Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.


3. Draw a best-fit straight line.
4. Extend the line to the zero-drawdown and read the value of the
intercept, r
0
.
Example: A water supply well is pumping at a constant discharge rate of
1000 m
3
/d (11,000 gpm). It happens that there are five observation wells
available. After pumping for 3 h, the drawdown at each observation well is
recorded as below. Estimate transmissivity and storativity of the aquifer
using the distance-drawdown method.
solution:
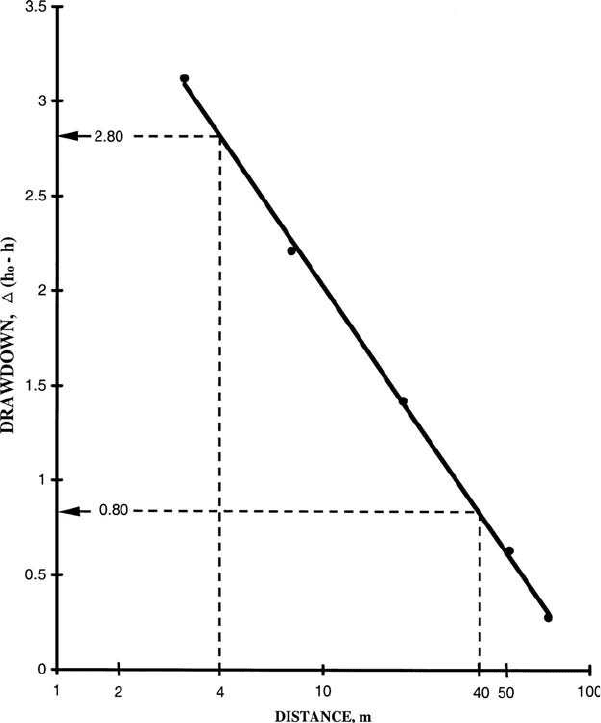
Step 1. Plot the distance-drawdown data on semilog paper as shown in
Fig. 3.7
Step 2. Draw a best-fit straight line over the observed data and extend the
line to the x-axis
Step 3. Read the drawdown value for one log cycle
From Fig. 3.7, for the distance for a cycle from 4 to 40 m, the value of the draw-
down, then ⌬(h
0
– h) is read as 2.0 m (2.8 m – 0.8 m).
Step 4. Read the intercept on the X axis for r
0
r
0
⫽ 93 m
Step 5. Determine T and S by Eqs. (3.41) and (3.43)
⫽ 183 m
2
/d
Time of pumping: t ⫽ 3 h/24 h/d
⫽ 0.125 days
⫽ 0.006
S 5
2.25Tt
r
2
0
5
2.25 3 183 m
2
/d 3 0.125 days
s93 md
2
T 5
0.366Q
⌬sh
0
2 hd
5
0.366 3 1000 m
3
/d
2.0 m
Distance
m ft Drawdown, m
3 10 3.22
7.6 25 2.21
20 66 1.42
50 164 0.63
70 230 0.28
Groundwater 205
5.4 Slug tests
In the preceding sections, the transmissivity T and storativity S of the
aquifer and permeability K of the soil are determined by boring one or
two more observation wells. Slug tests use only a single well for the
determination of those values by careful evaluation of the drawdown
curve and information of screen geometry. The tests involve either rais-
ing or lowering the water level in the well and measuring the return to
a static water level as a function of time.
206 Chapter 3
Figure 3.7 Plot of observed distance-drawdown data.
A typical test procedure requires introducing an object to record the
volume (the slug) of the well. The Hvorslev (1951) method using a
piezometer in an confined aquifer is widely used in practice due to it
being quick and inexpensive. The procedures of conducting and ana-
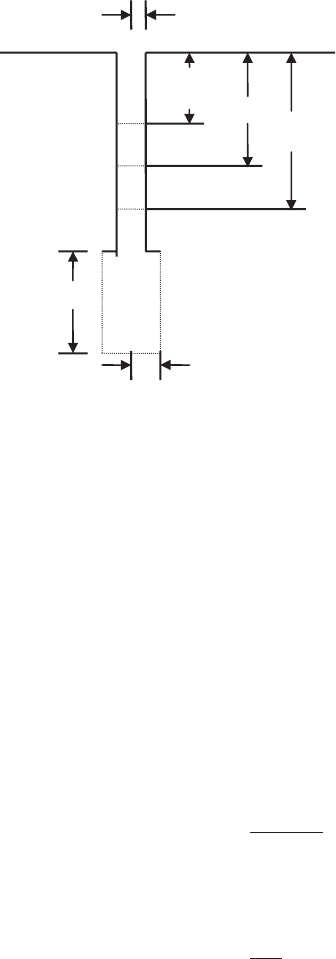
lyzing the Hvorslev test (Fig. 3.8) are as follows:
1. Record the instantaneously raised (or lowered) water level to the
static water level as H
0
.
