Leroy C., Rancoita P.-G. Principles Of Radiation Interaction In Matter And Detection
Подождите немного. Документ загружается.

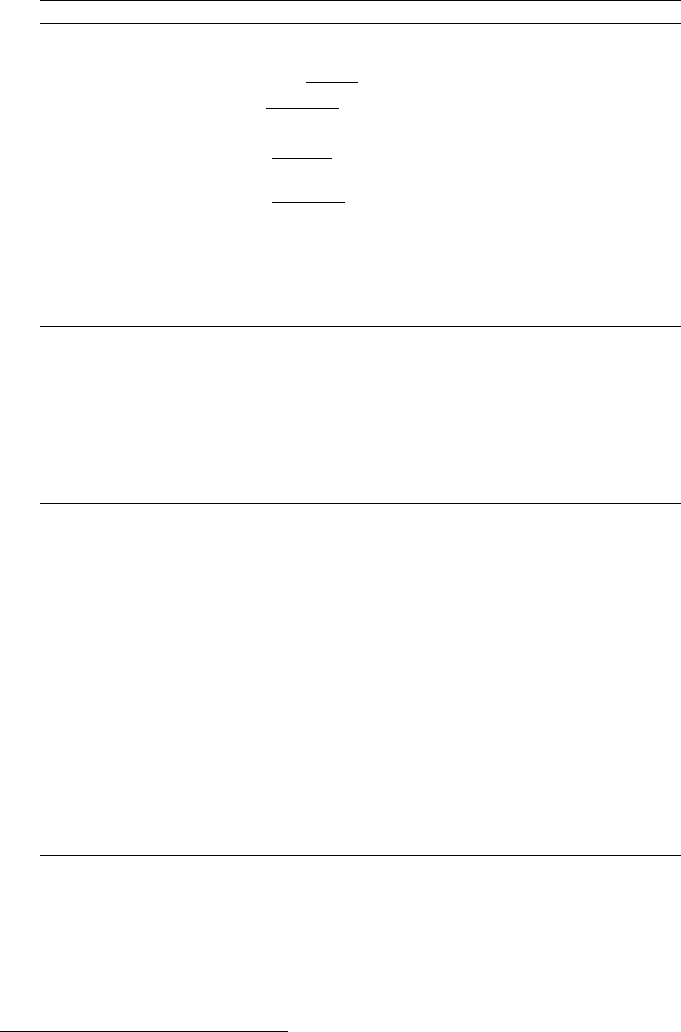
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
800 Principles of Radiation Interaction in Matter and Detection
continued from previous page
Quantity Symbol Value
Rydberg constant R
∞
=
m
e
c
2
α
2
2hc
for infinitely =
2π
2
e
4
m
e
(4π²
0
)
2
h
3
c
1.0973731568525 × 10
5
cm
−1
large R
∞
nuclear mass =
2π
2
e
4
m
e
h
3
c
, e in esu
Rydberg constant R
for =
R
∞
(1+m
e
/M )
nuclear mass M
Rydberg energy Ry = R
∞
hc
= (m
e
c
2
α
2
)/2 13.6056923 eV
classical Thomson 6.65245873 × 10
−25
cm
2
cross section σ
T h
= (8/3)πr
2
e
0.665245873 b
atomic radius in the
Thomas–Fermi
model a
Z
= a
0
/Z
1/3
Astronomical Unit
∗
AU 1.4959787 × 10
8
km
Parsec 3.08567758066631 × 10
13
km
2.06265 × 10
5
AU
pc 3.262 ly (light years)
volumetric radius 6.370998685023 × 10
3
km
of the Earth Re 6.370998685023 × 10
8
cm
solar mass
∗
m
J
1.989 × 10
33
g
solar radius
∗
R
J
6.955 × 10
5
km
solar mean density
∗
ρ
J
1.409 g/cm
3
Newtonian constant 6.67428×10
−11
m
3
/(kg s
2
)
of gravitation G 6.70881×10
−39
~c/(GeV/c
2
)
2
continued on next page
∗
Value from page 72 of [Aschwanden (2006)].
