Lax Alistair J. Bacterial protein toxins: Role in the interference with cell growth regulation (Бактериальные токсины белков: роль в регуляции роста клеток)
Подождите немного. Документ загружается.


P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
86
garret ihler, anita verma, and javier arevalo
importance. For example, Neisseria, which has similarities to Bartonella, can
utilize PorB to modulate apoptosis, because PorB is efficiently targeted to
mitochondria (Muller et al., 2002), where it can depolarize the mitochondrion.
Like Neisseria, Bartonella produces membranous blebs containing some but
not all of the outer membrane proteins (J. Arevalo and G. Ihler, unpublished).
These blebs have considerable pathogenic potential, since they can distribute
the outer membrane proteins widely and independently of viable bacteria.
Not only could these serve as decoys or as sinks for host antibacterial pro-
teins, but also if the proteins themselves have toxic properties, the blebs could
deliver them to host cells as efficiently as the live bacteria. Neisserial porins
can stimulate B cells and upregulate surface expression of host proteins via
an NF-κB–dependent mechanism (Massari et al., 2002).
Why Are Erythrocytes and Endothelial Cells the Two Primary
Targets for B. bacilliformis?
Bartonella are the only bacteria known to invade human erythrocytes. In-
fection by B. bacilliformis first involves colonization of the erythrocytes
(Figure 5.1), massive destruction of erythrocytes by spleen and liver, and
a resulting severe anemia, which is often fatal in the absence of antibiotic
treatment (Oroya fever). Invasion of endothelial cells and subsequent angio-
genesis results in the formation of verrugas. Verruga peruana has low or zero
mortality but involves formation of the numerous disfiguring skin lesions.
The net result is a two-phase disease (Carrion’s disease), although in some
cases the erythrocytic phase is inapparent.
It seems likely that there must be some underlying connection between
these two phases of the disease and the two cell types. But it does not seem
likely that B. bacilliformis uses the same mechanism of invasion for ery-
throcytes and nucleated cells because mature erythrocytes do not engage
in endocytosis or vesicle transport. Both infection and retransmission must
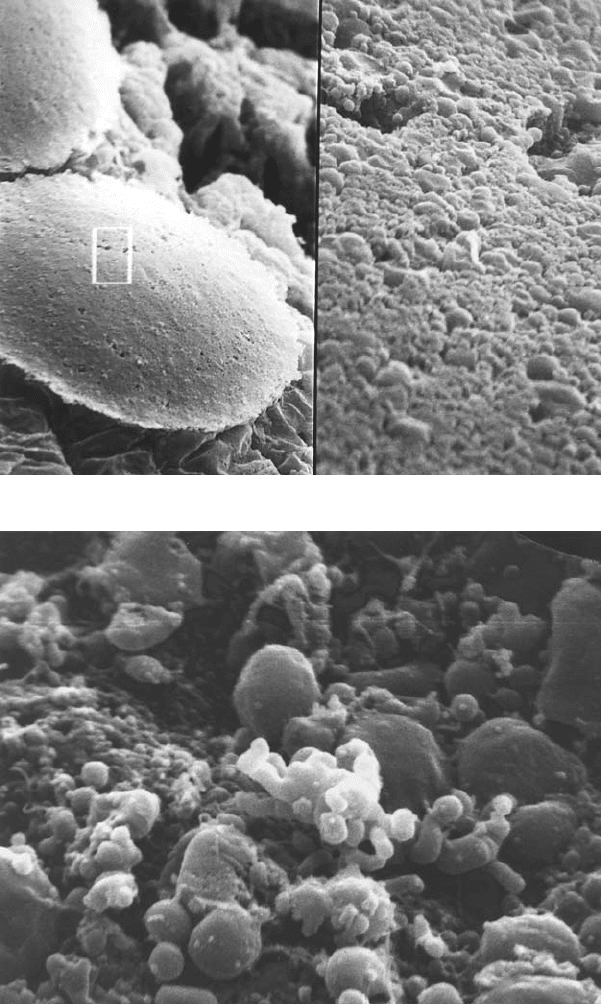
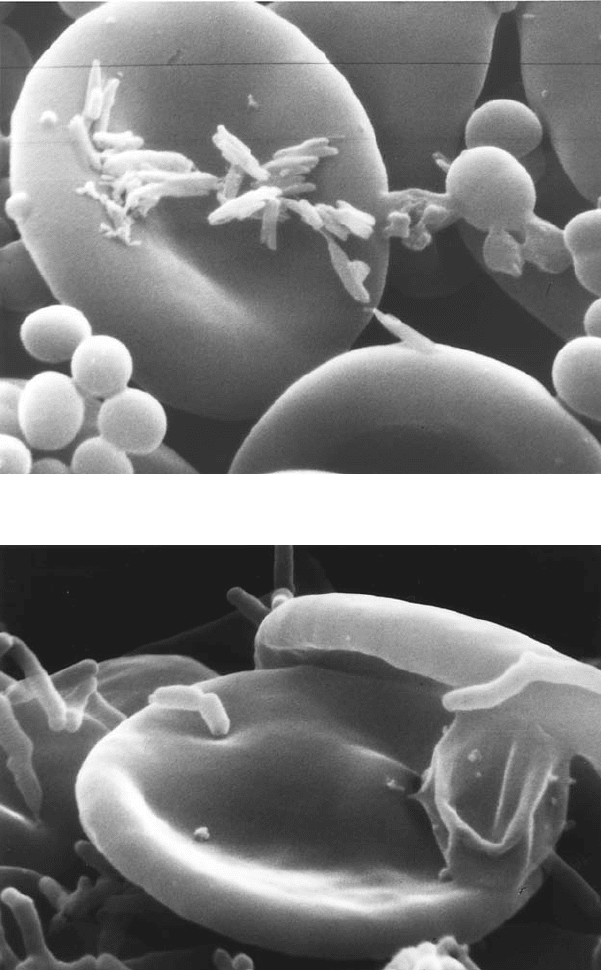
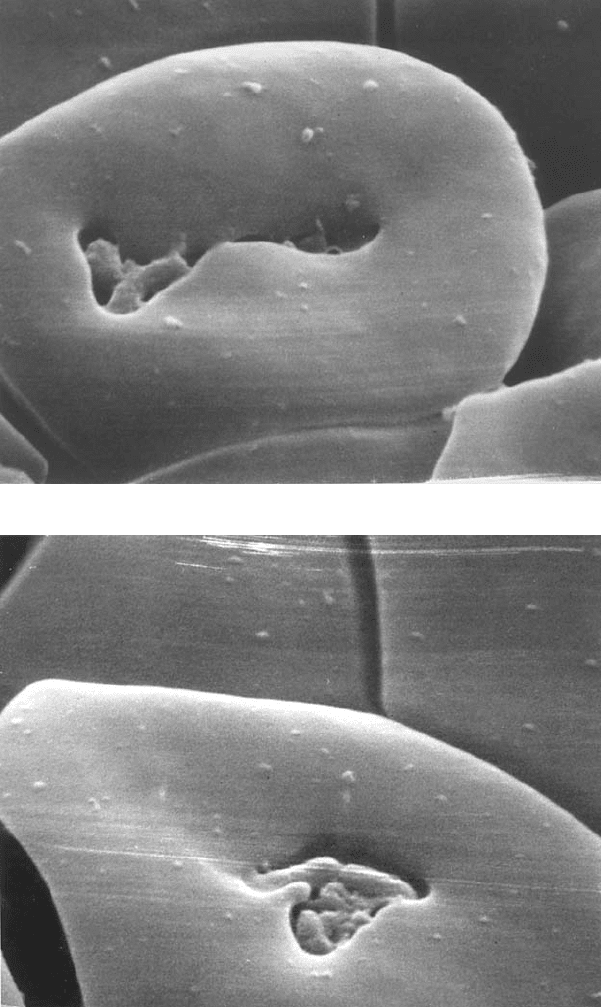
Figure 5.1 (Bacterial morphology and interaction with erythrocytes A. Colony morphology
at 14 days. Left: 440× Right: 4400×.B.Colony face split open. 9600× (A,B: G.
McLaughlin and G. Ihler, unpublished). C. Bacterial clump adherent to a red cell.