2. Read subsequently changing water levels with time as h. Thus h ⫽
H
0
at t ⫽ 0.
3. Measure the final raised head as H at infinite time.
4. There is a relationship as
(3.45)
where
(3.46)
⫽ Hvorslev-defined basic time lag
F ⫽ shape factor
T
0
5
pr
2
Fk
H 2 h
H 2 H
0
5 e
2t/T
0
Groundwater 207
r
H
o
h
H
L
R
Figure 3.8 Hvorslev slug test.

5. Calculate ratios (H – h)/(H – H
0
) as recovery.
6. Plot on semilogarithmic paper (H – h)/(H – H
0
) on the logarithmic
scale versus time on the arithmetic scale.
7. Find T
0
at recovery equals 0.37 (37% of the initial change caused by
the slug).
8. For piezometer intake length divided by radius (L/R) greater than 8,
Hvorslev evaluated the shape factor F and proposed an equation for
hydraulic conductivity K as
(3.47)
where K ⫽ hydraulic conductivity, m/d or ft/d
r ⫽ radius of the well casing, cm or in
R ⫽ radius of the well screen, cm or in
L ⫽ length of the well screen, cm or in
T
0
⫽ time required for water level to reach 37% of the initial
change, s
Other slug test methods have been developed for confined aquifers
(Cooper et al., 1967; Papadopoulous et al., 1973; Bouwer and Rice, 1976).
These methods are similar to Theis’s type curves in that a curve-matching
method is used to determine T and S for a given aquifer. Afamily of type
curves H
t
/H
0
versus Tr/r
2
c
were published for five values of the variable,
(defined as (r
2
s
/r
2
c
) S, to estimate transmissivity, storativity, and hydraulic
conductivity.
The Bouwer and Rice (1976) slug test method is most commonly used
for estimating hydraulic conductivity in groundwater. Although the
method was originally developed for unconfined aquifers, it can also be
applied for confined or stratified aquifers if the top of the screen is some
distance below the upper confined layer. The following formula is used
to compute hydraulic conductivity:
(3.48)
where K ⫽ hydraulic conductivity, cm/s
r ⫽ radius of casing, cm
y
0
, y
t
⫽ vertical difference in water levels between inside and
outside the well at time t ⫽ 0, and t ⫽ t, m
R ⫽ effective radius distance over which y is dissipated, cm
K 5
r
2
ln sR/r
w
d
2L
1
t
ln
y
0
y
t
K 5
r
2
lnsL/Rd
2LT
0
208 Chapter 3

r
w
⫽ radius distance of undisturbed portion of aquifer from well
centerline (usually r plus thickness of gravel).
L ⫽ length of screen, m
t ⫽ time, s
Example 1: The internal diameters of the well casing and well screen are
10 cm (4 in) and 15 cm (6 in), respectively. The length of the well screen is 2
m (6.6 ft). The static water level measured from the top of the casing is 2.50
m (8.2 ft). A slug test is conducted and pumped to lower the water level to 3.05
m (10 ft). The time-drawdown h in the unconfined aquifer is recorded every
3 s as shown in the following table. Determine the hydraulic conductivity of
the aquifer by the Hvorslev method.
solution:
Step 1. Calculate (h – H
0
)/(H – H
0
)
Given: H
0
⫽ 2.50 m, H ⫽ 3.05 m
Then H – H
0
⫽ 3.05 m – 2.50 m ⫽ 0.55 m.
