Kutz M. Handbook of materials selection
Подождите немного. Документ загружается.


2 ORTHOPEDIC BIOMATERIALS: TOTAL HIP ARTHROPLASTY 1179
Table 4 Summary of Mechanical Properties for Materials Commonly Used in Current
Total Joint Replacement Designs
a
Property HMWPE Titanium Alloy Co–Cr Alloy Alumina Cortical Bone
Elastic Modulus
[GPa]
2.2 110 220–234 350–400 10– 20
Comp. Strength
[MPa]
4000 130–280
Tensile Strength
[MPa]
3 860 600–1000 270 80–160
Endurance Limit
[MPa] at 10
7
cycles
620 500
Density [g/ cm
3
] 0.93–0.94 4.5 9.2 3.9 1.8
Hardness [MPa] 3500 3000–4000 20,000
a
Titanium alloy, Ti6Al4V; Co–Cr alloy, wrought CoNiCrMo.
Source: Data from ASTM-F136 (1998), ASTM-F562 (2000), ASTM-F648 (2000), Bernache-
Assolant (1991), Brunski (1996), Cowin (1989), and Park and Lakes (1992)
mechanical properties of these common materials and lists cortical bone for
comparison purposes.
In the selection of metals, cobalt–chromium alloys with molybdenum have
been preferentially chosen over those with nickel. Despite the fact that the me-
chanical properties are slightly reduced in the molybdenum alloy compared to
the nickel variant, it has been shown to have improved wear properties. Titanium
is often chosen due to the fact that its elastic modulus is half of that seen in
cobalt–chromium or stainless steel alloys, therefore reducing the stiffness of the
implant and the accompanying bone loss due to stress shielding.
During the past decade, substantial research has been conducted to develop
different pairs of materials for the bearing surface in order to reduce the wear
phenomenon commonly seen in HMWPE, which often leads to premature failure
of an implant. Metal-on-metal and ceramic-on-ceramic head–cup combinations
have shown promise in terms of long-term outcomes, especially when used in
younger patients for whom an implant life of greater than 20 years would be
desirable (Delaunay, 2000; Skinner, 1999; Wagner and Wagner, 2000). The ce-
ramics typically selected have been alumina and zirconia, while the metal is
generally a cobalt–chromium alloy. Titanium does not lend itself to metal–metal
bearing surfaces due to the fact that it tends to seize when in contact with other
metals. The success of these material combinations is due in large part to the
improvements in materials processing, machining, and polishing that have been
achieved in the last quarter century, allowing for the development of high-quality
materials, excellent geometric matches, and highly polished surfaces. This pro-
gress also indicates that designs that were tried out at an earlier point in history,
and were perhaps abandoned, may deserve to be reexamined. Metal-on-metal
bearings were originally used in total hip replacements in the 1950s, with limited
success. Complete ceramic bearings were seen as early as the 1970s, but fracture
of the ceramic components and high wear were attributed to problems in proc-
essing that resulted in defects and inadequate grain sizes (Plenk, et al., 1992;
Sedel, et al., 1991). In time, they were almost completely replaced in the clinical
world by metal–polymer combinations. However, their revival in the past few

1180 SELECTION OF MATERIALS FOR BIOMEDICAL APPLICATIONS
Fig. 6 Schematic diagram of an artery showing the three tissue layers:
adventitia (outer), media (middle), and intima (inner).
years, spurred on by developments in materials engineering, indicates that the
original developers had the right idea—they were just a few years ahead of their
time.
3 BLOOD-CONTACTING BIOMATERIALS: VASCULAR PROSTHESES
When blood vessels are damaged through injury or disease, they often must be
replaced or bypassed in order to maintain adequate blood flow to and from the
regions of the body. Disease-induced damage, such as atherosclerosis and an-
eurisms, occurs more often in arteries than in veins, due in large part to the
higher working pressure of the blood within these vessels. Injury can occur to
any blood vessel; however, collateral circulation typically eliminates the need to
replace small veins, and the low venous return pressure provides an environment
in the larger veins that is much more conducive to traditional surgical repair or
autograft use. As a result, this section will focus on the selection of materials
for the development of arterial prostheses.
