Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

LITHIUM-ION BATTERIES 35.69
Because Li-ion cells are degraded irreversibly by overcharge or overdischarge, and may
vent if overcharged, Li-ion batteries typically employ battery management circuitry to ensure
safe operation and prevent overcharge. This circuitry can also provide other features such as
a state-of-charge gauge and temperature monitoring. Many manufacturers recommend charg-
ing at low rates (less than 0.1C) if the cell voltage is below 2.5 V.
Many applications require batteries to retain capacity on continuous float (constant volt-
age) charge. In this scenario, the cells are charged whenever their open circuit voltage is
less than a predetermined threshold value. The ability of wound C/LiCoO
2
cells to retain
capacity on float charge is indicated in Fig. 35.85, which shows the capacity retained by
cells float charged for up to 21 months.
93
Capacity retention was temperature dependent and
followed an Arrhenius relationship. The capacity loss rate increased by a factor of
⬃1.3 for
each 10
⬚C rise in temperature.
0
20
40
60
80
100
(a ):( 0 ºC)
4.00V
4.05V
4.10V
Capacity retention / %
0
20
40
60
80
100
(b):(25 ºC)
4.00V
4.05V
4.10V
Capacity retention / %
0 3 6 9 12 15 18 21 24
0
20
40
60
80
100
(c):(45 ºC)
Float-charging period (months)
4.00V
4.05V
4.10V
Capacity retention / %
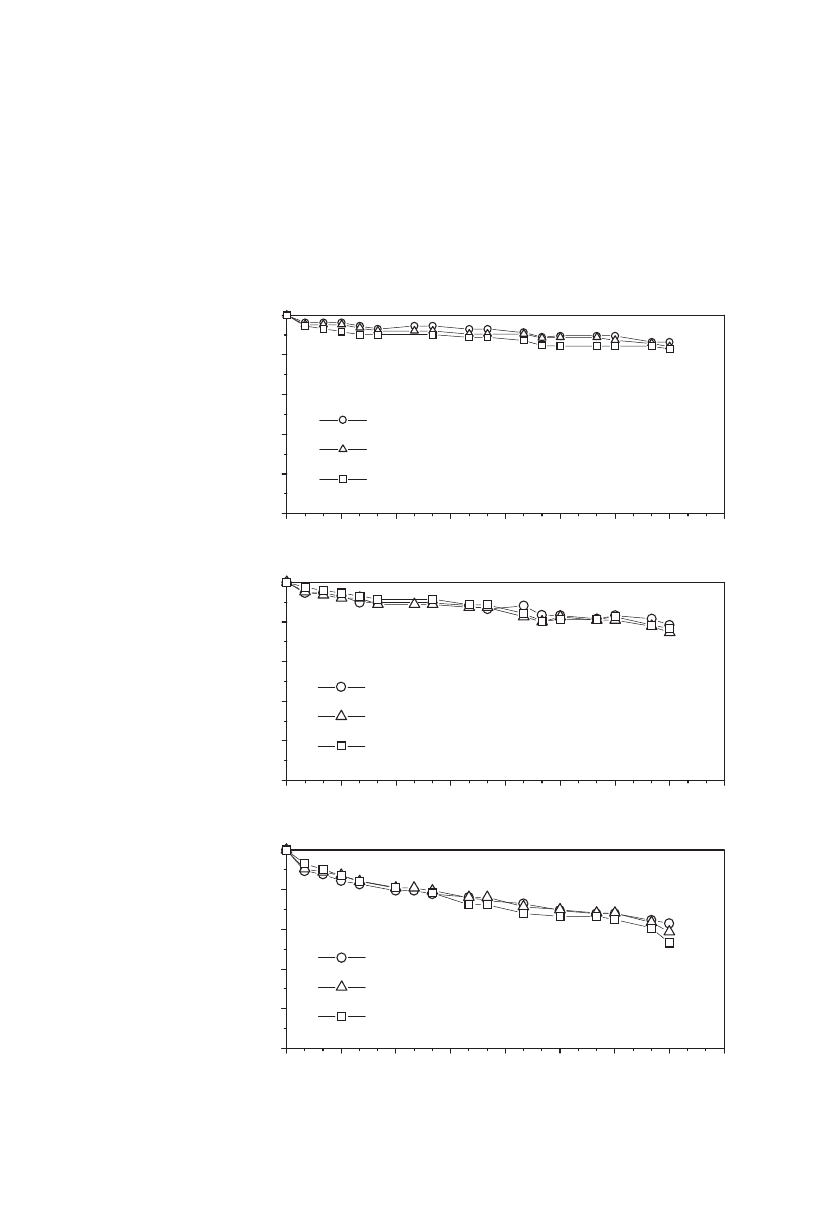
FIGURE 35.85 Capacity retention of wound ICP22846 C / LiCoO
2
Li-ion
cells float charged at 0⬚C, 25⬚Cor45⬚C at 4.0 V, 4.05 V or 4.1 V. (Courtesy
of Japan Storage Battery Co., Ltd.)
