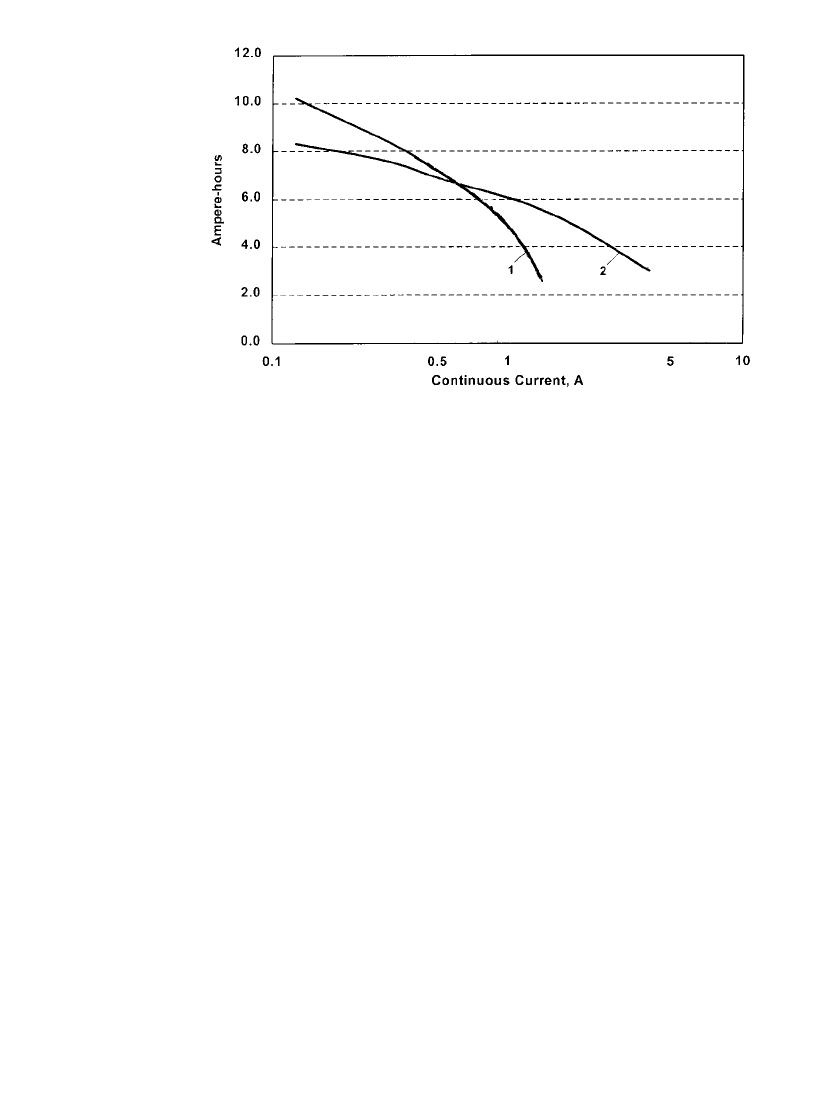
FIGURE 36.11 Comparison of 20⬚C performance of rechargeable zinc / alkaline
/ manganese dioxide D-size battery (curve 1) to output of four AA-cells connected
in parallel (in D-size can) to 0.9 V end-voltage (curve 2). (Courtesy of Battery
Technologies Inc.)
36.4.4 Bundle (Parallel) Multicell Batteries
Figure 36.11 also illustrates the advantage of using a multicell battery design, with smaller
cells in parallel, than a larger single cell battery. The battery with four AA-size cells in a
container having the same dimensions as a D-size battery weighs about 90 grams, compared
to a single D-size battery which weighs about 125 grams, but will outperform it at the higher
discharge currents. The beneficial effect of using several small diameter cylindrical cells in
parallel in a multicell battery instead of a single larger diameter battery also results in a gain
in total internal electrode interface, thereby decreasing the current density at a given load
and improving performance.