Arteries are three-layer hollow tubes (Fig. 6) composed of a combination of
elastin, collagen, and smooth muscle—with the proportions of each component
dependent on the size of the artery. The size of an artery also varies substantially
along the arterial tree, from a typical diameter of about 4 cm for the aorta in an
adult to a diameter of less than 1 mm for arterioles. The age and size of the
individual will also affect the dimensions of the arteries. Normal arterial pressure
is approximately 120 mmHg during the systolic phase (contraction) of the car-
diac cycle, decreasing to approximately 70 mmHg during the diastolic phase
(relaxation). However, pressures can rise as high as 200 mmHg or more in
individuals with either transient or chronic hypertension, and hypertension itself
is a risk factor for a number of the pathological processes that may require
arterial replacement or bypass.
Elastic arteries, such as the aorta, are expected to expand during systole as
blood is forced into them by the heart. The elastic recoil of these vessels, due
in large part to the high amount of elastin in the wall, acts to maintain arterial
pressure during the relaxation phase of the cardiac cycle, further transporting
blood away from the heart. Distributing arteries, which (as their name indicates)
distribute blood from the larger, elastic arteries to the various regions of the
body, have more collagen than elastin in their walls. However, the physiologi-
cally important component of these vessel walls is the smooth muscle, which

3 BLOOD-CONTACTING BIOMATERIALS: VASCULAR PROSTHESES 1181
allows the distributing arteries to adjust their diameter and therefore their resis-
tance. By controlling the arterial resistance to blood flow in various regions of
the body through vasodilation and constriction, blood can be directed to regions
that have the highest metabolic demand—the muscles during exercise or the
gastrointestinal system during digestion.
Vascular grafts were first introduced in the early twentieth century as solid
tubular structures. Fabric grafts were developed in the 1950s and provide the
basis for the grafts that are currently in use or under development. An arterial
graft or arterial prosthesis has one obvious function—to transport blood from
vessels that are proximal to the graft to those that are distal to the graft. In
addition, the vasodilation/constriction and elastic recoil capabilities of the var-
ious types of arteries assist the heart with transport and direction of blood to the
diverse tissues of the body. However, there are additional functional constraints
that are equally as important. First, the prosthesis must maintain hemostasis—
not allow blood to leak from the artery into the surrounding tissues. Second,
interaction of the blood cells with the vessel must not act to initiate coagulation,
which could result in thrombus formation and embolisms, nor hemolysis, the
destruction of red blood cells. These functional constraints will be considered
in the discussion on biocompatibility.
3.1 Function
Blood Transport
To transport blood between two connecting vascular segments, a tubular structure
is required. However, given modern material processing techniques, this require-
ment does not itself limit the selection of materials substantially. The graft must
also, however, be connected to the ends of the remaining vascular segments in
some way, either through ligatures or sutures.
Using a maximum arterial pressure of 200 mmHg (30 kPa), the circumfer-
ential and axial wall stresses within a graft (diameter of 1 cm, thickness of 0.5
mm) can be approximated to reach 270 and 135 kPa, respectively. This is well
within the mechanical limits of most undegraded artificial materials; however, it
may become a substantial constraint for vessels engineered from natural mate-
rials in the future. In addition, the physiological environment, combined with
the cyclic loading seen by the vessel due to the normal vascular pressure vari-
ations, will affect the properties of the graft material. As discussed above for
hip replacement, corrosion, absorption, and leaching processes that occur when
a material is placed in vivo will all negatively affect the ultimate strength of a
material.