10,100×.D.Adherent bacteria with small indentation. Individual bacteria are often seen
in deep invaginations of the erythrocyte membrane (Benson et al., 1986). E–F. Bacteria
associated with trenches and pits. The lips of the trench sometimes seem to fold over, as if
engulfing the bacteria. Possibly this could occur if the clump binds to two or more sites on
the erythrocyte surface as in Figure 1C and then contracts to pull the erythrocyte
membrane over the clump.
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
87
bartonell a
and endothelial cells
Figure 5.1A
Figure 5.1B (Continued )
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
88
garret ihler, anita verma, and javier arevalo
Figure 5.1C
Figure 5.1D
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
89
bartonell a
and endothelial cells
Figure 5.1E
Figure 5.1F
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
90
garret ihler, anita verma, and javier arevalo
involve the blood, so it is easy to see why attachment to erythrocytes might
be advantageous. The intense monitoring of erythrocytes in humans by
the spleen might be part of the reason why a tissue sanctuary is a feature of the
disease, and the long life span of the verruga (more than a year) relative to
the maximum life span of an erythrocyte (120 days) could be important as
well. B. henselae manages to cause formation in humans of lesions similar to
verrugas without a prominent erythrocytic phase, but there is no evidence that
B. henselae can be re-transmitted to an insect vector from humans (although
patients are certainly bacteremic). Bartonella tribocorum,arat pathogen, in-
vades and replicates in erythrocytes, but the erythrocytes are not markedly
cleared (Schulein et al., 2001).
Although B. bacilliformis can be internalized into erythrocytes and cir-
culate protected within the erythrocytes, it does not accomplish this in a
stealthy manner. Instead, clumps of bacteria adhere to the external surface of
the erythrocytes, where they are exposed to antibodies and the immune sys-
tem. The bacteria induce major deformations in the erythrocyte membrane,
including pits and trenches (Benson et al., 1986; Derrick and Ihler, 2001).
These changes cause erythrocytes to be recognized as defective, especially
in the splenic circulation, but also by liver macrophages if the damage is
less subtle. It is this massive colonization of erythrocytes and the deforma-
tion of the cells that results in the profound anemia that is seen in Oroya
fever because erythrocytes deformed by B. bacilliformis infection are readily
phagocytosed.
Phagocytosis of erythrocytes by macrophages might suggest that phos-
phatidylserine – a signal for phagocytosis not only in erythrocytes, but in
most cells – is exposed on the outer face of the erythrocyte membrane. In
normal erythrocytes, phosphatidylserine is absent from the external face be-
cause it is transported by an energy-dependent enzyme system from the outer
to the inner leaflet of the membrane bilayer. Exposure of phosphatidylser-
ine endows erythrocytes with the propensity of adhering to HUVECs (Closse
et al., 1999)aswell as to phagocytic cells, although normal erythrocytes are
generally non-adhesive to endothelial surfaces.
Endothelial cells can be readily infected in vitro by free bacteria, and pre-
sumably also within the verruga. But the initial colonization at the site of
a future verruga might be facilitated if the bacteria are bound to and circu-
lating with erythrocytes, either because the erythrocyte is phagocytosed or
because the bacteria are brought into close proximity to the endothelial cell.
An erythrocytic route is suggested by the fact that erythrocyte colonization
constitutes the first phase of the disease. One might speculate that the pres-
ence of bacteria on the surface of erythrocytes could facilitate invasion of
endothelial cells as a second protected site because erythrocytes fill small
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
91
bartonell a
and endothelial cells
capillaries and are deformed by their passage. Although endothelial cells are
sometimes described as wrapping around or lining capillaries, in fact the
capillary has no physical existence except as a hole through endothelial cells.
Thus the bacteria would “scrape” along the endothelial surface and could
perhaps attach more easily to the endothelial cells after being delivered by
carrier erythrocytes.