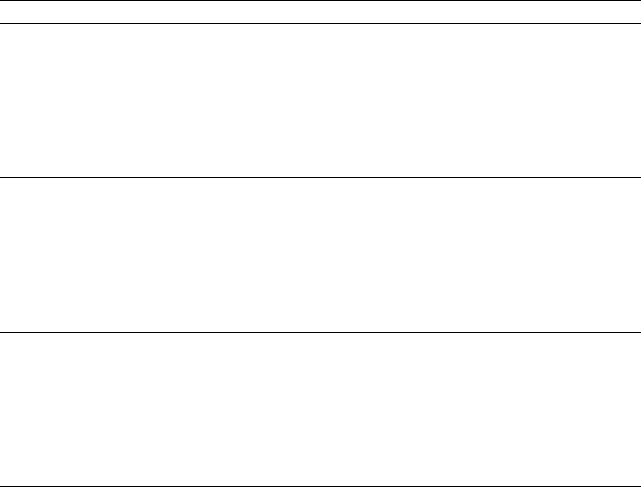
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Physical Constants 801
continued from previous page
Quantity Symbol Value
Fermi
coupling constant G
F
/(~c)
3
1.16637(1)×10
−5
GeV
−2
Stefan–Boltzmann σ
constant = π
2
k
4
B
/(60~
3
c
2
) 5.670400 ×10
−8
W/(m
2
K
4
)
air density at NTP
i.e., (20
◦
C, 1 atm) 0.001205 g cm
−3
air density at STP
i.e., (0
◦
C, 1 atm) 0.0012931 g cm
−3
unit of absorbed dose Gy 10
4
erg g
−1
for deposited energy 1 Gy = 100 rad 6.24 × 10
12
MeV kg
−1
unit of activity Bq
1 Bq = 1 dis. s
−1
1/(3.7 × 10
10
) Ci
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
802 Principles of Radiation Interaction in Matter and Detection
A.3 Periodic Table of Elements
This version of the Periodic Table is based on that recommended by the Commission
on the Nomenclature of Inorganic Chemistry and published in IUPAC Nomencla-
ture of Inorganic Chemistry, Recommendations (1990). The definition of (standard)
atomic weight for an element is given in Sect. 1.4.1. For more precise values of atomic
weights see the table of 1997 recommended values [Pure Appl. Chem. 71, 1593-1607
(1999)]. For elements with no stable nuclides the mass of the longest-lived isotope
has been quoted in brackets. The names for elements 110 to 118 are temp orary and
are based on the 1978 recommendations [Pure Appl. Chem. 51, 381-384 (1979)]. El-
ements marked with a ‡ have recently been reported (see, for instance, [Novov et
al. (1999); Oganessian et al. (1999a,b)]).
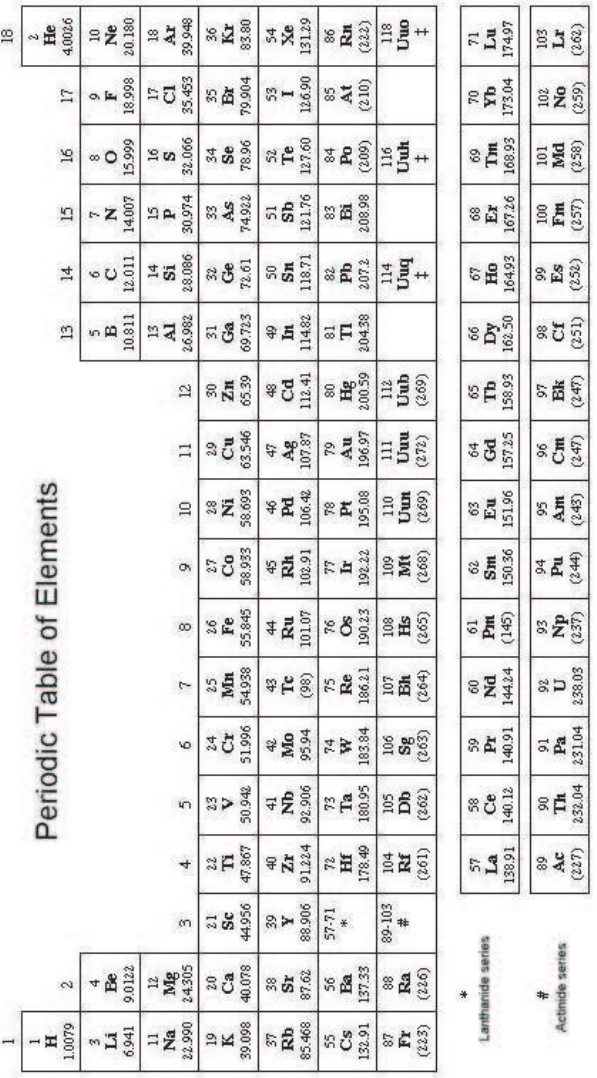