The expansion and recoil observed in elastic arteries must be taken into con-
sideration when selecting a material for grafts at these locations. It is reasonable
to assume that a relatively short length of the graft does not itself need to
dynamically change dimensions to maintain blood flow through the segment of
vasculature. However, any mismatch in the behavior of the graft to the connected
vessels will result in substantial stresses on the ligatures, sutures, or the vessel
itself. In certain applications, the graft may replace a substantial portion of an
artery, such as in the descending aorta. In this case, elastic recoil in the graft
will play an important role in maintaining the velocity of blood flow through
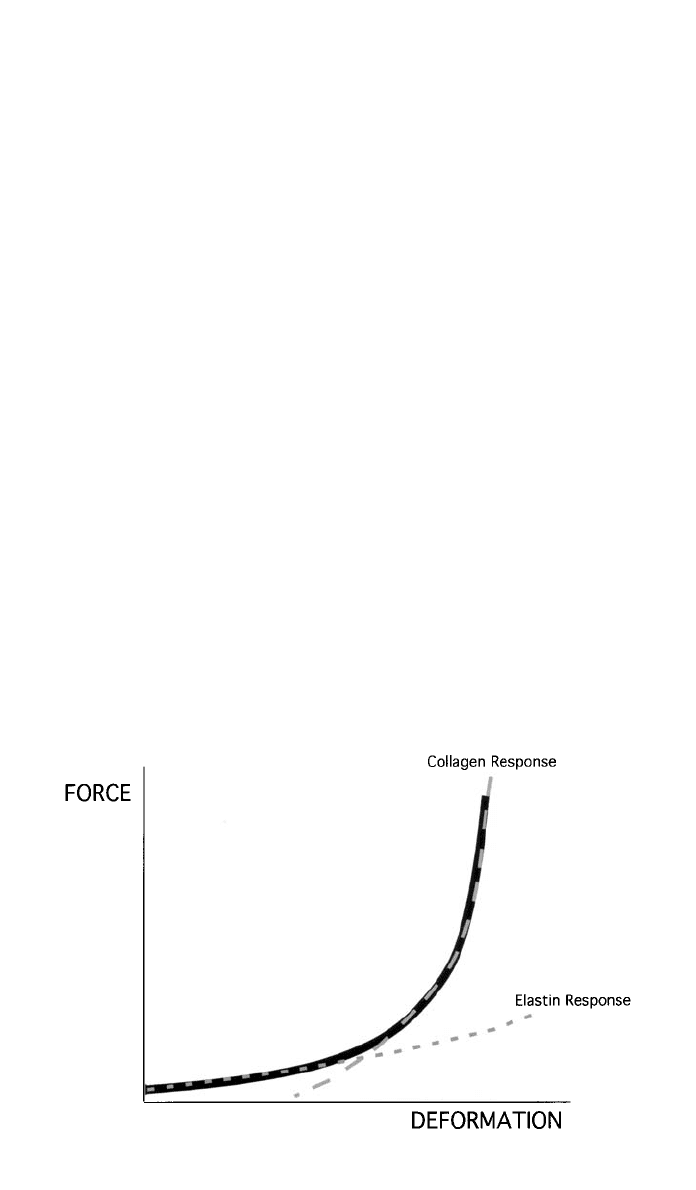
1182 SELECTION OF MATERIALS FOR BIOMEDICAL APPLICATIONS
Fig. 7 Schematic diagram showing the characteristic nonlinear force–deformation relationship
of an artery. Initial region of high compliance is characteristic of the elastin component in the
walls, while the later, low-compliance region exemplifies the deformation of the collagen fibers.
the distal vessels, and the pulsatile flow may be important to maintaining func-
tion in organs supplied by the arterial tree (Mergerman and Abbott, 1983). The
same philosophy would apply to distributing arteries. At the current time, no
artificial material exists that would respond to the physiologic control mecha-
nisms in order to dilate and constrict in conjunction with surrounding vessels.
However, a graft that is less compliant than the attached vessel would result in
substantial stress concentrations at the junction between the artificial and natural
materials. This elevation in stress could lead to failure of the sutures or ligatures
connecting the structure, as well as accelerate fatigue processes within the graft
ends (Wilkerson and Zalina, 1994). In addition, a less compliant graft can also
result in a stenosis in the vascular tree during systole, when the proximal seg-
ment of artery expands under pressure, but the blood then encounters the length
of reduced diameter graft that acts to retard flow (Herring, 1983). These issues,
along with the fact that blood vessels experience significant deformation due to
general body motion, essentially dictate that a soft, flexible material be used for
the graft.