Transfer of bacteria to endothelial cells could be additionally facilitated if
the erythrocytes or the bacteria that were bound to the erythrocytes adhered
to the capillary endothelium; reverse transfer of bacteria from the verrugas to
erythrocytes might also be possible if erythrocytes became arrested in capil-
laries or post-capillary venules. In sickle-cell anemia, adhesion pathways have
been identified as contributing to the sickle-cell crisis, in addition to, or as a
consequence of, erythrocyte deformation caused by HbS polymerization. It
is noteworthy that the sickle-cell crisis is very often associated with infection.
One possibility might be that infection systemically upregulates adhesive pro-
teins on endothelial cells, which become more effective in arresting abnormal
sickle-cell erythrocytes.
The importance of cell adhesion molecules in leukocyte trafficking and
in mediating immune and inflammatory responses ensures that cell–cell
recognition proteins would certainly be upregulated in verrugas, as in other
infections, but it is not known whether these adhesive proteins would help
to bind erythrocytes. B. henselae outer membrane proteins (and perhaps LPS)
are known to upregulate E-selectin and ICAM-1 on HUVECs (Fuhrmann
et al., 2001; Maeno et al., 2002). For ICAM-1, infection is not required, since
the nonpiliated strain, unable to invade endothelial cells, induced ICAM-1 as
well as the piliated strain, and since the bacteria could be inactivated in various
ways (including being sonicated) without loss of activity. The unidentified
factor was stable to boiling and unaffected by the addition of polymyxin B,
suggesting that it was not LPS.
B. bacilliformis and B. henselae Infect Endothelial Cells
Invasion of endothelial cells by B. bacilliformis is considered to be a crucial
and important step in establishing the proliferative lesions of the verruga.
Invasion of endothelial cells by different species of Bartonella has been studied
primarily in vitro using human umbilical vein endothelial cells (HUVECs).
B. bacilliformis infections in vivo are characterized by bacteria within and
outside endothelial cells. Bartonella is tropic for endothelial cells, and has
special growth effects on them. The significance of this tropism is somewhat
confused by the fact that Bartonella can infect many cell types in vitro,in
addition to endothelial cells, although growth of these cells is not stimulated.
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
92
garret ihler, anita verma, and javier arevalo
Thus, if a specialized mechanism of Bartonella entry into endothelial cells
exists, Bartonella must possess some additional, more general mechanism
for gaining access to different types of cells. Despite this caveat, it nevertheless
does appear that Bartonella has a special tropism for endothelial cells, and a
special mechanism of entry.
In vitro, intracellular B. bacilliformis were observed electron microscopi-
cally in small membrane-bound inclusions within endothelial cells after 1 h of
infection and by 12 h a large membrane-bound inclusion containing numer-
ous bacteria was present. This was described as being similar to the Rocha-
Lima inclusion, which is a prominent feature of in vivo infections (Garcia et al.,
1992). Cytochalasin-D reduced the invasiveness of B. bacilliformis into endo-
thelial cells in vitro (Hill et al., 1992), indicating host participation.
Activation of Rho-family Small GTPases
Verma et al. (2000, 2001) showed that a significant entry of B. bacilliformis
occurs within 2 hrs, and also that there is also a very large increase in entry
between 16 and 24 h. A massive reorganization of the actin network leading
to the formation of thick actin bundles, known as stress fibers, was visible in
the infected endothelial cells by 12–16 h. It has been shown that a member
of the small GTPase family of proteins, Rho, is a key signaling molecule for
mediating the formation of stress fibers. Members of the Rho family of small
GTP-binding proteins (Rho, Rac, and Cdc42) function as molecular switches
by cycling between an inactive state with bound GDP and an active state
with bound GTP (see Chapter 3 for more details). The active form of Rho
interacts with downstream effector proteins to produce biological responses,
which include actin reorganization. Rho is also necessary for internaliza-
tion of B. bacilliformis. Entry at both early and late times can be prevented
by pretreatment of the endothelial cells with C3 exoenzyme, a protein toxin
from Clostridium botulinum which inactivates intracellular Rho by ADP ri-
bosylation. Moreover, Rho was shown to be directly activated in the infected
HUVECs, with a similar time course to that observed for bacterial invasion. In
a later study, Verma and Ihler (2002) also showed that pretreatment with the
Clostridial toxin TcsL-1522 – which specifically inactivates another member of
the small GTP-binding protein family, Rac and, to a lesser extent, Cdc42, but
not Rho – also inhibits entry. All three Rho family proteins, Rac, Cdc42, and
Rho, are activated after incubation of endothelial cells with B. bacilliformis.