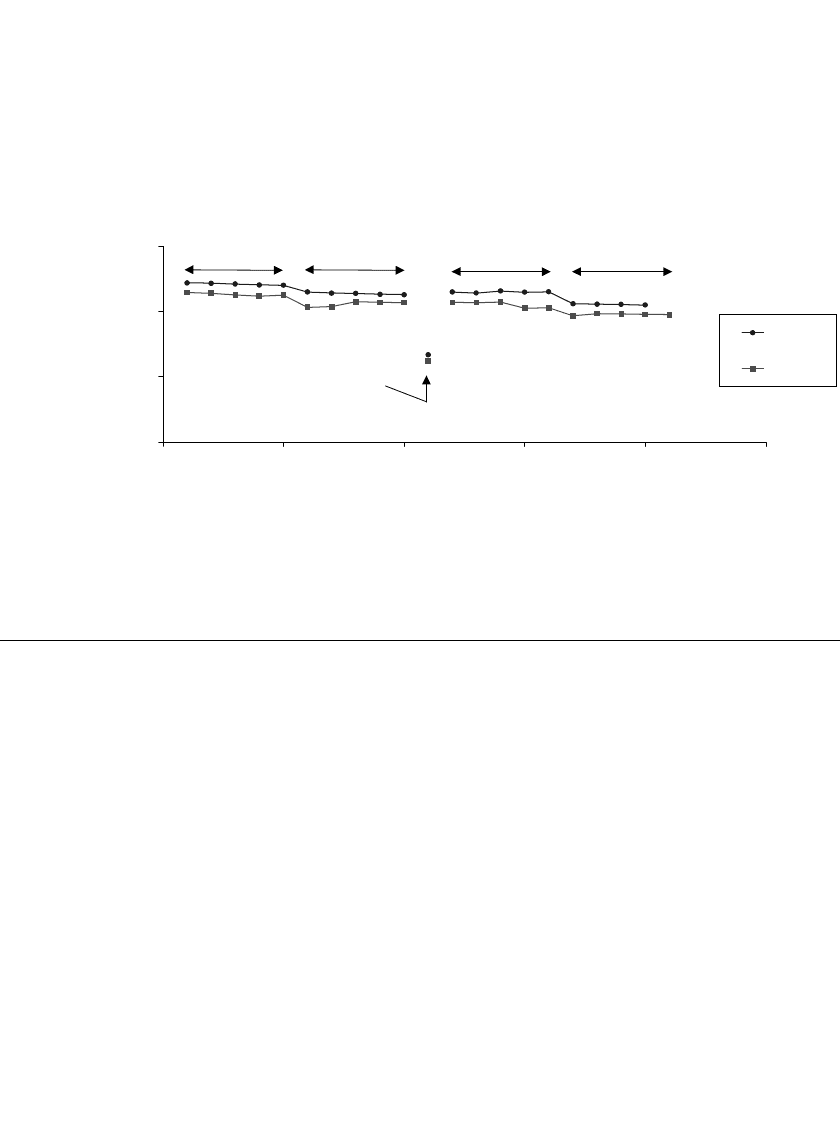
35.70 CHAPTER THIRTY-FIVE
The ability of flat plate prismatic designs to retain capacity on ‘‘float’’ is indicated by the
data shown in Fig. 35.86. After 12 weeks, discharge of the 5 Ah cells yielded 4.3 Ah,
indicating 18% self-discharge had occurred. Subsequent cycling demonstrated that they re-
tained over 98% of their reversible capacity.
3
4
5
6
0 5 10 15 20 25
Cycle Number
Discharge Capacity (Ah)
ZPB056
ZPB057
C/5 Charge,
C/5 Discharge
C/5 Charge,
1C Discharge
C/5 Charge,
C/5 Discharge
C/5 Charge,
1C Discharge
1
st
Discharge
after Storage
Before Stora
g
eAfter Stora
g
e
FIGURE 35.86 Float charge test of two different 5 Ah prismatic cells over 12 weeks at 25⬚C. (Courtesy of
Yardney Technical Products, Inc.)
35.6 SAFETY TESTING OF CYLINDRICAL C/ LiCoO
2
BATTERIES
Cylindrical 18650-type C/LiCoO
2
batteries have been exposed to a variety of safety test
regimes including UN, UL, IATA and military safety tests. These organizations, as well as
the IEC, are currently developing standards for Li-ion cells and batteries that will address
electrical testing, safety evaluation and transportation tests for Li-ion cells and batteries. The
safety evaluation tests include electrical, mechanical and environmental tests. The results are
summarized in Table 35.23.