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Periodic Table of Elements 803
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
804 Principles of Radiation Interaction in Matter and Detection
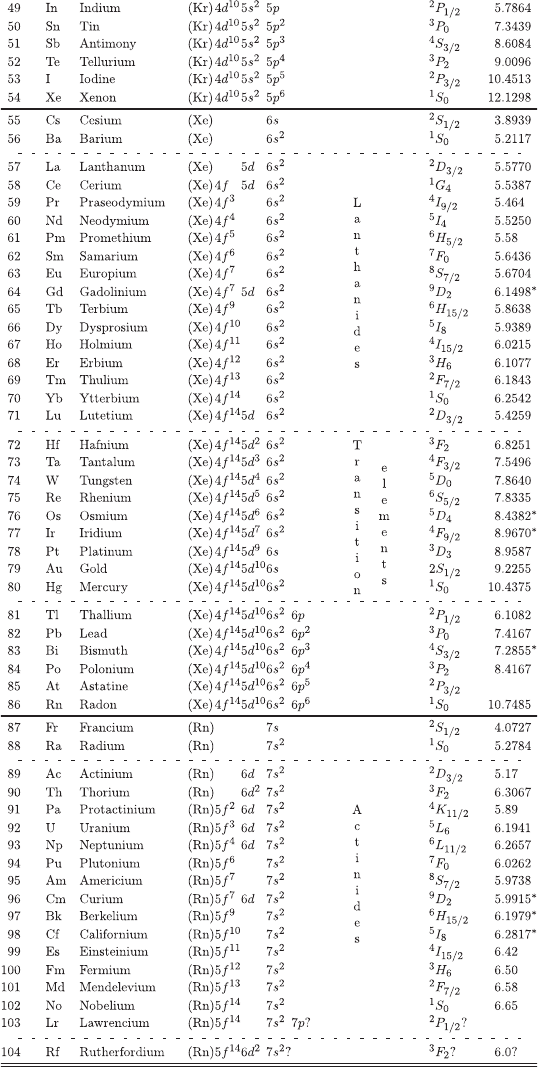
A.4 Electronic Structure of the Elements
For this version of the Electronic Structure of the Elements (reproduced with the
permission from Groom, D.E. et al. (2000), Table 5.1, pages 78-79, Review of
Particle Physics, Particle Data Group, The Eur. Phys. Jou. C 15, 1;
c
° by
SIF, Springer-Verlag 2000), the electronic configuration and ionization energies are
mainly taken (except those marked by *) from [Martin and Lise (1995)]. For in-
stance, the electron configuration for silicon indicates a neon electronic core (see
neon) plus two 3s electrons and two 3p electrons. The ionization energy is the least
necessary energy to remove to infinity one electron from an atomic element.
Electronic configurations of the elements are available on the web [Kotochigova,
Levine, Shirley, Stiles and Clark (1996)]. Furthermore in this Reference, data for
atomic electronic structure calculations have been generated to provide a standard
reference for results of specified accuracy under commonly used approximations. Re-
sults are presented there for total energies and orbital energy eigenvalues for all
atoms from H to U, at microHartree accuracy in the total energy, as computed
in the local-density approximation (LDA), the local-spin-density approximation
(LSD), the relativistic local-density approximation (RLDA), and scalar-relativistic
local-density approximation (ScRLDA).