15
36.4.5 Effect of Temperature
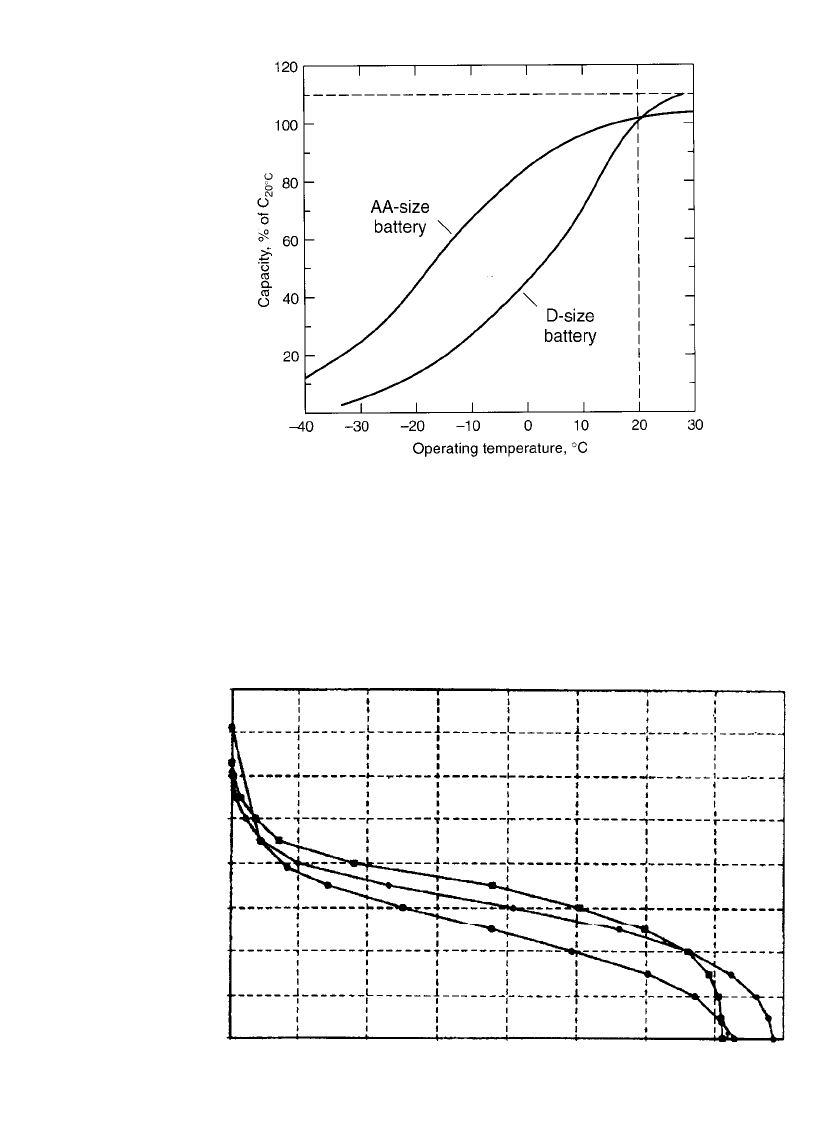
The performance of the rechargeable zinc/manganese dioxide batteries (AA and D-size) at
various temperatures is shown in Fig. 36.12. The relative performance of the AA-size battery
at low temperatures again is superior to that of the D-size cell because of its thinner cathode
and proportionately larger interface area. The performance of the AA-size cell at 45
⬚C and
65
⬚C is shown in Fig. 36.13. It should also be noted that the capacity and high current drain
capability are higher at the higher temperatures due to better diffusion and higher MnO
2
utilization.
16,17
36.12 CHAPTER THIRTY-SIX
FIGURE 36.12 Comparison of performance of rechargeable zinc/
alkaline / manganese dioxide D and AA-size batteries at various tem-
peratures. Discharge at C/ 20 rate, to 0.9-V end voltage. (Courtesy
of Battery Technologies, Inc.)