Table 35.24 gives the results of typical safety tests performed on commercial 18650-type
C/ LiCoO
2
Li-ion batteries. On short circuit, performed by discharging through a minimum
length of 16 AWG wire, the voltage dropped to zero as the temperature rose to a maximum
of 69
⬚C after 130 minutes, after which the temperature returned to room temperature. Similar
results are reported for nail penetration. When the battery was exposed to heat, up to 150
⬚C,
leakage resulted. On crush, the cell voltage dropped to 0.8 V within 30 seconds as the
temperature rose to 116
⬚C over 5 minutes after which the temperature returned to ambient
as the voltage decayed to 0 V. Performance on impact was similar to crush, a maximum
temperature of 117
⬚C was reached after 4 minutes after which the temperature decayed to
ambient as the voltage decayed to 0 V. In both the crush and impact tests, leakage resulted.
On forced discharge at 1.6 A, the battery voltage reached a minimum of
⫺2 V after 8 minutes
then recovered to
⫺0.6 V, the maximum temperature on forced discharge was 58⬚C. In the
overcharge test reported here, the cell was overcharged to a maximum of 12 V at 4.8 A
(3C). In this test, the cell reached its maximum temperature of 56
⬚C two minutes after it
was fully charged. After full charge was achieved, the voltage quickly reached the prescribed
maximum of 12 V while the charging current went to zero for the remaining duration of the
test (30 minutes). Note that in none of these tests did the cell spontaneously disassemble,
and pressure was released through the vent mechanism when required.
LITHIUM-ION BATTERIES 35.71
TABLE 35.23 Summary of Safety Tests Included for Li-ion Batteries
Type Test Conditions
Electrical Continuous charge Charge at 20⬚C per manufacturers recommendation, hold at end of charge
voltage for 28 days.
Short circuit
⬍50 m⍀ to 0.1 V at 20⬚C and 50⬚C.
Forced discharge Discharge at 0.2C, for 12.5 h.
Over charge Charge at manufacturer recommend rate to 2.5 times rated capacity
High rate charge Charge at three times manufacturer’s recommended rate.
Mechanical Shock 3 axis, minimum 75 g, peak 125 g to 175 g.
Vibration 0.8 mm amplitude, 10 Hz to 55 Hz
Crush Between flat surfaces to 13 kN
Free fall 1 m to hard wood floor, 6 times
Environmental High temp. storage Store at 75
⬚C for 48 h
Thermal shock 75
⬚C for 48 h, 20⬚C for 30 h
Altitude 11.6 kPa for 6 h
Exposure Heat to 130
⬚C, hold at 130⬚C for 60 min.
TABLE 35.24 Typical Safety Tests Performed on Commercial 18650 Li-ion Batteries. (Courtesy of
Sanyo.)
Test Method Result
Short circuit 16 AWG wire, 20⬚C No event, Tmax ⫽ 69⬚C
Short circuit 16 AWG wire, 60
⬚C No event
Heat Heat at 5
⬚C/ min to 150⬚C, soak at 150⬚C for 10 min. Leakage
Crush Flat surface to 2500 psig (17.2 Mpa). Leakage, Tmax
⫽ 116⬚C
Impact 20 lbs from 2 ft on a 5 / 16 in bar Leakage, Tmax
⫽ 117⬚C
Humidity 65
⬚C, ⬎95% humidity No event
Vibration 0.03 in amplitude, 10 to 55 Hz No event
Drop 6 ft, for ten times onto concrete surface No event
Forced discharge 1.6 A,
⫺12 V minimum No event, Tmax ⫽ 58⬚C
Over charge 12 V max, 4.8 A max No event, Tmax
⫽ 56⬚C
Fire exposure Expose to fire Fire, leakage, smoke
35.7 POLYMER Li-ION BATTERIES
Polymer Li-ion batteries provide the performance characteristics of Li-ion batteries, including
their high specific energy and high energy density, in a thin, high aspect-ratio form factor.