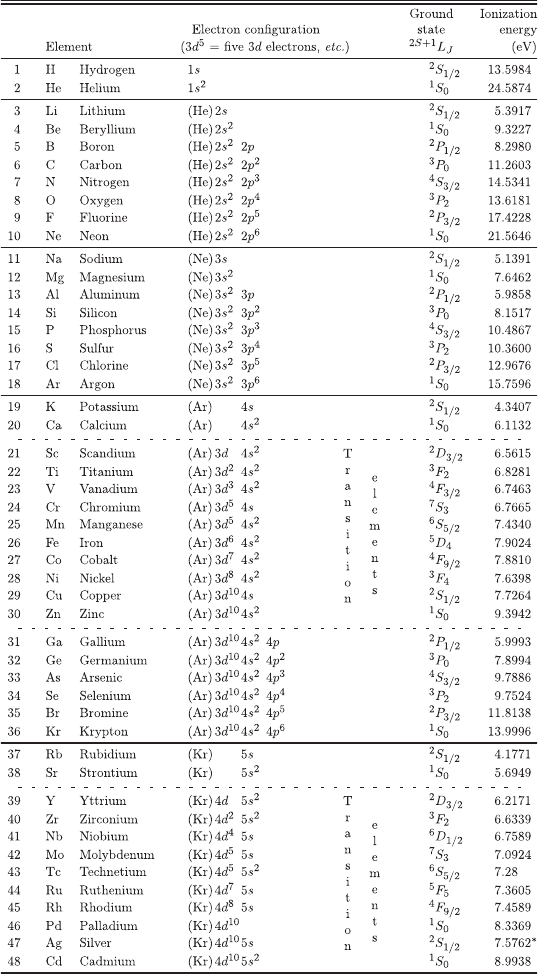
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electronic Structure of the Elements 805
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
806 Principles of Radiation Interaction in Matter and Detection

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Isotopic Abundances 807
A.5 Isotopic Abundances
IUPAC recommended isotopic compositions in percentage: uncertainties are shown
by the last decimals in italic (reprinted from Int. J. of Mass Spectrom., formerly
Int. J. of Mass Spectrom. and Ion Proc. 123, De Bi`evre, P. and Taylor, P.D.P.,
Table of the Isotopic Compositions of the Elements, 149-166, Copyright (1993),
with permission from Elsevier).
Isot. Comp.% Isot. Comp.% Isot. Comp.% Isot. Comp.% Isot. Comp.%
1
H 99.9851
54
Fe 5.81
96
Ru 5.526
136
Ce 0.191
180
W 0.134
2
H 0.0151
56
Fe 91.7230
98
Ru 1.886
138
Ce 0.251
182
W 26.32
57
Fe 2.21
99
Ru 12.71
140
Ce 88.4810
183
W 14.31
3
He 0.0001373
58
Fe 0.281
100
Ru 12.61
142
Ce 11.0810
184
W 30.6715
4
He 99.9998633
101
Ru 17.01
186
W 28.62
55
Mn 100
102
Ru 31.62
138
La 0.09022
6
Li 7.52
104
Ru 18.72
139
La 99.90982
184
Os 0.021
7
Li 92.52
58
Ni 68.0779
186
Os 1.5830
60
Ni 26.2238
102
Pd 1.021
141
Pr 100
187
Os 1.63
9
Be 100
61
Ni 1.1401
104
Pd 11.148
188
Os 13.37
62
Ni 3.6342
105
Pd 22.338
142
Nd 27.1312
189
Os 16.18
10
B 19.92
64
Ni 0.9261
106
Pd 27.333
143
Nd 12.186
190
Os 26.412
11
B 80.12
108
Pd 26.469
144
Nd 23.8012
192
Os 41.08
59
Co 100
110
Pd 11.729
145
Nd 8.306
12
C 98.903
146
Nd 17.199
185
Re 37.402
13
C 1.103
63
Cu 69.173
103
Rh 100
148
Nd 5.763
187
Re 62.602
65
Cu 30.833
150
Nd 5.643
14
N 99.6349
106
Cd 1.254
190
Pt 0.011
15
N 0.3669
64
Zn 48.63
108
Cd 0.892
144
Sm 3.11
192
Pt 0.796
66
Zn 27.92
110
Cd 12.4912
