Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

LITHIUM-ION BATTERIES 35.79
Cycle number
0 100 200 300 400
Capacity, (Ah)
0.00
0.05
0.10
0.15
2C discharge capacities
C/5 discharge
residual capacity at 1C
FIGURE 35.94 Capacity of a 0.136 Ah C / LiCoO
2
polymer Li-ion battery when
charged at the 1C rate to 4.2 V (CCCV, 90 min.), and discharged at the 1C rate to
2.8 V, with periodic discharge at the 0.2C and 2C rates, at 21⬚C. (Courtesy of
Telcordia.)
Discharge time, h
012345
Voltage, V
2.5
3.0
3.5
4.0
4.5
C/5
2C
1C
20
280
cycles
FIGURE 35.95 Battery voltage when discharged at the 0.2C, C or 2C rates at
cycle numbers from 20 to 290, at 21⬚C. (Courtesy of Telcordia.)
35.80 CHAPTER THIRTY-FIVE
0
100
200
300
400
500
600
700
0 100 200 300 400 500
Cycle Number
Discharge Capacity (mAh)
UPF36 3562
FIGURE 35.96 Capacity of a 0.57 Ah polymer Li-ion battery when
charged at 0.57 A in a CCCV regime to 4.2 V (0.028 A cutoff), and
discharged at 0.57 A to 2.57 V. (Courtesy of Sanyo.)
3.4
3.5
3.6
3.7
3.8
3.9
4.0
4.1
0 100 200 300 400 500
Cycle Number
Cell Thickness (mm)
0
10
20
30
40
50
60
70
Internal Resistance (mOhms)
UPF363562
Cell Thickness
Internal Resistance
FIGURE 35.97 Battery internal resistance and thickness for a 0.57 Ah
polymer Li-ion battery charged at 0.57 A in a CCCV regime to 4.2 V (0.028 A
cutoff) and discharged at 0.57 A to 2.75 V. (Courtesy of Sanyo.)
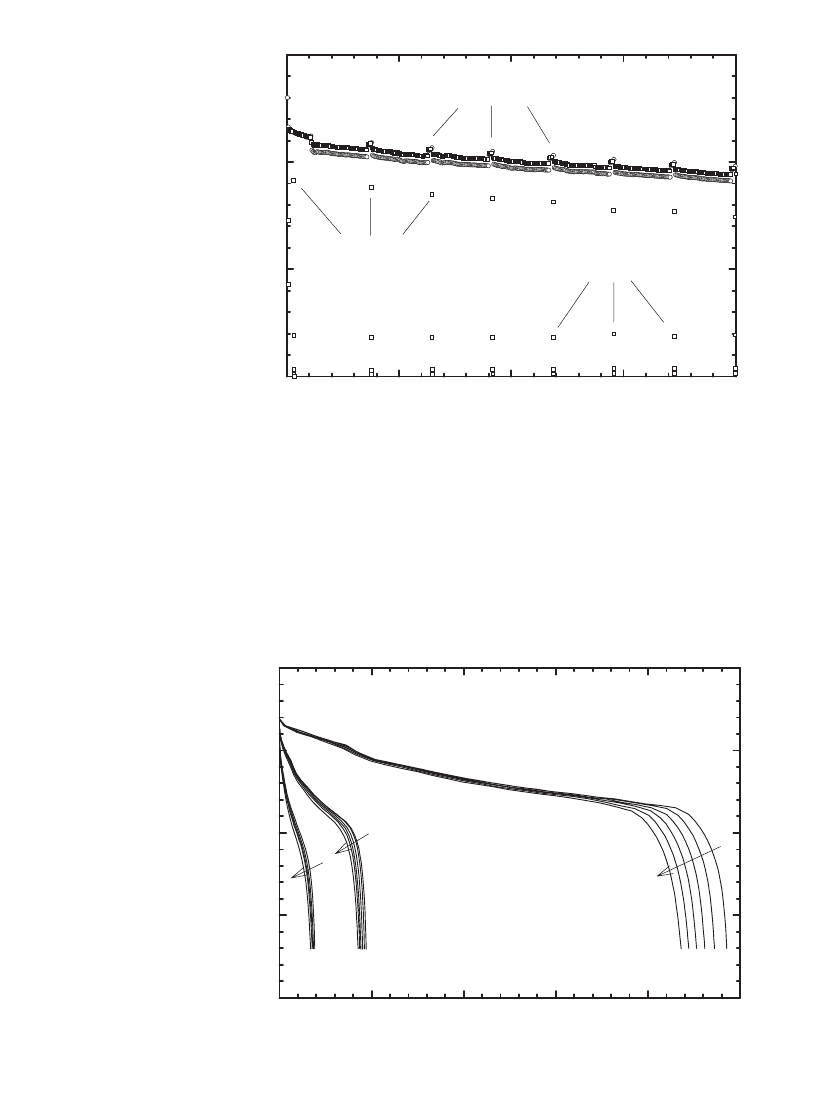
Storage Stability of Polymer C /LiCoO
2
Batteries. Polymer C/ LiCoO
2
Li-ion batteries can
sustain storage at elevated temperature, as illustrated in Fig. 35.98, for storage at 60
⬚C, and
in Fig. 35.99 for storage at 80
⬚C. In these tests, batteries were cycled at the C/5 rate, stored,
then cycled at either the C/2 or C/5 rates. As shown, after storage at 60
⬚Cor80⬚ C, ap-
proximately 7% capacity loss was observed.