0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6
Capacity (Ah)
65°C
45°C
21°C
1.6
1.5
1.4
1.3
1.2
1.1
1.0
0.9
0.8
Volts
FIGURE 36.13 Discharge of Rechargeable AA-size zinc/ alkaline / manganese dioxide batter-
ies at different temperatures at a 3.9 ohm load. (From Ref. 16)
RECHARGEABLE BATTERIES 36.13
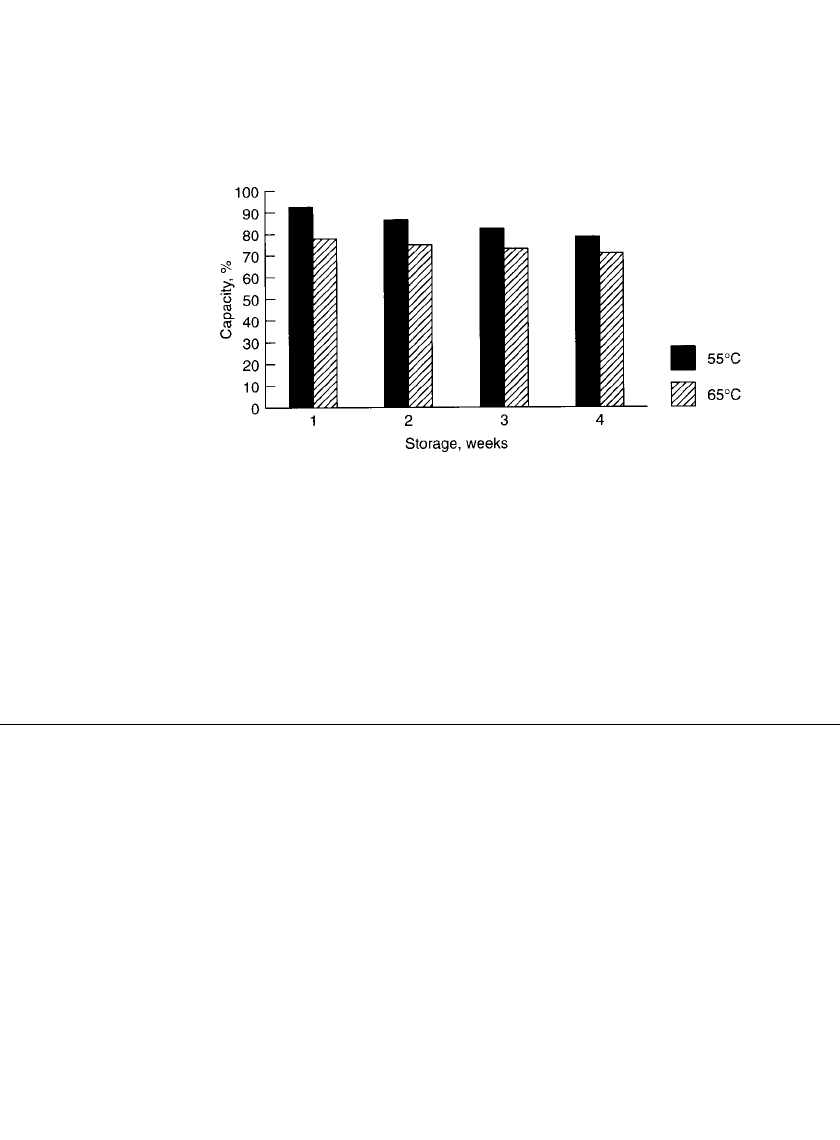
36.4.6 Shelf Life
The shelf life of fresh, unused (charged) rechargeable alkaline-manganese dioxide batteries
is about the same as that of the primary batteries (20 to 25% loss after 3 to 4 years when
stored at room temperature). The data for capacity losses after high-temperature storage are
shown in Fig. 36.14.
FIGURE 36.14 Capacity retention of mercury-free rechargeable zinc /
alkaline / manganese dioxide AA-size batteries at 20⬚C. (Courtesy of Battery
Technologies, Inc.)
The shelf life of cycled cells depends on whether they are stored in a charged or a
discharged condition. Batteries stored in a charged state after cycling show about the same
losses as a fresh uncycled battery. Storage of batteries in a discharged condition, particularly
at elevated temperatures (65
⬚C) may be detrimental to the anode performance on subsequent
cycles. However, under normal usage, batteries can be recharged close to the capacity level
of the previous cycle.
36.5 CHARGE METHODS
In the charging process for the zinc/ alkaline/ manganese dioxide cell, the discharged positive
active material, manganese oxyhydroxide (MnOOH), is oxidized to manganese dioxide
(MnO
2
) and the zinc oxide (ZnO) in the negative is reduced to metallic zinc. Manganese
dioxide can be further oxidized to higher oxides (Mn
⫹
6
compounds) which are soluble,
resulting in loss of rechargeability. Therefore proper recharging is important to obtain opti-
mum life. Charging over 1.72 V per cell for days or over 1.68 V per cell for weeks can
damage the battery. Batteries should not be charged after 105% of the ampere-hours removed
have been replaced. Batteries can be float-charged for extended periods at 1.65 V per cell.