The technology addresses applications, in particular portable communications and computing
devices which require a thin, large footprint rechargeable battery. While polymer Li-ion
cells utilize the same active materials as cylindrical or prismatic Li-ion cells, in polymer Li-
ion cells, flat, bonded electrodes are used to enable the fabrication of thin cells packaged
within a barrier film, in contrast to the steel or aluminum cell case used in other Li-ion
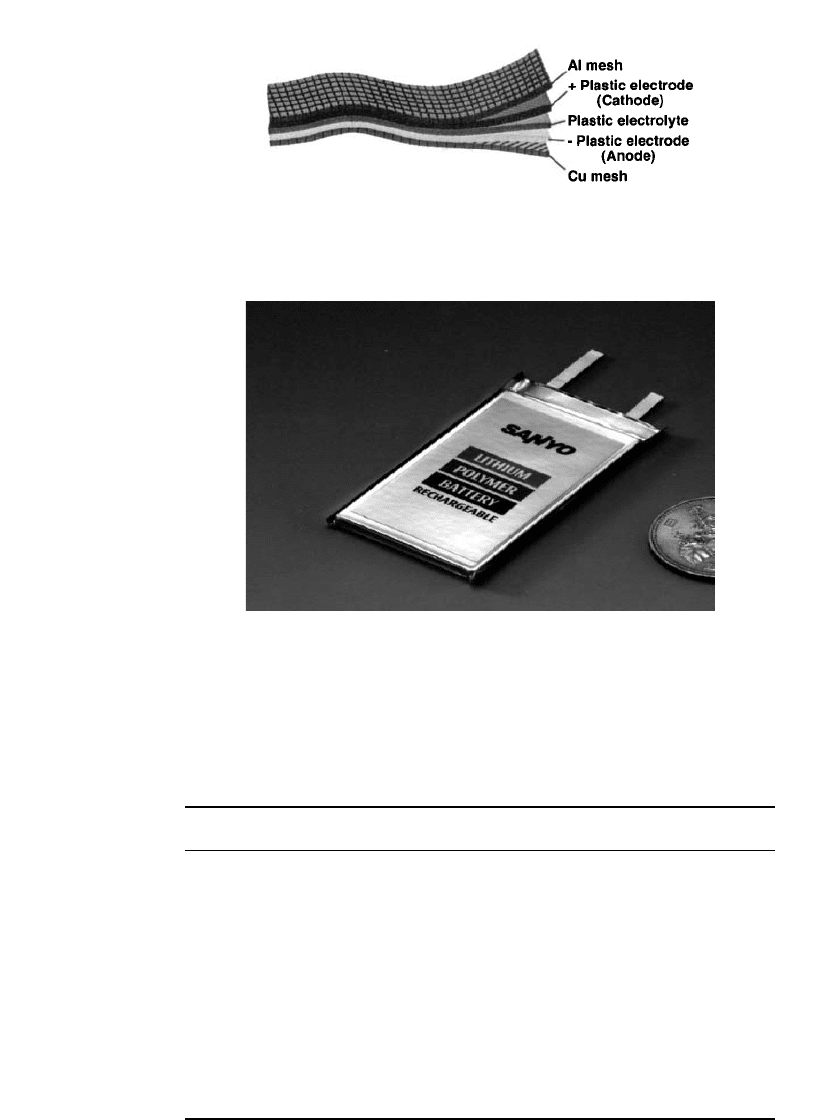
technologies. This construction is shown schematically in Fig. 35.87, which illustrates the
various layers of the construction. A commercial product is shown in Fig. 35.88.
An attractive feature of C/LiMn
2
O
4
polymer Li-ion cells is their ability to sustain abuse.
Safety and abuse tests passed by C /LiMn
2
O
4
polymer Li-ion cells are listed in Table 35.25.
In addition, C/ LiMn
2
O
4
polymer Li-ion cells can sustain nail penetration in the fully charged
state or the overcharged state without explosion or fire.
35.72 CHAPTER THIRTY-FIVE
FIGURE 35.87 Schematic diagram showing the construction of
a polymer Li-ion cell. (Courtesy of Telecordia.)
FIGURE 35.88 A 0.57 Ah polymer Li-ion battery. (Courtesy of
Sanyo.)
TABLE 35.25 Safety and Abuse Tests Passed by C/ LiMn
2
O
4
Polymer Li-ion
Batteries
Test Description
Flat crush 13 kN between flat plates
External short, 23
⬚C ⬍50 mOhm, 23⬚C
External short, 60
⬚C ⬍50 mOhm, 60⬚C
Forced discharge Discharge to
⫺12 V, 250% of rated capacity
Shock 75 G initial, 125 to 175G peak
Vibration 10-55-10 Hz, 90 min, 1.6 mm
Drop Ten times, 6 ft onto concrete
Impact 20 lbs from 2 ft onto a 8 mm insulated bar
Thermal cycling
⫺40⬚Cto70⬚C, ten times
Altitude Six hours at 11.6 kPa
Heating 5
⬚C/ min to 150⬚C, soak
Abnormal charge Three times recommended rate to 250% of rated capacity
Humidity Cycle 30
⬚Cto65⬚C, 85% to 95% relative humidity

LITHIUM-ION BATTERIES 35.73
The polymer Li-ion cells described here may be more accurately described as employing
a gel electrolyte, as the electrolyte contains a monomeric, volatile liquid component absorbed
into a polymeric host, in contrast to technologies which do not employ a volatile, liquid
component, such as solid polymer electrolyte batteries.
94
Because of the poor conductivity
of currently available solid polymer electrolytes (solid polymer lithium batteries developed
to date operate at 40⬚Cto80⬚C to accommodate the low conductivity of the electrolyte) (see
Sec. 34.4.2), current ‘‘polymer’’ Li-ion batteries incorporate less viscous, liquid components
to improve the conductivity of the electrolyte, enabling their use at ambient temperatures.