An ideal graft would exactly match the compliance of the attached blood
vessel, allowing it to expand and recoil to the same extent as the natural struc-
ture. This would require a nonlinear response, with an initial region of highly
compliant stretch, attributed to the unkinking and realigning of the collagen
fibres and a simultaneous stretching of the elastin. After the collagen in the
vessel wall is straightened, its higher elastic modulus dominates the behavior of
the vessel, and the compliance is significantly reduced. It is this behavior (Fig.
7) that is thought to limit the overexpansion of arteries during acute episodes of
elevated blood pressure. The compliance of each segment of artery therefore
depends on the proportion of collagen, elastin, and smooth muscle (which has
a negligible effect on compliance in its passive state) within the vessel wall.

3 BLOOD-CONTACTING BIOMATERIALS: VASCULAR PROSTHESES 1183
Typical values for compliance (in units of percent radial change per millimeter
of mercury) for cadaveric human arteries range between 10.9 at 60 mmHg and
3.8 at 150 mmHg (Mergerman and Abbott, 1983).
3.2 Biocompatibility
The primary functional need of a blood vessel—transport of blood—can easily
be met through general implant design. However, due to the delicate nature of
blood cells and the ease at which the clotting cascade can be initiated, biocom-
patibility issues place substantial limitations on material selection for this ap-
plication. The natural vessel provides an optimal environment for blood flow,
and the mimicking or replacing of its intimal layer is one of the underlying ideas
in work to improve biocompatibility in vascular grafts.
Neointima Formation
Natural blood vessels consist of three layers: the outer adventicia, the middle
media, and the inner intima. The intima has a layer of endothelial cells that
contact the moving blood in order to minimize damage to the cellular elements
of the fluid. Some artificial materials that are implanted into the body as vascular
grafts have been shown to develop a neointima—fibrin and fibroblasts that de-
velop as a lining on the inside surface of the tube (Herring, 1983).
The rate of neointima formation and its constituents may depend on the ma-
terial used in the vascular graft. A carbon–ceramic composite with a large degree
of surface porosity showed almost instantaneous (
⬍10 s) development of a fibrin
mesh with limited platelet adhesion (Chignier et al., 1987). This developed into
a 5- to 7-cell thick layer of fibroblasts, collagen, and elastin by 15 days, with
endothelial-like cells present and fully developed by 2 months. Herring (1983)
provides a good description of the ‘‘healing’’ of vascular grafts and the devel-
opment of the neointima. In most human vessels, complete endothelialization
occurs only in the vicinity of the anastomoses with the natural vessels, as cells
grow in from the ends of the connected tissue. To expand the area of vessel that
experiences complete healing—such that the lining resembles that of a natural,
healthy vessel—research is being conducted into seeding the vessel wall with
endothelial cells (Consigny, 2000; Herring, 1983). This can either be done during
the preclotting process, in the operating room, using a small number of cells
taken from a vein that is exposed during the surgical procedure, or preoperatively
using cells from the jugular vein that are cultured to provide a greater volume
for the seeding process. In both cases, the use of autologous (patient specific)
cells is important to eliminate issues of rejection.
The geometry of the vessel also affects neointima formation. The initial in-
teraction between the blood and the graft is the formation of a clot, or thrombus,
on the inner surface of the vessel. The thickness of the implant wall is directly
proportional to the thickness of the thrombus formed on its surface (Park and
Lakes, 1992). As the thrombus must be remodeled to form the mature neointima,
a smaller clot results in faster organization of the neointima. Neointima forma-
tion must be limited, however, to prevent vessel occlusion. This is one of the
issues with small diameter vascular grafts, where the fibrin layer may continue
to grow past 1 mm in thickness (Wilkerson and Zalina, 1994). Finally, it is
important to ensure that the neointimal layer that develops maintains its integrity

1184 SELECTION OF MATERIALS FOR BIOMEDICAL APPLICATIONS
and does not change in such a way, either through mineralization or adsorption
of additional organic components, that it no longer promotes the continued pa-
tency of the vessel (Hufnagel, 1983).
Hemostasis
For vessels to adequately transport blood, the blood must remain within the
lumen of the vessel and not leak into the surrounding tissues in any great
amount. The obvious answer to this problem is to employ solid materials for
vascular grafts, nonporous structures that will adequately contain the blood.
However, the development of a neointima within a vessel provides a second
design option, as it will act to seal a porous blood vessel against blood leakage.