147
Sm 15.02
194
Pt 32.96
16
O 99.76215
67
Zn 4.11
111
Cd 12.808
148
Sm 11.31
195
Pt 33.86
17
O 0.0383
68
Zn 18.84
112
Cd 24.1314
149
Sm 13.81
196
Pt 25.36
18
O 0.20012
70
Zn 0.61
113
Cd 12.228
150
Sm 7.41
198
Pt 7.22
114
Cd 28.7328
152
Sm 26.72
19
F 100
69
Ga 60.1089
116
Cd 7.4912
154
Sm 22.72
191
Ir 37.35
71
Ga 39.8929
193
Ir 62.75
20
Ne 90.483
107
Ag 51.8397
151
Eu 47.815
21
Ne 0.271
70
Ge 21.234
109
Ag 48.1617
153
Eu 52.215
196
Hg 0.151
22
Ne 9.253
72
Ge 27.663
198
Hg 9.978
73
Ge 7.731
112
Sn 0.971
152
Gd 0.201
199
Hg 16.8710
23
Na 100
74
Ge 35.942
114
Sn 0.651
154
Gd 2.183
200
Hg 23.1016
76
Ge 7.442
115
Sn 0.341
155
Gd 14.805
201
Hg 13.188
24
Mg 78.993
116
Sn 14.531
156
Gd 20.474
202
Hg 29.8620
25
Mg 10.001
74
Se 0.892
117
Sn 7.687
157
Gd 15.653
204
Hg 6.874
26
Mg 11.012
76
Se 9.3611
118
Sn 24.2311
158
Gd 24.8412
77
Se 7.636
119
Sn 8.594
160
Gd 21.864
197
Au 100
27
Al 100
78
Se 23.789
120
Sn 32.5910
80
Se 49.6110
122
Sn 4.633
156
Dy 0.061
203
Tl 29.52414
28
Si 92.231
82
Se 8.736
124
Sn 5.795
158
Dy 0.101
205
Tl 70.47614
29
Si 4.671
160
Dy 2.346
30
Si 3.101
75
As 100
113
In 4.32
161
Dy 18.92
204
Pb 1.41
115
In 95.72
162
Dy 25.52
206
Pb 24.11
31
P 100
78
Kr 0.352
163
Dy 24.92
207
Pb 22.11
80
Kr 2.252
120
Te 0.0962
164
Dy 28.22
208
Pb 52.41
continued on next page

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
808 Principles of Radiation Interaction in Matter and Detection
continued from previous page
Isot. Comp.% Isot. Comp.% Isot. Comp.% Isot. Comp.% Isot. Comp.%
32
S 95.029
82
Kr 11.61
122
Te 2.6034
33
S 0.754
83
Kr 11.51
123
Te 0.9082
159
Tb 100
209
Bi 100
34
S 4.218
84
Kr 57.03
124
Te 4.8166
36
S 0.021
86
Kr 17.32
125
Te 7.1396
162
Er 0.141
232
Th 100
126
Te 18.951
164
Er 1.612
35
Cl 75.777
79
Br 50.697