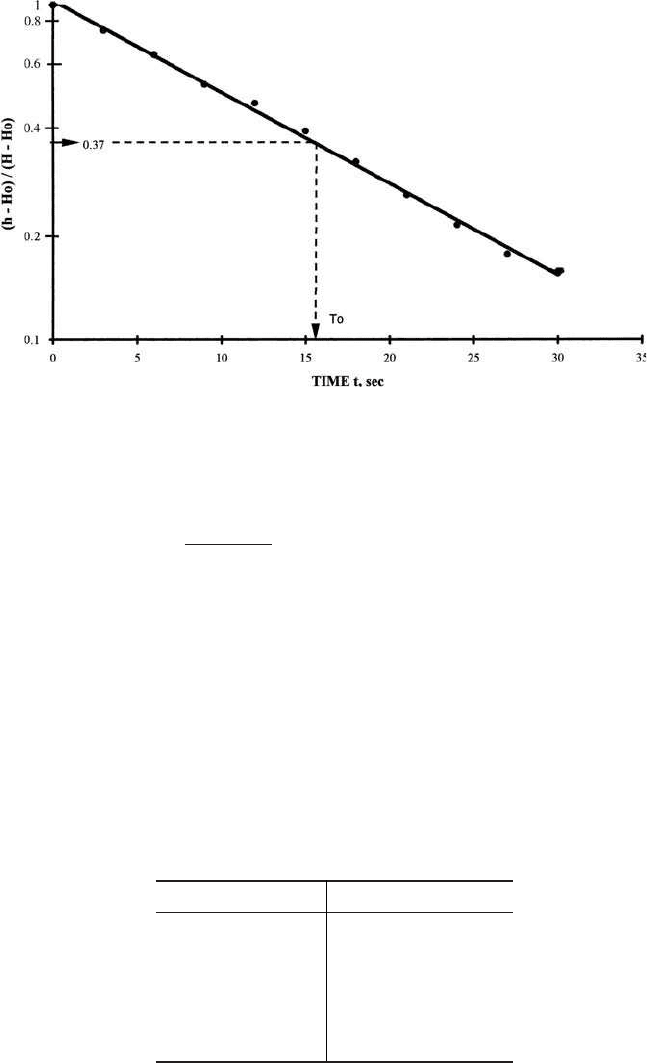
Step 2. Plot t versus (h – H
0
)/(H – H
0
) on semilog paper as shown in Fig. 3.9,
and draw a best-fit straight line
Step 3. Find T
0
From Fig. 3.9, read 0.37 on the (h – H
0
)/(H – H
0
) scale and note the time for
the water level to reach 37% of the initial change T
0
caused by the slug. This
is expressed as T
0
. In this case T
0
⫽ 16.2 s
Step 4. Determine the L/R ratio
R ⫽ 15 cm/2 ⫽ 7.5 cm
L/R ⫽ 200 cm/7.5 cm ⫽ 26.7 ⬎ 8
Thus Eq. (3.47) can be applied
Time t, s h, m h – H
0
, m (h – H
0
)/0.55
0 3.05 0.55 1.00
3 2.96 0.46 0.84
6 2.89 0.39 0.71
9 2.82 0.32 0.58
12 2.78 0.28 0.51
15 2.73 0.23 0.42
18 2.69 0.19 0.34
21 2.65 0.15 0.27
24 2.62 0.12 0.22
27 2.61 0.10 0.18
30 2.59 0.09 0.16
Groundwater 209
Step 5. Find K by Eq. (3.47)
r ⫽ 10 cm/2 ⫽ 5 cm
⫽ (5 cm)
2
ln 26.7/(2 ⫻ 200 cm ⫻ 16.2 s)
⫽ 0.0127 cm/s
⫽ 0.0127 cm/s ⫻ (1 m/100 cm) ⫻ 86,400 s/d
⫽ 11.0 m/d
⫽ 36 ft/d
Example 2: A screened, cased well penetrates a confined aquifer with gravel
pack 3.0-cm thickness around the well. The radius of casing is 5.0 cm and the
screen is 1.2 m long. A slug of water is injected and water level raised by m. The
effective radial distance over which y is dissipated is 12 cm. Estimate hydraulic
conductivity for the aquifer. The change of water level with time is as follows:
t, s y
t
, cm t,s y
t
, cm
1 30 10 4.0
2 24 13 2.0
3 17 16 1.1
4 14 20 0.6
5 12 30 0.2
6 9.6 40 0.1
8 5.5
K 5
r
2
ln sL/Rd
2LT
0
210 Chapter 3
Figure 3.9 Plot of Hvorslev slug test results.