However, the time course for neointima formation is not short enough to provide
the immediate seal needed during surgery and postoperatively. Therefore, many
vascular grafts are preclotted in the operating room before they are implanted
by exposing both the inner and outer surfaces to the patient’s blood. This pro-
vides an initial surface that serves as the basis for neointima formation.
Hemolysis
Hemolysis, or the damage and destruction of red blood cells, occurs continually
within the body. The cells are constantly replaced by new erythrocytes produced
by the bone marrow. However, chronic damage to these cells and the release of
their cellular contents into the plasma of the bloodstream can result in anemia,
kidney failure, and other toxic reactions (Hershko, et al., 1998). Hemolysis due
to nonphysiologic mechanisms typically occurs due to high shear stresses. A
shear stress as low as 400 Pa can damage or rupture a red blood cell that comes
in contact with another solid surface (Sallam and Hwang, 1984). Turbulent flow
can also result in sufficient shear stresses to cause hemolysis. Therefore, two
goals of vascular graft design are to minimize the contact between red blood
cells and the graft surfaces and minimize the turbulence that may occur at
branches or divisions of blood vessels. The latter falls within the domain of fluid
mechanics, rather than biomaterial selection.
To minimize the contact between blood cells and graft walls that may lead
to hemolysis, it is desirable to form a natural tissue layer between the two. Graft
materials with high surface tensions tend to initially attract platelets and fibrin
molecules, which aggregate and form the natural boundary that is then remod-
eled to become the neointima. The elements of the tissue layer also exhibit a
slight negative electrical charge (Collins, 1983), which may act to repel the
negatively charged red blood cells, therefore additionally reducing the contact
between the cells and the vessel wall.
Coagulation
Blood clotting, or coagulation, is a necessary physiologic process that allows
the body to heal and maintain its blood pressure through hemostasis. However,
the formation of stationary clots within blood vessels (thromboses) and the
movement of those clots with the blood flow (emboli) can result in vascular
blockage, tissue damage, and even death. Therefore, it is important to select
materials for graft use that will minimize the initiation of the clotting cascade.
The effect of a material on blood clotting is not easy to assess, particularly
as it is difficult to separate the effect of the material from the natural physiologic

3 BLOOD-CONTACTING BIOMATERIALS: VASCULAR PROSTHESES 1185
process of coagulation. Blood will also behave slightly differently in vivo than
it will in vitro and, like other tissues, can differ between individuals. Therefore,
an in vitro coagulation test with blood from a dog may not be fully indicative
of how that material will behave when implanted into a human. Standards have
been developed, however, for coagulation tests (Bruck, 1980), and these progress
from static, in vitro tests to dynamic, in vivo tests.
Surface tension, surface charge, and surface roughness are properties of a
material that will affect the rate and amount of coagulation that takes place when
it is in contact with blood. Polymeric materials tend to adsorb a mixture of
proteins to their surfaces in the first 30–60 s of contact with physiologic fluid
(Baier, 1975), the composition of which depends on the polar or nonpolar nature
of the polymer (Herring, 1983). Adsorbtion and activation of key molecules from
blood, including Hageman factor, factor XI, and others, will trigger the clotting
cascade (Forbes, 1993). Rougher surfaces, including crimped grafts, have been
shown to increase the rate of coagulation that occurs when in contact with blood
(Collins, 1983). This is probably due to the larger surface area that can come in
contact with the blood. A rough surface may be desirable, however, in order to
promote preclotting on the surface of a porous graft. In the case of crimping,
the process also prevents kinking of the vessels during surgery or prolonged
implantation, which itself can lead to occlusion. Surface charge can help to
minimize the contact between the graft and the blood elements. The formed
elements of the blood—red and white blood cells and platelets—have been
shown to have a negative surface charge. As a result, a vascular surface with a
slight negative charge, through the presence of either a neointima or a naturally
or artificially induced surface charge, will act to repel the blood cells and plate-
lets away from the vascular wall (Collins, 1983). When platelets do not come
in contact with the vascular wall or foreign bodies, they are less prone to initiate
the clotting cascade.