LITHIUM-ION BATTERIES 35.81
Cycle number
0 20406080
Capa city, Ah
0.0
0.1
0.2
0.3
C/5
discharge rate: C/2
storage loss
C/5
C/5
FIGURE 35.98 Capacity fade of a C/ LiCoO
2
polymer Li-ion battery
when cycled at 21⬚C after storage fully charged at 60⬚C for seven days.
(Courtesy of Telcordia.)
Cycle number
020406080
Capacity, (Ah)
0.0
0.1
0.2
0.3
C/5 discharge rate: C/2
storage loss
C/5 C/5
FIGURE 35.99 Capacity fade of a C / LiCoO
2
polymer Li-ion battery
when cycled at 21⬚C after storage fully charged at 80⬚C for seven days.
(Courtesy of Telcordia.)
35.7.4 Performance of C /LiMn
2
O
4
-type Polymer Li-ion Batteries
The electrical performance of polymer Li-ion batteries that employ the C/LiMn
2
O
4
chemistry
is comparable to other Li-ion batteries. The voltage of a C /LiMn
2
O
4
battery when discharged
at 0.5C then charged in a CCCV regime at the 0.5C rate to 4.2 V is indicated in Fig. 35.100.
As shown, the batteries provide slightly higher average voltage than C/LiCoO
2
-type batteries,
3.8 V, although their specific energy and energy density are lower than comparable batteries

35.82 CHAPTER THIRTY-FIVE
0123456
3.0
3.2
3.4
3.6
3.8
4.0
4.2
Charge 4.2V
Constant Voltage
Charge C/2
Constant Current
Rest
Open Circuit
C/2 Discharge
Voltage (Volts)
Test Time (hrs)
FIGURE 35.100 Voltage as a function of time for a 3 Ah C /LiMn
2
O
4
polymer Li-ion battery discharged and charged at the 0.5C rate. (Courtesy
of Valence Technology.)
FIGURE 35.101 Polymer C/ LiMn
2
O
4
Li-ion batteries. (Courtesy of Valence Technology.)
that employ a cobalt-based chemistry. The batteries also provide a low self discharge rate,
2.5% per month at 23
⬚C and 6% per month at 40⬚C. The manganese-based cathode materials
these batteries employ are regarded as environmentally more benign than their cobalt or
nickel-based analogs, and the batteries are claimed to offer improved safety properties. Figure
35.101 is an illustration of several polymer C /LiMn