17
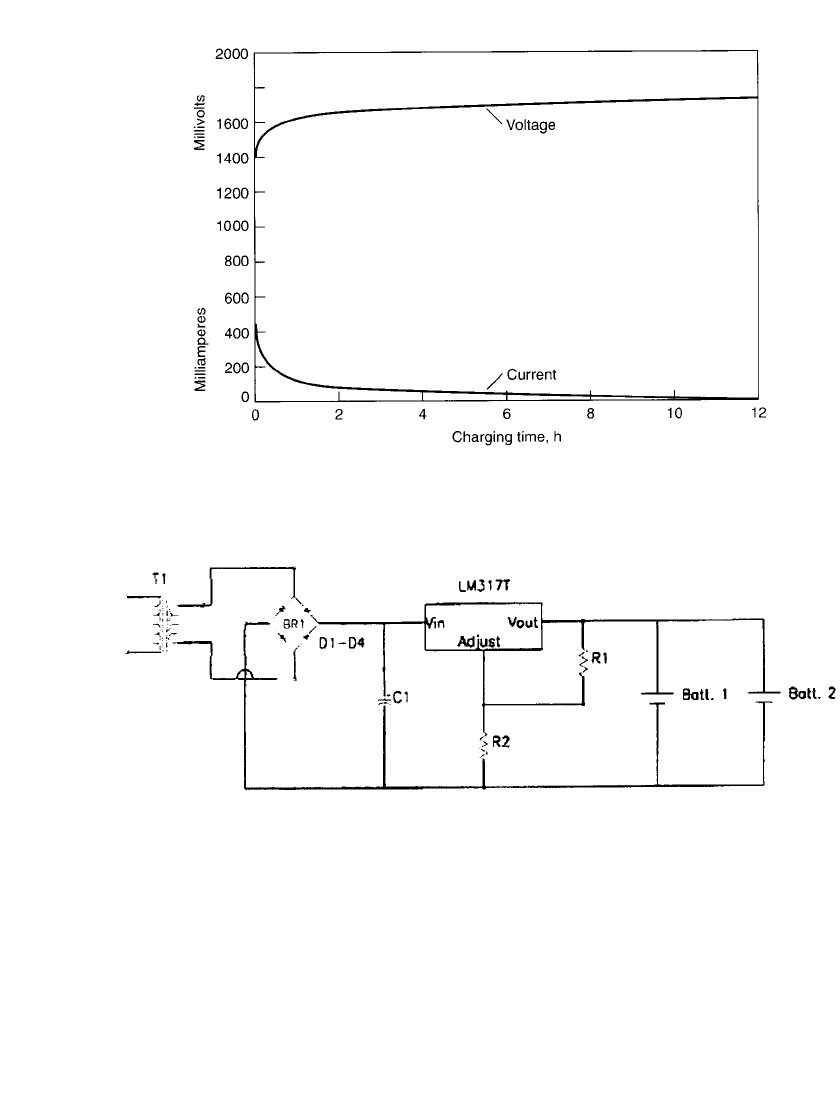
36.5.1 Constant-Potential Charging
Constant-potential charging is the preferred method. This is equivalent to a taper current
charge method. The voltage on charge should not exceed 1.65 to 1.68 V. If charging is
continued at higher voltages, current will continue to flow and some damage to the cell can
be expected due to increased anode corrosion by the soluble Mn
⫹
6
species. If the end voltage
is set to below 1.65 V, the charging takes longer and the cell may not be fully charged
overnight, but the cycle life of the battery is improved. Figure 36.15 shows the voltage and
current profiles during the charge. A constant potential charger using a voltage regulator
such as a LM317 device is shown in Fig. 36.16.
36.14 CHAPTER THIRTY-SIX
FIGURE 36.15 Constant-potential charging of AA-size rechargeable zinc / alkaline /
manganese battery at 20⬚C. (Courtesy of Battery Technologies, Inc.)
FIGURE 36.16 Principle circuit diagram for a constant potential charger utilizing a LM317T voltage reg-
ulator. (Courtesy of Battery Technologies, Inc.)
36.5.2 Constant-Current Charging
Uncontrolled constant-current charging over an extended period of time leads to electrolysis
of the electrolyte, which causes a buildup of internal gas pressure and results in the rupturing
of the safety vent, allowing the release of the gases. Constant-current charging is feasible if
the charge voltage is limited to 1.65 V per cell (resistance-free) and a shutoff control is
incorporated in the charge circuit.