The natural lining of blood vessels possesses unique properties that cannot
be easily mimicked. It was originally believed that the smooth surface and the
negative surface charge were the properties of an endothelial lining that pre-
vented clot formation. However, studies have shown this to be more complex.
In particular, the presence of endothelial cells allows for the secretion of para-
crine agents that act to break down small thromboses and interfere with clot
formation (Bruck, 1980). Smooth muscle and fibroblasts did not exhibit the same
function, and in some cases precipitated platelet activation. Thus, as was the
case in bone’s resistance to fatigue, the living function of vascular tissue cannot
be fully replaced using current technologies and artificial materials.
To reduce clotting in artificial materials, several approaches have been taken.
Heparin, a negatively charged polysaccharide that is commonly used to prevent
clottting in many clinical applications, has been coated on implants. Using the
same logic, anionic radicals have been included in an artificial material to pro-
duce the negative surface charge that only naturally can occur in polymers.
Taking a different approach, materials with low surface tensions have been pro-
posed, as they are less likely to attract the formed blood elements to the material
surface and initiate a clotting cascade. This last tact is, of course, in contradiction
to the suggested use of high surface tension materials to minimize hemolysis!
This latest dilemma is indicative of many decisions that must be made in
selecting a material for use in biomedical applications. No material—besides

1186 SELECTION OF MATERIALS FOR BIOMEDICAL APPLICATIONS
possibly the original tissue—will perfectly match all of the goals and constraints
of the design. It is generally necessary to weigh the benefits and drawbacks of
each material that makes the short list and to select the one that shows the best
balance. While this is frustrating from a design aspect, it is what spurs material
development and allows for the continuing work in the area of material synthesis
and implant design.
Property Degradation
Once a material is selected that does not seem to have a significant, adverse
effect on the blood itself, it is necessary to evaluate how the properties of the
graft are affected during the expected contact with the physiological environ-
ment. As discussed above for orthopedic implants, polymers can react to the
physiologic environment by either leaching smaller molecules into the surround-
ing tissue or absorbing water or other materials from the tissue. In the case of
vascular grafts, the constant flow of blood past the implant surface will eliminate
the possibility to develop an equilibrium between the molecule concentrations
inside and outside of the implant material. As an example, nylon has been found
to absorb water from the surrounding environment when implanted (Edwards,
1983). The water molecules act as plasticizers, which reduce the cohesion and
bonding between the chains of the polymer. The final result is a reduction in
strength.
Whether property degradation will be a determining factor in material selec-
tion for vascular implants depends largely on the planned time of implantation
for the device. For a graft that will permanently replace a section of blood vessel,
it is extremely important to maintain the tissue properties at an appropriate level.
However, many blood contacting applications involve short-term use—for in-
stance intravenous catheters for administering blood or pharmaceuticals. In these
cases, the length of intended use is typically less than a few days, and the
catheter can be relatively easily replaced if necessary. Therefore, for short-term
implants, the prevention of blood clot formation and blood cell damage, along
with ease of use and cost, become the determining factors in material selection.
3.3 Current Material Selection
Since the 1950s, polymer fabrics have been the primary material used for long-
term vascular grafts. Nylon was introduced in 1955, but was withdrawn from
use after the occurrence of aneurisms within the grafts indicated a loss of me-
chanical integrity in vivo (Edwards, 1983). Teflon (PTFE, Gore-Tex) and poly-
ethylene terephthalate (PET, Dacron) have been shown to have acceptable
amounts of property degradation when implanted for 20 years or more (Snyder,
1983). Table 5 shows results on the loss of strength in various polymers that
were implanted for 100 days. The Dacron showed an initial drop in strength that
then stablized at an acceptable level.
Typical vascular grafts are constructed of a woven or knitted fabric and are
crimped, to both prevent kinking and to allow for longitudinal expansion (Fig.
8). The response of the graft in vivo will depend not only on the constituent
material selected, but also on the weave of the fabric and on its processing
(Sawye, et al., 1983). Grafts are typically tested to validate their tensile or burst-
ing strength, and the values obtained for the constructed vessel will differ from
those of the bulk material due to the knitted or woven nature of the fabric.