2
O
4
Li-ion batteries.
LITHIUM-ION BATTERIES 35.83
Discharge Rate Capability. The rate capability of a C/ LiMn
2
O
4
batteries charged in a
CCCV regime at the 0.5C rate to 4.2 V is illustrated in Fig. 35.102. As shown, the batteries
provide 98% of their capacity at the 0.5C rate, and 85% at the 1C rate. Also, these batteries
provide a flat voltage profile with average voltage of 3.8 V at low rates (0.2C); at higher
rates (1C) the average voltage is lower, typically 3.6 V.
FIGURE 35.102 Rate capability of a C / LiMn
2
O
4
polymer Li-ion battery charged
at the 0.5C rate to 4.2 V in a CCCV regime at 23⬚C. (Courtesy of Valence Technology.)
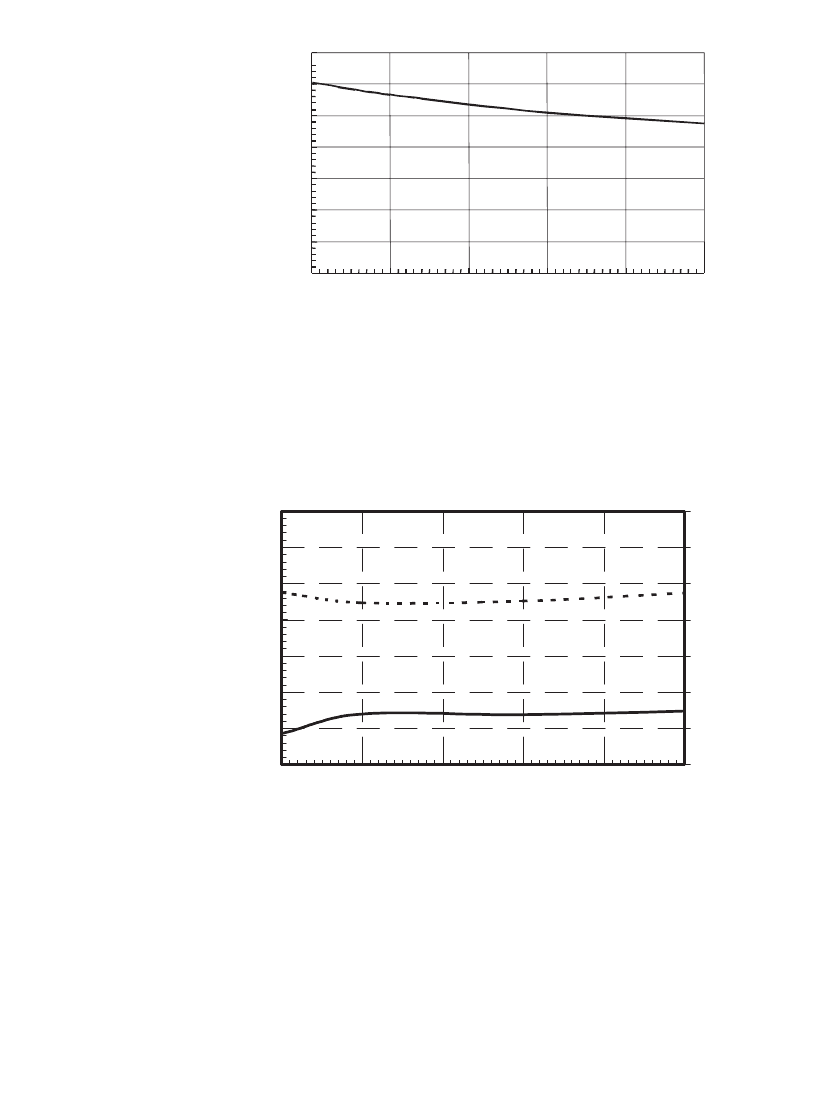
High and Low Temperature Discharge Capability. The ability of C/LiMn
2
O
4
polymer Li-
ion batteries to provide 0.2C discharge capability at temperatures from 60
⬚Cto⫺20⬚Cis
illustrated in Fig. 35.103. As shown, at temperatures at or above 23
⬚C, the battery voltage
and capacity was little changed. At 0
⬚C, the battery provided over 95% of its capacity; at
⫺10⬚ the battery provided 86% of its capacity, and at ⫺20⬚C the battery provided 46% of
the capacity delivered at 23
⬚C. At ⫺20⬚C the average voltage was 3.5 V, 0.3 V lower than
provided at 23
⬚C. The ability of a C /LiMn
2
O
4
polymer Li-ion cell to provide 1C discharge
capability at temperatures from 23
⬚Cto⫺20⬚C is indicated by the data shown in Fig. 35.104.
As shown, at 0
⬚C the 0.6 Ah cell provided 40% of its capacity, and at ⫺10⬚C, 23%.
2
Voltage (V)
FIGURE 35.103 Voltage of a 0.6 Ah C / LiMn
2
O
4
polymer Li-ion battery when dis-
charged at the 0.2C rate at temperatures from 60⬚Cto⫺20⬚C. The battery was charged in
a CCCV regime at 0.5C to 4.2 V at 23⬚C. (Courtesy of Valence Technology.)
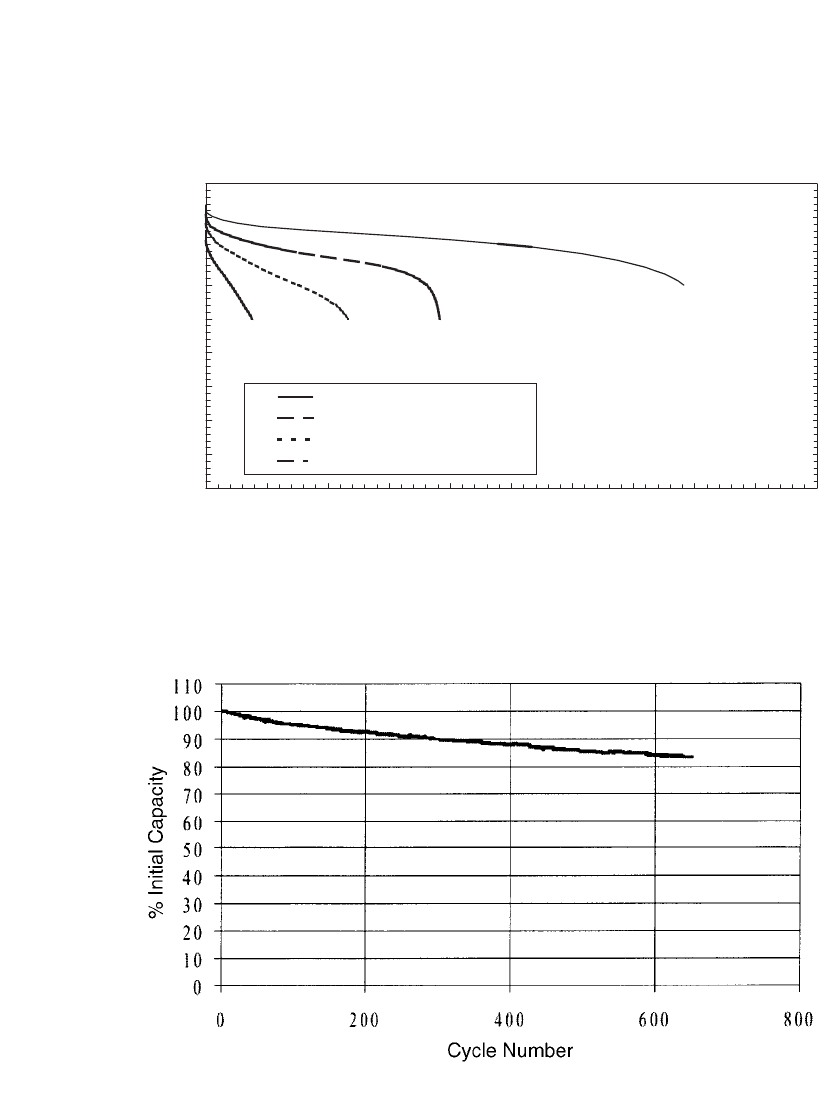
35.84 CHAPTER THIRTY-FIVE
Cycle Life. Polymer C/LiMn
2
O
4
batteries also provide long cycle life, as illustrated in Fig.