solution:
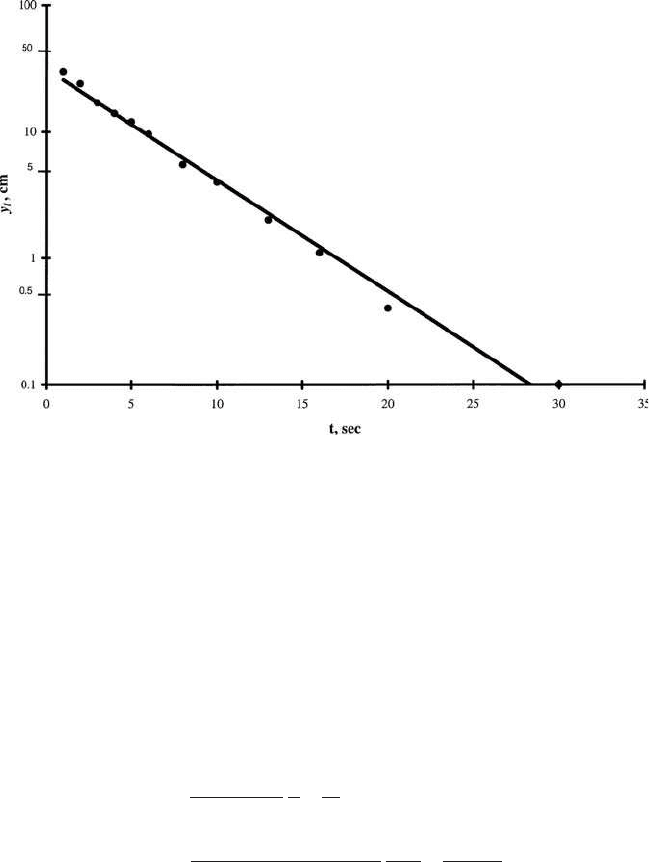
Step 1. Plot values of y versus t on semilog paper as shown in Fig. 3.10
Draw a best-fit straight line. The line from y
0
⫽ 36 cm to y
t
⫽ 0.1 cm covers
2.5 log cycles. The time increment between the two points is 26 s.
Step 2. Determine K by Eq. (3.48), the Bouwer and Rice equation
r ⫽ 5 cm
R ⫽ 12 cm
r
w
⫽ 5 cm ⫹ 3 cm ⫽ 8 cm
⫽ 9.56 ⫻ 10
–3
cm/s
6 Groundwater Contamination
6.1 Sources of contamination
There are various sources of groundwater contamination and various
types of contaminants. Underground storage tanks, agricultural activity,
municipal landfills, abandoned hazardous waste sites, and septic tanks
5
s5 cmd
2
lns12 cm/8 cmd
2s120 cmd
1
26 s
ln
36 cm
0.1 cm
K 5
r
2
ln sR/r
w
d
2L
1
t
ln
y
0
y
t
Groundwater 211
Figure 3.10 Plot of y
t
versus t.

are the major threats to groundwater. Other sources may be from indus-
trial spill, injection wells, land application, illegal dumps (big problem
in many countries), road salt, saltwater intrusion, oil and gas wells,
mining and mine drainage, municipal wastewater effluents, surface
impounded waste material stockpiles, pipelines, radioactive waste dis-
posal, and transportation accidents.
Large quantities of organic compounds are manufactured and used by
industries, agriculture, and municipalities. These man-made organic
compounds are of most concern. The inorganic compounds occur in
nature and may come from natural sources as well as human activities.
Metals from mining, industries, agriculture, and fossil fuels also may
cause groundwater contamination.
Types of contaminants are classified by chemical group. They are
metals (arsenic, lead, mercury, etc.), volatile organic compounds
(VOCs) (gasoline, oil, paint thinner), pesticides and herbicides, and
radionuclides (radium, radon). The most frequently reported ground-
water contaminants are the VOCs (Voelker, 1984; Rehfeldt et al.,
1992).
Volatile organic compounds are made of carbon, hydrogen, and oxygen
and have a vapor pressure less than one atmosphere. Compounds such
as gasoline or dry cleaning fluid evaporate when left open to the air.
They are easily dissolved in water. There are numerous incidences of
VOCs contamination caused by leaking underground storage tanks.
Groundwater contaminated by VOCs poses cancer risks to humans either
by ingestion of drinking water or inhalation.
6.2 Contaminant transport pathways
The major contaminant transport mechanisms in groundwater are
advection, diffusion, dispersion, adsorption, chemical reaction, and
biodegradation. Advection is the movement of contaminant(s) with the
flowing groundwater at the seepage velocity in the pore space and is
expressed as Darcy’s law:
(3.49)
where v
x
⫽ seepage velocity, m/s or pps
K ⫽ hydraulic conductivity, m/s or fps
n ⫽ porosity
dh ⫽ pressure head, m or ft
L ⫽ distance, m or ft
Equation (3.49) is similar to Eq. (3.10b).
v
x
5
K
n
dh
L
212 Chapter 3

Diffusion is a molecular-scale mass transport process that moves
solutes from an area of higher concentration to an area of lower con-
centration. Diffusion is expressed by Fick’s law:
(3.50)
where F
x
⫽ mass flux, mg/m
2
⭈ s or lb/ft
2
⭈ s
D
d
⫽ diffusion coefficient, m
2
/s or ft
2
/s
dc/dx ⫽ concentration gradient, mg/(m
3
⭈ m)
or lb/(ft
3
⭈ ft)
Diffusive transport can occur at low or zero flow velocities. In a tight soil
or clay, typical values of D
d
range from 1 to 2 ⫻ 10
9
m
2
/s at 25⬚C (Bedient
et al. 1994). However, typical dispersion coefficients in groundwater are
several orders of magnitude greater than that in clay.