3 BLOOD-CONTACTING BIOMATERIALS: VASCULAR PROSTHESES 1187
Table 5 Average Changes in Tensile
Properties of Synthetic Grafts Implanted
for 100 Days
Material Loss of Strength (%)
Nylon ⫺81
Orlon
⫺6.9
Dacron
⫺10.1
Teflon
⫹3.2
Source: Data from (Edwards, 1983)
Fig. 8 Left and right: Examples of fabric arterial grafts, illustrating the crimped structure that
prevents kinking in vivo. Center: Extruded PTFE graft lined with pyrolytic carbon.
Pyrolitic or LTI (low-temperature isotropic) carbon has been found to have
excellent anticoagulation properties (Bruck, et al., 1973), without the need for
heparin coating of the material. The original LTI formulation required a solid
substrate and so was more conducive for use on shunts or leaflets of heart valves.
A newer, ultra-low-temperature iostropic carbon (ULTI) can be vapor deposited
onto fabrics as well (Bruck, 1980), allowing for its use in regions that require
flexibility (Fig. 8). ULTI carbon, which can be deposited with a thickness of
less than 1
m, has been shown not to affect the compliance of the underlying
fabric (Sharp, 1983), indicating that it will maintain its flexibility when deposited
on a fabric graft.
For short-term implants, generally in the form of catheters, silicone rubber
has become the standard. Its optical translucence makes it easy to monitor fluid
transfer, it is easy to use, and it is highly biocompatible.
Fabric grafts and silicone tubes, however, are not adequate for the small-vessel
replacement that may be required during reconstructive surgery. As a result,

1188 SELECTION OF MATERIALS FOR BIOMEDICAL APPLICATIONS
tissue engineering techniques are currently being employed to construct small-
diameter vessels from natural materials, such as chitosan (Chupa, et al., 2000).
Research such as this is working to expand the possibilities for material selec-
tion, not only for vascular prostheses but also for all biomedical implants.
4 SPACE-FILLING BIOMATERIALS: BREAST IMPLANTS
Implants designed to fill voids where tissue has either been removed or destroyed
are commonly used in reconstructive surgery. Hard tissue, soft tissue, and com-
bination materials have been utilized to replace or augment cranial defects, the
loss of an ear, nose, or eye due to trauma or disease, and congenital facial
abnormalities, among others. Perhaps the most familiar and infamous space-
filling implant was, and is, the breast implant. Originally designed for recon-
structive surgery following mastectomies, breast implants became increasingly
popular for cosmetic enhancement during the 1970s and 1980s. The question of
whether the implants were the cause of the illnesses and disabilities reported in
the 1990s has never been answered to everyone’s satisfaction, and probably
never will be. It is an example of a biocompatibility issue that, if true, went
unnoticed for many years. It also serves notice to all biomedical implant de-
signers that because an implant does not indicate any problems after 5 years
does not mean that monitoring of potential complications should stop.
Breast implants were designed with one function in mind—to replace tissue
that had either been removed through surgery or that an individual felt nature
had left lacking. There was no other physiologic role for the implant, which
made its design much simpler. The first breast augmentation utilized injections
of silicone, paraffin wax, or bees wax directly into the tissue surrounding the
breast. This method was banned by the Food and Drug Administration (FDA)
in the 1960s as the injected material was seen to migrate and lose its shape. In
addition, because of the large contacting surface area between the injected ma-
terial and surrounding tissue, adverse tissue reactions were seen (Frisch, 1983).
Since that time, breast implants have utilized confined volumes of materials
(Figure 9).
4.1 Function
Space Filler
As stated above, the single role of a breast implant is to fill up a given volume
of space within the body. However, cosmetic appearance and ‘‘feel’’ have also
dictated much of the development of the implants. The density of the implant
should be similar to or less than the surrounding tissue, so that tissue damage
does not occur due to increased weight. The consistency of the material should
also be somewhat similar to the composite of fatty and connective tissue that it
has replaced. Thus, metals, ceramics, and solid pieces of polymer would be
inappropriate.
4.2 Biocompatibility
Capsule Formation
No artificial material that is implanted into the body will be ignored completely.
The degree of the reaction from the immune system will depend on a number