35.105 for a battery cycled at the 0.5C rate. As shown, the battery provided 90% of its initial
capacity after 300 cycles, and 84% after 600 cycles.
0
0.5
1
1.5
2
2.5
3
3.5
4
4.5
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
Capacity [%]
Voltage [V]
1C Discharge at 23°C
1C Discharge at 0°C
1C Discharge at -10°C
1C Discharge at -20°C
FIGURE 35.104 Voltage of a 0.6 Ah C / LiMn
2
O
4
polymer Li-ion battery when discharged at the 1C rate
at temperatures from 23⬚Cto⫺20⬚C. The battery was charged in a CCCV regime at 0.5C to 4.2V at 23⬚C.
(Courtesy of Valence Technology.)
FIGURE 35.105 Capacity of a C / LiMn
2
O
4
polymer Li-ion battery when charged in a CCCV regime
at the 0.5C rate to 4.2 V and discharged to 3.0 V at the 0.5C rate, at 23⬚C. (Courtesy of Valence
Technology.)

LITHIUM-ION BATTERIES 35.85
35.7.5 Conclusions: Polymer Li-ion Batteries
Polymer Li-ion batteries offer specific performance comparable to cylindrical and prismatic
Li-ion batteries within a unique, thin form factor. In addition to providing a thin form factor,
the batteries offer many properties desirable for commercial applications including good rate
capability, low self-discharge, long storage stability and the ability to safely sustain physical
or electrical abuse. These products are now being produced in high volume and are being
used in increasing quantities in cell phones and PDAs.
35.8 THIN-FILM, SOLID-STATE Li-ION BATTERIES
A specialized type of Li-ion battery developed for semi-conductor and printed circuit board
(PCB) applications are thin-film, solid-state devices. These batteries which employ ceramic
negative, solid electrolyte and positive electrode materials, can sustain high temperatures
(250
⬚C), and can be fabricated by high volume manufacturing techniques on silicon wafers
which are viable as on-chip or on-board power sources for microelectronics.
97,98
Batteries of
this type can be very small, 0.04 cm
⫻ 0.04 cm ⫻ 2.0
m. For microelectronics applications,
all components must survive solder re-flow conditions, nominally 250
⬚C in air or nitrogen
for 10 minutes. Cells with liquid or polymer electrolytes cannot sustain these conditions
because of the volatility or thermal stability of organic components. Further, cells that employ
lithium metal also fail as solder re-flow conditions exceed the melting point of lithium
(180.5
⬚C).
A schematic drawing of a thin-film, solid-state Li-ion cell is shown in Fig. 35.106. These
cells are fabricated by sequential layer deposition of the cell components using rf magnetron
sputtering, except for the metallic current collector components which are deposited by DC
magnetron sputtering. The deposition conditions for LiCoO
2
99
and lithium phosphorous ox-
ynitride (LiPON) electrolyte are reported in the literature.
98
The cells are fabricated on a
substrate, typically alumina, quartz, soda-lime glass, or silicon. Positive current collectors of
gold or platinum (0.1
mto0.3
m), over a layer of cobalt (0.01
m to 0.05
m, to
improve adhesion), have been used. Cells using either LiCoO
2
or LiMn
2
O
4
positive electrode
materials have been fabricated. The positive electrode layer of laboratory test cells is typically
0.05
mto5
m thick and 0.04 cm
2
to 25 cm
2
in area, depending on the capacity required
by the application. The electrolyte layer, LiPON, is typically 0.7
mto2
m thick.
Cells with and without negative electrode materials have been fabricated.
100
In cells with
a negative electrode material, SiSn
0.87
O
1.20
N
1.72
(SiTON), SnN
x
, InN
x
or Zn
3
N
2
have been
used. To accommodate the difference in volumetric capacity of positive and negative elec-
trode materials, the thickness of the negative electrode is typically 7% of the positive elec-
trode. Negative current collectors of copper, titanium, or titanium nitride (0.1
mto0.3
m)
are typical. To enhance the hermeticity of the cell, protective overlayers of LiPON (1
m)
or parylene (6
m) and titanium or aluminum (0.1
m) have been used.