128
Te 31.691
166
Er 33.62
234
U 0.00555
37
Cl 24.237
81
Br 49.317
130
Te 33.801
167
Er 22.9515
235
U 0.720012
168
Er 26.82
238
U 99.274560
170
Er 14.92
36
Ar 0.3373
84
Sr 0.561
121
Sb 57.368
38
Ar 0.0631
86
Sr 9.861
123
Sb 42.648
40
Ar 99.6003
87
Sr 7.001
165
Ho 100
88
Sr 82.581
124
Xe 0.101
39
K 93.258144
126
Xe 0.091
168
Yb 0.131
40
K 0.01171
85
Rb 72.16520
128
Xe 1.913
170
Yb 3.056
41
K 6.730244
87
Rb 27.83520
129
Xe 26.46
171
Yb 14.32
130
Xe 4.11
172
Yb 21.93
40
Ca 96.94118
89
Y 100
131
Xe 21.24
173
Yb 16.1221
42
Ca 0.6479
132
Xe 26.95
174
Yb 31.84
43
Ca 0.1356
90
Zr 51.453
134
Xe 10.42
176
Yb 12.72
44
Ca 2.08612
91
Zr 11.224
136
Xe 8.91
46
Ca 0.0043
92
Zr 17.152
169
Tm 100
48
Ca 0.1874
94
Zr 17.384
127
I 100
96
Zr 2.802
174
Hf 0.1623
45
Sc 100
130
Ba 0.1062
176
Hf 5.2065
92
Mo 14.844
132
Ba 0.1012
177
Hf 18.6064
46
Ti 8.01
94
Mo 9.253
134
Ba 2.41727
178
Hf 27.2974
47
Ti 7.31
95
Mo 15.925
135
Ba 6.59218
179
Hf 13.6296
48
Ti 73.81
96
Mo 16.685
136
Ba 7.85436
180
Hf 35.1007
49
Ti 5.51
97
Mo 9.553
137
Ba 11.234
50
Ti 5.41
98
Mo 24.137
138
Ba 71.707
175
Lu 97.412
100
Mo 9.633
176
Lu 2.592
50
V 0.2502
133
Cs 100
51
V 99.7502
93
Nb 100
180
Ta 0.0122
181
Ta 99.9882
50
Cr 4.34513
52
Cr 83.78918
53
Cr 9.50117
54
Cr 2.3657
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Commonly Used Radioactive Sources 809
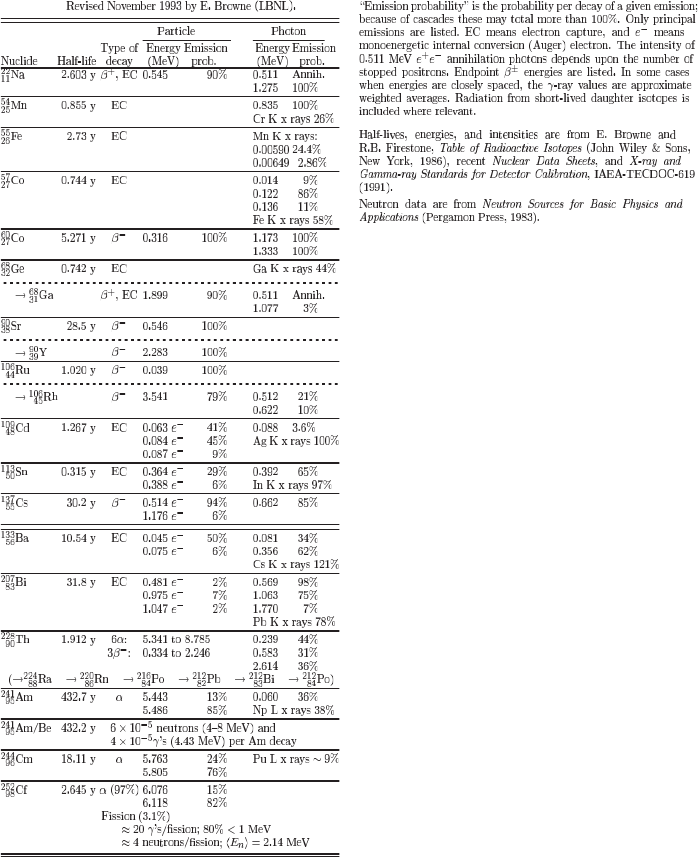
A.6 Commonly Used Radioactive Sources
In this table (reproduced with the permission from Groom, D.E. et al. (2000), Table
26.1, page 190, Review of Particle Physics, Particle Data Group, The Eur. Phys.
Jou. C 15, 1;
c
° by SIF, Springer-Verlag 2000), half-lives and energy (or end-point
energy) emissions are shown for some commonly used radioactive β
+
, β
−
, α and
γ sources.