The electrochemistry of polymer Li-ion cells covers a wide range of active materials and
electrolyte compositions, comparable to those used in liquid-electrolyte Li-ion cells. Active
materials include lithiated manganese oxides, such as Li
1.05
Mn
1.95
O
4
, LiCoO
2
, LiNiO
2
and
its Co-doped derivatives, as positive (cathode) active materials, and graphitic and non-
graphitic carbons capable of reversibly intercalating lithium as the negative (anode) active
materials. Liquid electrolytes used in polymer Li-ion cells are comparable to those used in
cylindrical or prismatic Li-ion cells, typically ca. 1 M solutions of LiPF
6
in mixtures of
ethylene carbonate (EC), dimethyl carbonate (DMC), and other carbonate esters or other co-
solvents.
35.7.1 Electrode and Battery Fabrication
The electrodes for polymer Li-ion cells may be cast from a viscous mixture (a slurry) com-
posed of an active material (e.g., LiMn
2
O
4
, LiCoO
2
, LiAl
0.05
Co
0.15
Ni
0.8
O
2
, etc. for the positive
electrode, and a microbead mesophase graphite, artificial graphite or milled graphite fiber
for the negative electrode); a conductive additive (e.g., Super P carbon); a dissolved poly-
meric binder (e.g., PVDF-HFP, such as Kynar FLEX
2801); a medium-to-low-volatility
plasticizer, such as dibutyl phthalate or propylene carbonate, and processing aids, including
surfactants, antioxidants etc.; and a volatile solvent such as acetone or methyl ethyl ketone
(MEK). A plastic separator film may be cast from a homogenized slurry of the same poly-
meric binder (PVDF-HFP), silanized fumed silica, or alumina, and a plasticizer, in a similar
volatile solvent.
95
In the Telcordia (formerly Bellcore) technology, an ancillary plasticizer such as dibutyl
phthalate is incorporated into the resin that temporarily remains in the separator and/ or
electrode layers after their respective films are cast using a more volatile solvent. The plas-
ticizer facilitates the densification of the electrodes under lower temperature and /or pressure
than is required for the full densification of the electrode compositions typical in liquid-
electrolyte Li-ion cells. This, in turn, prevents damage to the delicate expanded-metal current
collector grids typically used in polymer Li-ion cells. The plasticizer also acts as a porosity
modifier and preserver in the electrodes and the separator during processing. Further, the
plasticizer facilitates formation of a bond between the electrodes and the separator. Often,
identical or similar polymeric binders, typically based on commercially available
poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) copolymers, are used in both
electrodes and the separator, facilitating the bonding of layers during the lamination step.
The use of other polymers, copolymers and their mixtures, e.g., PVDF-CTFE (CTFE
⫽
chlorotrifluoroethylene), poly(vinyl chloride) (PVC) and polyacrylonitrile (PAN) has also
been described.
96
Electrode tapes are then densified and bonded to their corresponding expanded-metal
current collector grids, aluminum in the case of the positive electrode and copper for the
negative, using heated double-roll laminators or parallel-platen presses. The current collector
grid can be either embossed into the outside surface of the electrode, or preferably, embedded
between two layers of electrode tape. Finally, the electrodes are laminated to a plastic or
microporous separator interposed between the electrodes.

35.74 CHAPTER THIRTY-FIVE
The electrode-separator bonding can be achieved by lamination (calendering), again be-
tween a pair of preheated rollers or platens, followed by removal of the plasticizer. The
plasticizer is removed by either supercritical or solvent extraction with an appropriate low-
boiling solvent of the plasticizer, but non-solvent of the polymeric binder, such as propane,
carbon dioxide, methanol, ether, or hexanes. Alternatively, the plasticizer is removed by
simple evaporation, preferably under reduced pressure and at elevated temperature.
The de-plasticized (extracted or otherwise) cells are then packaged into vapor-
impermeable, flexible, multilayer polymer-aluminum bags, dried under reduced pressure and
elevated temperature to remove any adsorbed water, activated with a measured amount of
liquid electrolyte solution, and sealed. The liquid electrolyte is rapidly absorbed into the
microporous structure of the electrodes and separator, thus making the spillage of the liquid
electrolyte from an open battery highly improbable.
A larger-capacity cell can contain one or more flat plates, each plate composed of one
positive electrode and one negative electrode bonded to two opposite sides of an ionically
conductive separator. The plates can be stacked as individual plates, Z-folded, or folded in
other ways as permitted by the mechanical properties of the individual component layers. A
popular option is a ‘‘bicell’’ configuration, where the central plate is a double-thickness
electrode, typically a negative electrode, sharing a single current collector grid. Both faces
of the central electrode are bonded via two separator layers to two slightly smaller counter-
electrodes, typically positive electrodes, each having its separate current collector.