Alternatively, the negative electrode materials may be omitted. A schematic diagram of
this type of cell prior to and after initial charge is illustrated in Fig. 35.107. In these cells,
lithium metal is plated onto the negative current collector when the cell is charged. The
lithium plating-stripping process is efficient with this technology. Thus cells with a negative
electrode material are typically engineered to oversaturate the negative electrode material, or
the negative electrode material is omitted completely.
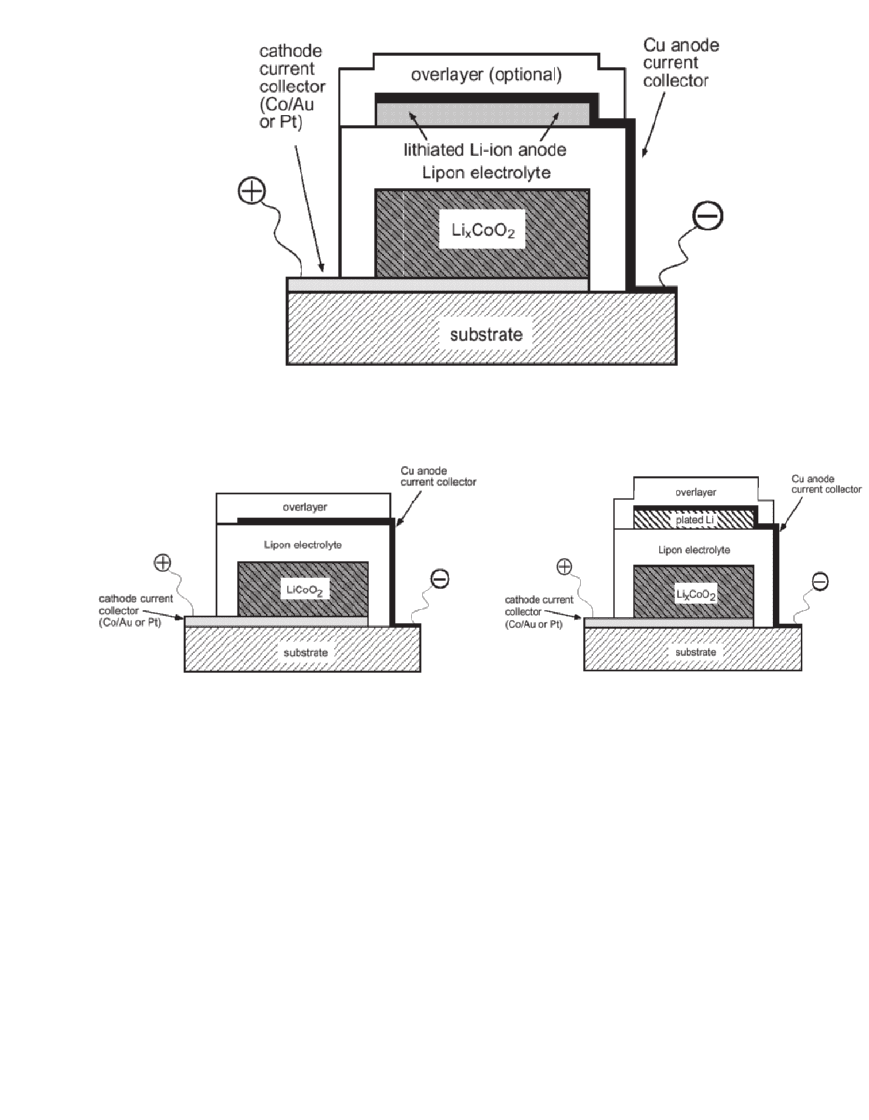
35.86 CHAPTER THIRTY-FIVE
FIGURE 35.106 Schematic drawing of the construction of a thin-film,
solid-state Li-ion cell after an initial charge that lithiates the negative elec-
trode material. (Courtesy of Oak Ridge National Laboratory.)
FIGURE 35.107 Schematic diagram of a thin-film ‘‘lithium free’’ solid-state battery prior to and after initial
charge. (Courtesy of Oak Ridge National Laboratory. Reproduced by permission of The Electrochemical Society,
Inc. From Ref. 100.)