Within 30 min, levels of activated Rac and activated Cdc42 were increased.
Relocalization of other Rho family proteins also occurs. Within 30 min,
filopodia (filamentous actin extensions) formed; within 1 h lamellipodia
(membrane rufflings) formed. Clumps of bacteria could be seen adhering
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
93
bartonell a
and endothelial cells
to or in the close vicinity of both the filopodia and lamellipodia. F-actin was
associated with both filopodia and lamellipodia, and Rac was shown to be
associated with the lamellipodia (Verma and Ihler, 2002). Activation of Rac is
known to induce formation of lamellipodia and activation of Cdc42 induces
formation of filopodia. Activated Rho is translocated from a cytoplasmic to a
membrane-associated location. Rho induces formation of actin-lined invagi-
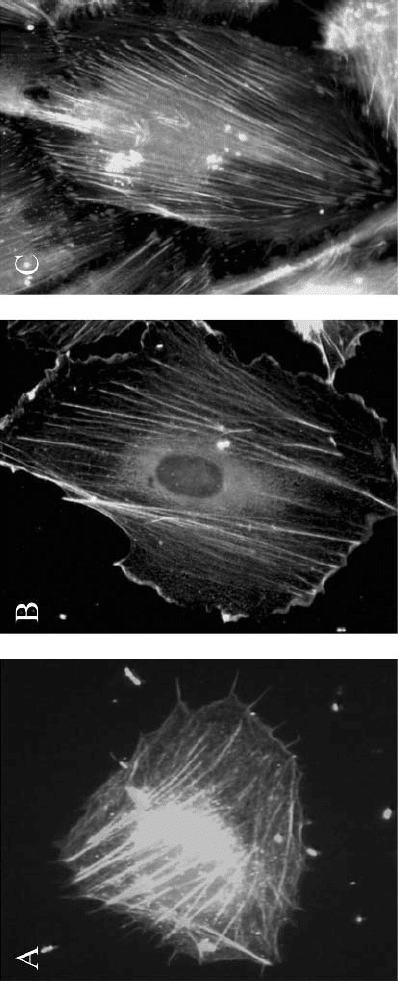
nations in the plasma membrane. Figure 5.2 shows microspikes (Cdc42),
membrane ruffling (Rac), and stress fibers (Rho) related to the activation of
Rho-GTPases.
These results indicate that Bartonella, like other pathogenic bacteria, es-
pecially the intracellular pathogens, utilize host signaling and response mech-
anisms as indispensable components of the infectious process. Many other
bacteria also facilitate their entry into the host cells by inducing a rearrange-
ment of the actin cytoskeletal network (see also Chapter 6). Shigella entry is
dependent on Cdc42, Rac, and Rho (Adam et al., 1996; Dumenil et al., 2000).
Salmonella also uses Rac and Cdc42 in the entry process. Salmonella encodes
a protein, the product of sopE, which binds to and activates Cdc42 and Rac by
promoting exchange of GTP for GDP, acting as nucleotide exchange factor. A
Salmonella typhimurium strain carrying a null mutation in sopE was deficient
in its ability to enter cells after short infection times. A dominant negative
mutant of Cdc42 prevents entry of S. typhimurium (Chen et al., 1996), but
neither dominant negative Rac nor inhibition of Rho with C3 exoenzyme pre-
vented membrane ruffling (Jones et al., 1993). For Salmonella, Cdc42 would
seem to be the more important target.