A prismatic battery of larger capacity can be assembled from one or more stacked bicells,
whose current collector tabs are welded inside the packaging and thus present only a single
metal foil feed-through tab connected to each of the two multi-plate electrodes. To facilitate
multi-plate battery assembly and alignment, individual bicells may be Z-folded to prevent
electrodes and current collectors from breaking at the fold line; they may also be connected
by common current collector(s), separator(s), electrode layer(s), etc.
35.7.2 Energy Density of Polymer Li-ion Batteries
Current implementations of polymer Li-ion batteries technology achieve specific energy and
energy density values that are somewhat higher than those reported for liquid-electrolyte Li-
ion batteries. Since the electrochemistry as well as the volume and weight fractions of various
components in these two technologies are similar, this gain may be attributed to the lighter,
thinner packaging materials used in flat, bonded-electrode batteries, and possibly more ef-
ficient utilization of space.
The physical dimensions, specific energy and energy density of a selection of C /LiCoO
2
and C /LiMn
2
O
4
polymer Li-ion batteries are listed in Table 35.26. Polymer Li-ion batteries
offer a thin form factor, batteries with thickness from 1.2 mm to 8.4 mm are available,
although 3.5 mm to 4.0 mm thick batteries are typical; available capacities range from 0.5
Ah to 8 Ah. The practical lower limit to polymer battery thickness is 0.5 mm. The batteries
offer specific energy and energy density comparable to that delivered by cylindrical and
prismatic Li-ion batteries, C/ LiCoO
2
batteries deliver 145 to 190 Wh/kg, and 270 to 400
Wh/ L, whereas C /LiMn
2
O
4
batteries deliver 130 to 144 Wh/kg and 235 to 300 Wh/L.
35.75
TABLE 35.26 Electrical and Physical Characteristics of C / LiCoO
2
or C / LiMn
2
O
4
Polymer Li-ion Batteries
Battery type
ICP*
36/35/62
ICP*
36/34/50
ICP*
48/50/80
ICP*
60/ 100 / 100
IMP
30/ 25 / 110
IMP
30/34/48
IMP
30/ 103 / 103
IMP
30/36/65
IMP
12/70/140
IMP
57/70/140
Shape C/ LiCoO
2
(Source: Sony, Sanyo, Telcordia) C / LiMn
2
O
4
(Source: Valence Technologies)
Length, mm 62 50 80 100 110 48 103 65 140 140
Width, mm 35 34 50 100 25 34 103 36 70 70
Thickness, mm 3.6 3.6 4.8 6.0 3.0 3.0 3.0 3.0 1.2 5.7
Volume, ml. 7.8 6.1 19.2 60 8.25 4.9 31.8 7.0 11.8 55.9
Mass, g. 14.5 15 48 150 16 9.0 63 14 23 116
Capacity (Ah) 0.57 0.70 2.40 7.7 0.55 0.30 2.29 0.47 0.73 4.39
Specific energy (Wh/ kg) 145 170 180 190 130 130 138 127 120 144
Energy density (Wh/ L) 270 360 380 400 253 236 273 254 235 300
* Prototypes, not available in quantity.

35.76 CHAPTER THIRTY-FIVE
35.7.3 Performance of C /LiCoO
2
Polymer Li-ion Batteries.
Charge Performance. Polymer Li-ion batteries, like cylindrical or prismatic Li-ion batter-
ies, can be charged using either a constant current (CC), or constant current-constant voltage
(CCCV) regime. The charge current and battery voltage during the charge of a 0.120 Ah C/
LiCoO
2
polymer Li-ion battery at the 1.3C, 1C, and 0.7C rates is illustrated in Fig. 35.89.
In each case, the batteries charged at constant current until the battery voltage reached the
4.2 V voltage limit, at which time the current was reduced to maintain 4.2 V. At the 1C
rate, 40% of the two hour regime was at constant current.
Voltage, V
3.0
3.5
4.0
4.5
Charging time, (h)
0.0 0.5 1.0 1.5 2.0 2.5
Charging current, A
0.00
0.05
0.10
0.15
0.20
0.7C
1.3C
1C
FIGURE 35.89 Charge current and battery voltage during the charge of a 0.120 Ah
C / LiCoO
2
polymer Li-ion battery at the 1.3C, 1.0C and 0.7C rates, at 21⬚C. (Courtesy of
Telcordia.)
Discharge Rate Capability. The rate capability of a C /LiCoO
2
polymer Li-ion battery at
rates from 3C to 0.2C is illustrated in Fig. 35.90. As shown, the battery provided over 95%
of its capacity at the 1C rate, 87% at 2C, and 77% at 3C. At low rates, the battery provided
an average voltage of 3.8 V; at the 2C rate, 3.55 V; and at the 3C rate, 3.45 V on average.