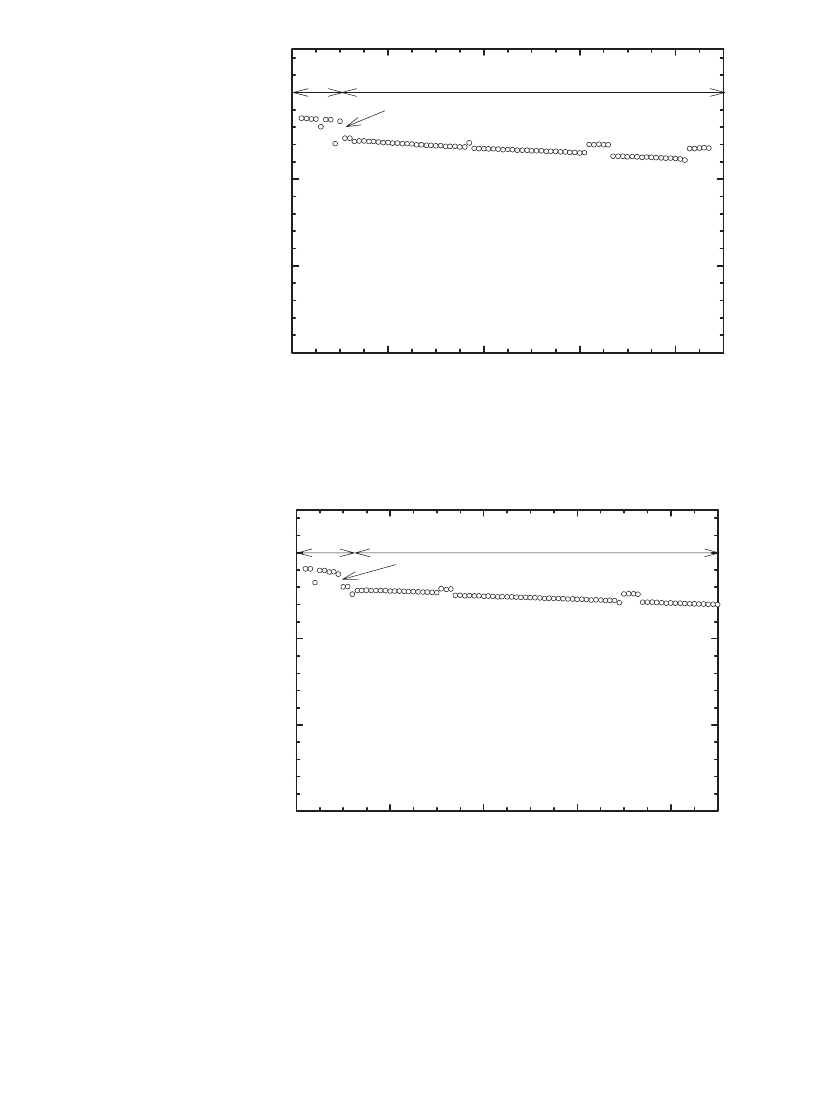
35.8.1 Electrical Performance of Thin-film, Solid-state Li-ion Batteries
SiTON/LiCoO
2
Batteries. The rate capability of a SiTON/LiCoO
2
battery before any ther-
mal treatment is illustrated in Fig. 35.108. As shown, the battery provided a discharge current
density up to 5 mA/ cm
2
, comparable to cylindrical Li-ion batteries, within a voltage range
of 4.2 V to 2.7 V. The utilization of the negative electrode material (anode) was high, 600
mAh/g at 2 mA/cm
2
.
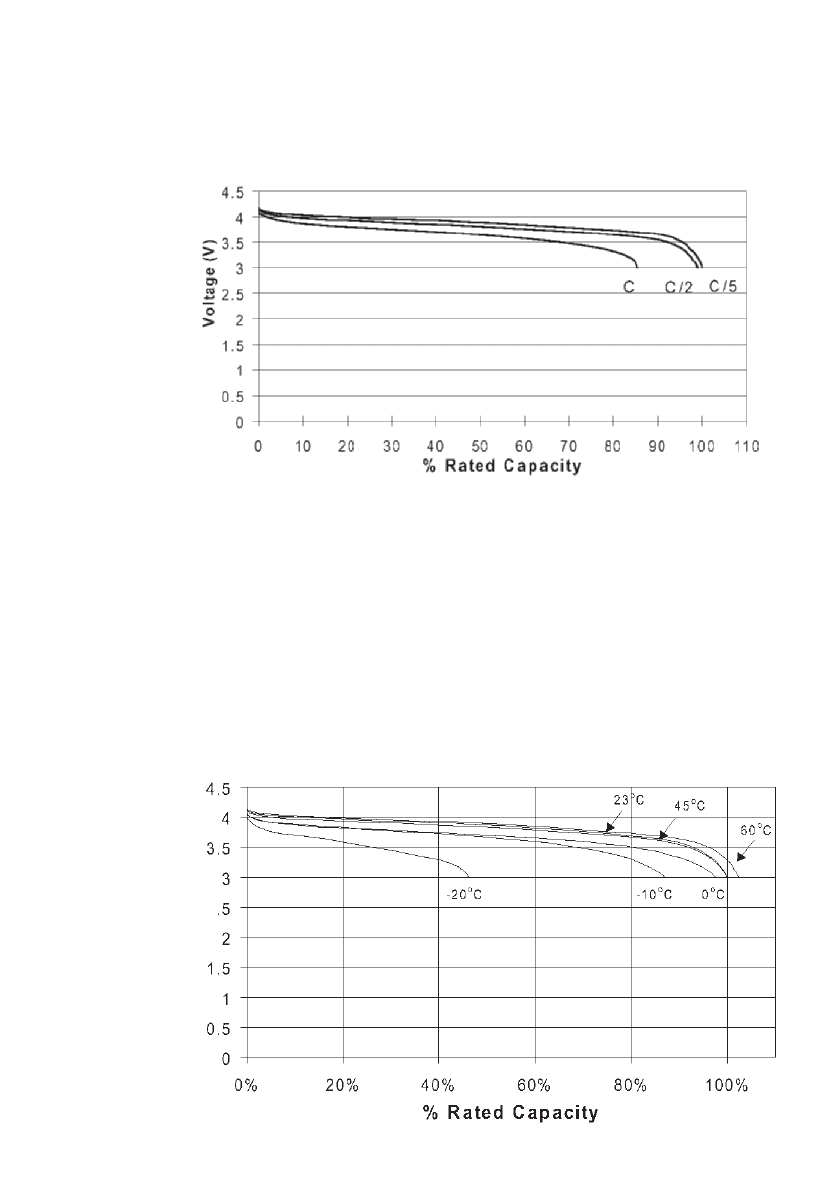
These batteries are designed for use in microelectronics applications where all components
must sustain solder reflow processes, typically a heat treatment at 250
⬚C for 10 minutes. To
demonstrate the ability to sustain these conditions, Figure 35.109 shows discharge curves
before and after heat treatment at 250
⬚C for 10 minutes or 1 hour. As shown, the capacity
of the battery increased by 20% as a result of the 10 minute treatment, indicating that solder
re-flow processes improve battery performance. This improvement has been suggested to
result from improved ionic conductivity and decreased charge transfer resistance of the
LiPON electrolyte as a result of the heat treatment.