Dispersion is a mixing process caused by velocity variations in porous
media. Mass transport due to dispersion can occur parallel and normal
to the direction of flow with two-dimensional spreading.
Sorption is the interaction of a contaminant with a solid. It can be
divided into adsorption and absorption. An excess concentration of con-
taminants at the surfaces of solids is called adsorption. Absorption refers
to the penetration of the contaminants into the solids.
Biodegradation is a biochemical process that transforms contami-
nants (certain organics) into simple carbon dioxide and water by microor-
ganisms. It can occur in aerobic and anaerobic conditions. Anaerobic
biodegradation may include fermentation, denitrification, iron reduction,
sulfate reduction, and methane production.
Excellent and complete coverage of contaminant transport mecha-
nisms is presented by Bedient et al. (1994). Theories and examples are
covered for mass transport, transport in groundwater by advection, dif-
fusion, dispersion, sorption, chemical reaction, and bio-degradation.
Some example problems of contaminant transport are also given by
Tchobanoglous and Schroeder (1985). Mathematical models that analyze
complex contaminant pathways in groundwater are also discussed else-
where (Canter and Knox, 1986; Willis and Yeh, 1987; Canter et al., 1988;
Mackay and Riley, 1993; Smith and Wheatcraft, 1993; Watson and
Burnett, 1993; James, 1993; Gupta, 1997).
The transport of contaminants in groundwater involves adsorption,
advection, diffusion, dispersion, interface mass transfer, biochemical
transformations, and chemical reactions. On the basis of mass balance,
the general equation describing the transport of a dissolved contaminant
through an isotropic aquifer under steady-state flow conditions can be
mathematically expressed as (Gupta, 1997).
F
x
52D
d
dc
dx
Groundwater 213

(3.51)
where c ⫽ solute (contaminant in liquid phase)
concentration, g/m
3
S ⫽ concentration in solid phase as mass of
contaminant per unit mass of dry soil, g/g
t ⫽ time, day
b
⫽ bulk density of soil, kg/m
3
x
⫽ effective porosity
k
l
, k
s
⫽ first-order decay rate in the liquid and soil phases,
respectively, day
–1
x, y, z ⫽ Cartesian coordinates, m
D
x
, D
y
, D
z
⫽ directional hydrodynamic dispersion coefficients, m
2
/d
V
x
, V
y
, V
z
⫽ directional seepage velocity components, m/d
There are two unknowns (c and S) in Eq. (3.51). Assuming a linear
adsorption isotherm of the form
S ⫽ K
d
c (3.52)
where K
d
⫽ distribution coefficient due to chemical reactions and bio-
logical degradation, and substituting Eq. (3.52) into Eq. (3.51), we obtain
(3.53)
where R ⫽ 1 ⫹ k
d
(
b
/)
⫽ retardation factor which slows the movement of
solute due to adsorption
k ⫽ k
l
⫹ k
s
K
d
(
b
/)
⫽ overall first-order decay rate, day
–1
The general equation under steady-state flow conditions in the x direc-
tion, we modify from Eq. (3.53).
6.3 Underground storage tank
There are 3 to 5 million underground storage tanks (USTs) in the United
States. It is estimated that 3% to 10% of these tanks and their associ-
ated piping systems may be leaking (US EPA 1987). The majority of
R
'c
't
1 kc 5 D
x
'
2
c
'x
2
1 D
y
'
2
c
'y
2
1 D
z
'
2
c
'z
2
2 V
x
'c
'x
2 V
y
'c
'y
2 V
z
'c
'z
5 D
x
'
2
c
'x
2
1 D
y
'
2
c
'y
2
1 D
z
'
2
c
'z
2
2 V
x
'c
'x
2 V
y
'c
'y
2 V
z
'c
'z
'c
't
1
r
b
r
'S
't
1 ck
l
1 Sk
s
r
b
w
214 Chapter 3