The battery provided over 80% of its available energy at 2C.
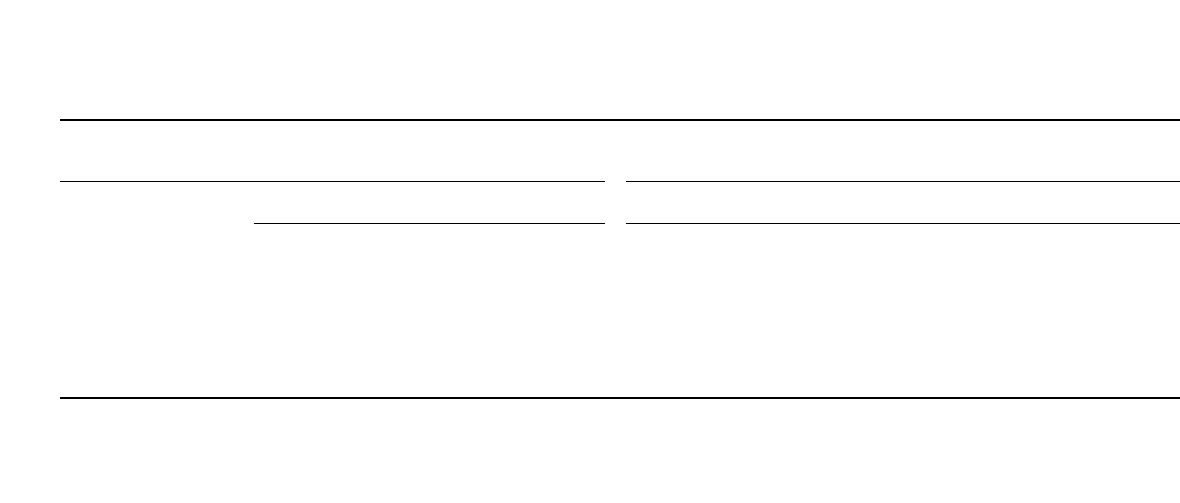
Polymer Li-ion batteries offer similar capability when discharged at constant power, as
illustrated in Fig. 35.91, which shows discharge curves for a 0.136 Ah (0.5 Wh) C/LiCoO
2
polymer Li-ion battery discharged at rates from 2 E to 0.25 E. At low rates (0.25 E), the
battery provided 0.51 Wh. At the 1 E rate, the battery provided 0.46 Wh, and 0.3 Wh at
2 E, or 58% of the available energy. As shown, the average voltage at low rates was 3.75
V, whereas at the 2 E rate, 3.5 V was obtained.
LITHIUM-ION BATTERIES 35.77
capacity utilization, %
0 20406080100
Voltage, V
2.5
3.0
3.5
4.0
4.5
C/5
3C
2C
1C
C/2
FIGURE 35.90 Discharge rate capability of a 0.12 Ah C / LiCoO
2
polymer Li-ion
battery at 3C to 0.2C rates, at 21⬚C. (Courtesy of Telcordia.)
Energy, Wh
0.0 0.1 0.2 0.3 0.4 0.5
Voltage, V
2.5
3.0
3.5
4.0
4.5
2E
E/2
3/2E
1E
E/4
FIGURE 35.91 Discharge rate capability of a 0.136 Ah C / LiCoO
2
polymer
Li-ion battery at 2 E to 0.25 E, at 21
⬚C. (Courtesy of Telcordia.)