98
Heat treatment also improved the rate
capability of the battery, as illustrated in Fig. 35.110. As shown, at 5 mA/ cm
2
, between 4.2
V and 2.7 V, the utilization of the SiTON was 450 mAh/g, 30% higher that observed before
heat treatment. In addition, discharge at 10 mA /cm
2
and 20 mA/cm
2
was demonstrated.
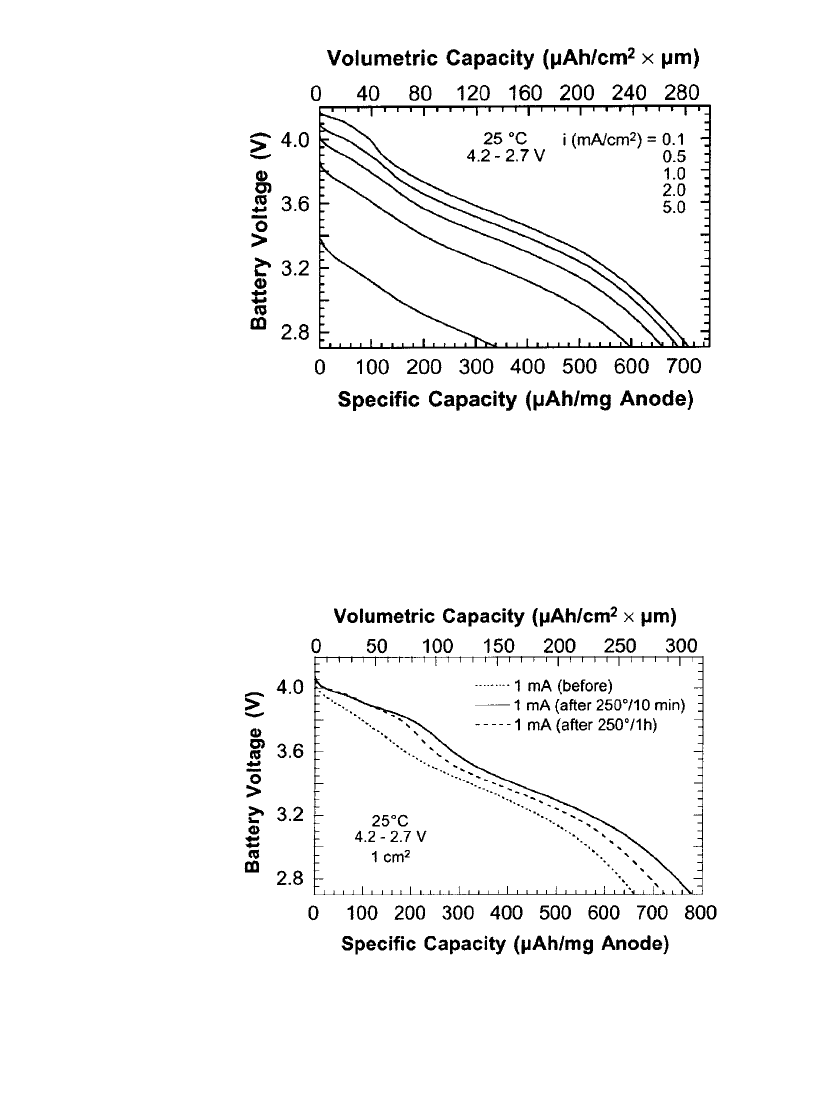
LITHIUM-ION BATTERIES 35.87
FIGURE 35.108 Rate capability of a SiTON / LiPON / LiCoO
2
bat-
tery. Specific capacity and volumetric capacity are based on the as-
deposited mass and volume of SiTON. (Courtesy of Oak Ridge Na-
tional Laboratory. Reproduced from the Journal of Power Sources, Vol.
81, 1999, with permission from Elsevier Science.)