Presumably activation of Rho family proteins is directly or indirectly ac-
complished by a Bartonella toxin or toxins. If it acts directly on Rho family
GTPases, this hypothetical toxin could be analogous to toxins (CNF1, DNT)
known in E. coli, Yersinia, and other bacteria that activate Rho proteins (for a
review, see Lerm et al., 2000; Chapter 3). CNF1 readily enters cultured cells,
probably by endocytosis, and is activated in the cytosol, after which it acti-
vates Rho by deamidation of glutamine 63 and has physiological effects on
endothelial cells that are similar to those seen after B. bacilliformis infection
(Vouret-Craviari et al., 1999). Epithelial cells activated with CNF1 demonstrate
membrane ruffling and membrane protrusions that lead to entrapment of
bacteria (or even latex particles (Falzano et al., 1993)) in large endocytic vesi-
cles (macropinosomes). These similarities, however, arise from the fact that
Rho is activated in Bartonella invasion and as a consequence activates down-
stream effector proteins, and do not necessarily imply that Bartonella has
a toxin similar to CNF1. Indeed, other toxins, for example the Pasteurella
multocida toxin, activate Rho indirectly by affecting upstream signaling (see
Chapter 2), and Bartonella may operate like this.
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
94
garret ihler, anita verma, and javier arevalo
Figure 5.2.
B. bacilliformis
infected endothelial cells showing the structures associated
with the activation of Rho-GTPases. A. Infected endothelial
cell stained with F-actin (green) and anti-Cdc42 antibodies
(red) showing microspikes related to activation of Cdc42
after 30 min of infection.
B. Infected endothelial cell stained with F-actin (green) and anti-Rac
antibodies (red) showing membrane ruffling and colocalization
of F-actin and
Rac in the membrane ruffles (yellow) related to activation of Rac
after 1 h of infection. C. Infected endothelial cell stained with
F-actin (green) and
anti-paxillin antibodies (red) showing formation of thick
stress fibers terminating in paxillin-rich focal adhesions (red)
after 24 hrs of infection with
the activation of Rho. (See
color section.)
P1: IwX
052182091Xc05.xml CB786/Lax 0 521 82091 X November 4, 2005 1:37
95
bartonell a
and endothelial cells
Both Bartonella and the closely related Brucella carry virB genes, coding
for Type IV protein translocation systems, which are closely related to those of
Agrobacter (Schmiederer and Anderson, 2000, Sieira et al., 2000). Inactivation
of Rho, Rac, and Cdc42 with Clostridial toxins reduces uptake of B. abortus
by HeLa cells, whereas CNF1 increases internalization. Dominant negative
Rho, Rac, and Cdc42 inhibit uptake; the dominant positive forms promote
uptake (Guzman-Verri et al., 2001).
Two Mechanisms of Entry – Conventional Endosomes
and Macropinocytosis?
Caron and Hall (1998) identified two distinct pathways of macrophage phago-
cytosis, an immunoglobin receptor and Cdc42/Rac-dependent pathway, and
a complement receptor and Rho-dependent pathway. The existence of sepa-
rate Rho-family GTPase-dependent pathways is consistent with the possibility
that B. bacilliformis might activate multiple pathways for internalization be-
cause B. bacilliformis activates all three Rho-GTPases. Alternatively Bartonella
could cause the formation of a unique endocytic apparatus not directly used
by the host cell, as suggested for Shigella (Nobes and Hall, 1995).
B. henselae has been shown to enter endothelial cells either as individual
or as a few bacteria enclosed within vacuoles, or alternately in large clumps
(Dehio et al., 1997; Kempf et al., 2000). Up to 24 h is required to complete
internalization of the clumps, but significant numbers of individual bacteria
were found to have entered within 2 h. Entry of individual B. henselae is not
inhibited by Cytochalasin D (Kempf et al., 2001), but internalization of large
clumps of bacteria by endothelial cells is prevented. These observations seem
to indicate that two mechanisms of entry must be considered, entry of indi-
vidual bacteria in small endosomes and entry of large clumps of bacteria by
macropinocytosis. The latter mechanism might be specialized to endothelial
cells.
Entry of Clumps by Macropinocytosis
A fascinating descriptive study of a unique sequence of events in the inva-
sion process of endothelial cells by large clumps of B. henselae was reported
by Dehio et al. (1997). First the leading lamella of migrating endothelial
cells established contact with the bacteria and moved the bacterial aggre-
gate on the cell surface by retrograde transport. Subsequently, the bacterial
aggregate consisting of hundreds of bacteria was engulfed and eventually
internalized in a well-defined host cell structure, called the invasome. The