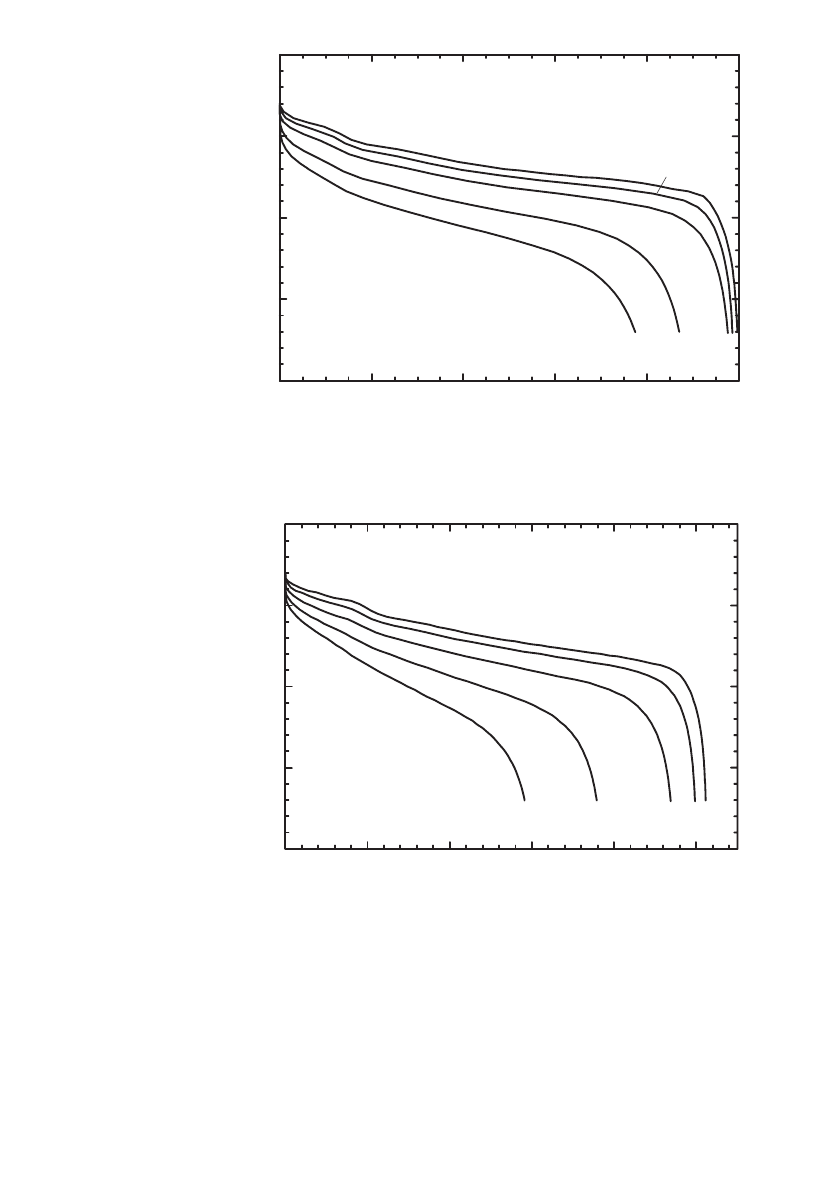
Low Temperature Performance. Discharge curves for a 0.57 Ah Sanyo C /LiCoO
2
Li-ion
polymer battery are shown at the 1C rate at temperatures from
⫺20⬚Cto⫹25⬚C in Fig.
35.92. As shown, the battery provided 58% its rated capacity at
⫺10⬚C.
The low temperature discharge capability of C/LiCoO
2
polymer Li-ion batteries is sum-
marized in Fig. 35.93 for rates from 0.125C to 2C at temperatures from
⫺20⬚Cto21⬚C. At
0
⬚C, when discharged at the 1C rate, the batteries provided 72% of their capacity. At ⫺20⬚C,
at low rates (0.125C) the batteries provided 90% of their capacity, whereas at the C /2 rate
at
⫺20⬚C, the battery provided 37% of its capacity. After the low temperature discharge, the
battery provided its original capacity when discharged at 21
⬚C indicating the system was not
degraded by thermal cycling.
35.78 CHAPTER THIRTY-FIVE
2.5
3.0
3.5
4.0
0 100 200 300 400 500 600 700
Discharge Capacity / mAh
Voltage / V
UPF363562 Bare cell
–20°C
–10°C
0°C +25°C
FIGURE 35.92 Battery voltage on 0.57 A discharge of a 0.57 Ah
polymer Li-ion battery. The battery was charged in a CCCV regime at
0.57 A to 4.2 V (0.028 A cutoff). (Courtesy of Sanyo.)
capacity utilization, %
0
20
40
60
80
100
test temperature,
o
C
2C
1C
C/2
C/4
C/8
21 0 -20 21
di
After discharge
at 0 and –20°C
scharge rate
FIGURE 35.93 Low temperature discharge capability of C / LiCoO
2
polymer
Li-ion batteries. Batteries were charged at 21⬚C. (Courtesy of Telcordia.)
Cycle Life. Polymer C /LiCoO
2
batteries offer high cycle life, as illustrated in Fig. 35.94
for a 0.136 Ah battery. This battery was charged at the 1C rate to 4.2 V (CCCV, 90 min.),
then discharged at either the 0.2C, C, or 2C rate. After discharge at 2C, the battery was
further discharged at 1C. Over 300 cycles, the battery capacity faded nominally 10%. The
effect of repeated cycling on the battery voltage and capacity is illustrated in Fig. 35.95,
which shows discharge curves at the 0.2C, C or 2C rates at cycles 20, 80, 130, 180, 240
and 290. As shown, little change in average voltage was observed, less than 0.1 V change
was observed, although the effect of capacity fade was more significant at lower rates. The
decrease in voltage is due to the increase in internal resistance with repeated cycling. Also,
the thickness of polymer Li-ion batteries increases 2 to 3% over the first 100 cycles. The
effect of these changes is illustrated in Fig. 35.96, which shows the capacity of a 0.57 Ah
polymer Li-ion battery when cycled at the 1C rate for 500 cycles, and Fig. 35.97, which
shows the internal resistance of the battery and the battery thickness during this regime.