FIGURE 35.109 Voltage of a SiTON/ LiCoO
2
battery when discharged
at 25⬚Cat1mA/cm
2
before and after heat treatment at 250⬚C for 10
minutes or 1 hour. (Courtesy of Oak Ridge National Laboratory. Repro-
duced from the Journal of Power Sources, Vol. 81, 1999, with permission
from Elsevier Science.)
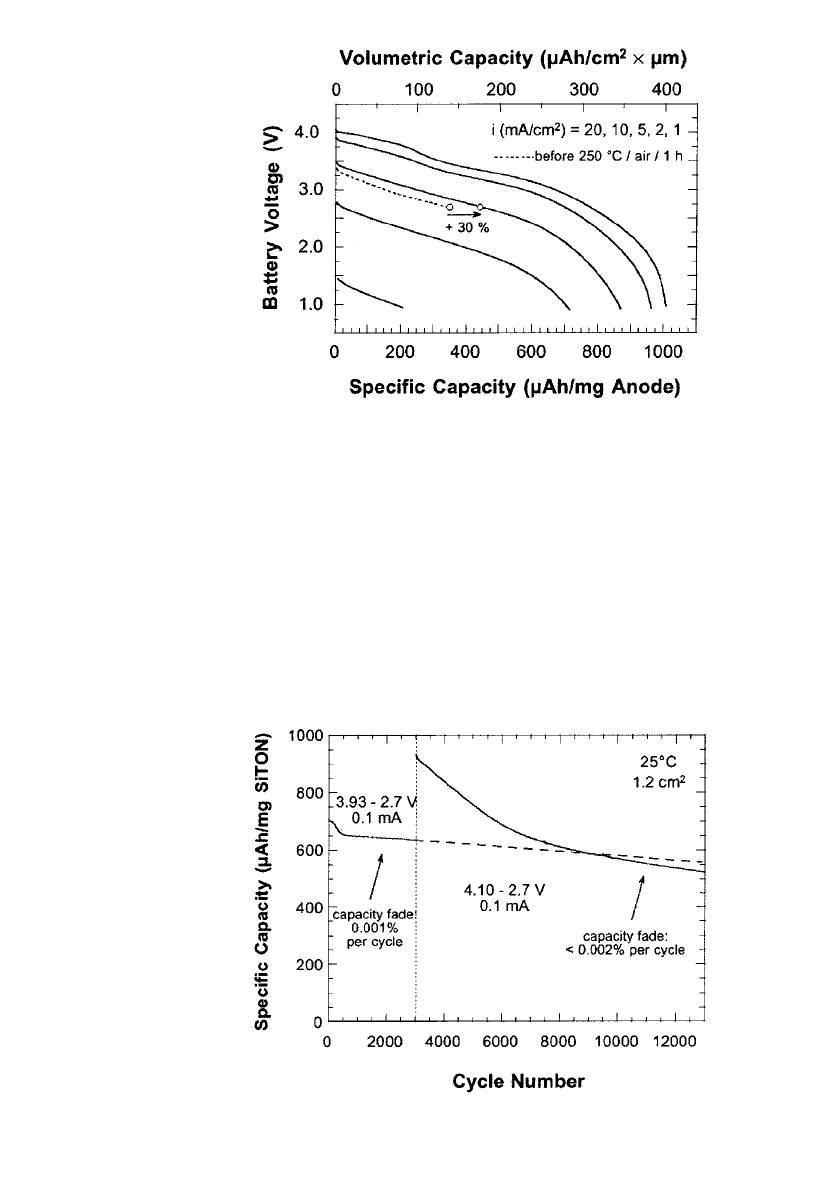
35.88 CHAPTER THIRTY-FIVE
FIGURE 35.110 Rate capability of a SiTON/ LiPON/ LiCoO
2
battery after treatment at 250⬚C in air for 1 hour. Specific capacity
and volumetric capacity are based on the as-deposited mass and
volume of SiTON. (Courtesy of Oak Ridge National Laboratory.
Reproduced from the Journal of Power Sources, Vol. 81, 1999, with
permission from Elsevier Science.)
Solid-state SiTON/ LiCoO
2
batteries offer high cycle life, as illustrated in Fig. 35.111 for
a cathode heavy cell cycled initially between 3.93 V and 2.7 V for 3000 cycles, then between
4.1 V and 2.7 V for 10,000 cycles, all at 0.08 mA/cm
2
at 25⬚C. When the upper voltage
limit was set to 3.93 V, no Li-plating occurred, whereas with the upper voltage limit set to
4.1 V, 30% of the capacity occurred while the negative electrode was at 0 V vs. lithium. In
the initial 3000 cycles, the capacity fade rate was 0.001% per cycle, whereas with the higher
upper voltage limit, the capacity fade rate was 0.002% per cycle while the cell capacity was
initially increased over 40%.
FIGURE 35.111 SiTON specific capacity when cycled in a SiTON/
LiCoO
2
battery between either 3.93 V and 2.7 V or 4.1 V and 2.7 V,
at 0.08 mA / cm
2
at 25⬚C. (Courtesy of Oak Ridge National Labora-
tory. Reproduced from the Journal of Power Sources, Vol. 81, 1999,
with permission from Elsevier Science.)
