King M.R., Mody N.A. Numerical and Statistical Methods for Bioengineering: Applications in MATLAB
Подождите немного. Документ загружается.


elimination for square n × n matrices. MATLAB assesses the structural properties of
A before deciding which algorithm to use for solving A \ b.IfA is a triangular matrix,
then backward or forward substitution is applied directly to obtain x.IfA is singular
or nearly singular, MATLAB issues a warning message so that the result produced
may be interpreted by the user accordingly. The function mldivide executes the
left-division operation that is represented by the \ operator. Type help mldivide
for more information on the backslash operator.
We use the backslash operator to solve Equations (E1)–(E3):
44
A=[2−43;12−3; 3 1 1];
44
b = [1; 4; 12];
44
x = A\b
The output produced is
x=
3
2
1
The backslash operator is capable of handling overdetermined and underdeter-
mined systems as well, e.g. systems described by a rectangular m × n matrix A.
2.7 Ill-conditioned problems and the condition number
One may encounter a system of equations whose solution is considerably sensitive to
slight variations in the values of any of the coefficients or constants. Such systems exhibit
large fluctuations in the solutions despite comparatively small changes in the parametric
values. Any solution of these equations is usually not reliable or useful since it is often far
removed from the true solution. Such systems of equations are said to be ill-conditioned.
Often coefficient values or constants of equations are derived from results of experi-
ments or from estimations using empirical equations and are only approximations to the
true values. Even if the problem at hand contains an approximate set of equations, we
wish the solution to be as close as possible to the true solution corresponding to the set of
equations having the correct coefficients and constants. Equations that show little
variability in the solution despite modest changes in the parameters are said to be
well-conditioned. Even in cases where the coefficients and constants of the equations
are known exactly, when performing numerical computations finite precision will
introduce round-off errors that will perturb the true value of the parameters. Such
small but finite perturbations will produce little change in the solutions of well-
conditioned problems, but can dramatically alter the result when solving ill-conditioned
systems of equations. Therefore, when working with ill-conditioned problems, or with
problems involving numbers of disparate magnitudes, it is always recommended to
carry out numerical computations in double precision, since powerful computational
resources – high speeds and large memory – are easily available.
Ill-conditioning is an inherent property of the system of equations and cannot be
changed by using a different solution algorithm or by using superior solution
techniques such as scaling and pivoting. The value of the determinant of the
coefficient matrix indicates the conditioning of a system of linear equations. A set
of equations with a coefficient matrix determinant close to zero will always be ill-
conditioned. The effect of the conditioning of the coefficient matrix on the solution
outcome is demonstrated below.
97
2.7 Ill-conditioned problems and the condition number

Example 2.6
Let’s consider a 2 × 2 well-conditioned coefficient matrix and 2 × 1 constants vector:
44
A=[23;41];
44
b = [5; 5];
44
x = A\b
x=
1
1
Now let’s change the constants vector to
44
bnew = [5.001; 5];
44
xnew = A\bnew
xnew =
0.9999
1.0004
The relative change in the first element of b = 0.001/5 = 0.0002. The relative change in the solutions is
calculated as 0.0001 and 0.0004 for both unknowns, which is of the same order of magnitude as the slight
perturbation made to the constants vector. This is representative of the behavior of well-conditioned
coefficient matrices.
Now let’s study the behavior of an ill-conditioned coefficient matrix:
44
A = [4 2; 2.001 1];
44
b = [6; 3.001];
44
x = A\b
x=
1.0000
1.0000
The answer is correct. Let’s introduce a slight modification in the first element of b:
44
bnew = [6.001; 3.001];
44
xnew = A\bnew
xnew =
0.5000
2.0005
A relative perturbation of 0.001/6 = 0.000167 (0.0167%) in only one of the elements of b resulted in a
relative change of 0.5 (50%) and 1.0005 (100%) in the magnitudes of the two variables. The changes
observed in the resulting solution are approximately 3000–6000 times the original change made in the first
element of b!
Next, we calculate how well xnew satisfies the original system of equations (b = [6; 3.001]). To
determine this, we calculate the residual (see Section 2.9 for a discussion on “residuals”), which is
defined as krk¼kb Axk. The residual is an indicator of how well the left-hand side equates to the
right-hand side of the equations, or, in other words, how well the solution x satisfies the set of equations:
4
4
r=b– A*xnew
r=
−0.0010
0
The solution
to the perturbed set of equations satisfies the original system of equations well, though not
exactly. Clearly, working with ill-conditioned equations is most troublesome since numerous solutions of
a range of magnitudes may satisfy the equation within tolerable error, yet the true solution may remain
elusive.
98
Systems of linear equations

In ord er to understand the origin of ill-conditioning, we ask the following question:
how is the magnitude of the slight perturbations in the constants in b or the
coefficients in A related to the resulting change in the solution of the equations?
Consider first small variations occurring in the individ ual elements of b only.
Consider the original equation Ax = b. Let Δb be a small change made to b, such
that the solution changes by Δx. The new system of equations is given by
Axþ ΔxðÞ¼b þ Δb:
Subtracting off Ax = b, we obtain
AΔx ¼ Δb
or
Δx ¼ A
1
Δb:
Taking the norms of both sides yields
kΔxk¼kA
1
ΔbkkA
1
kkΔbk: (2:21)
Now we take the norm of the original equation Ax ¼ b to obtain
kbk¼kAxkkAkkxk
or
1
kAkkxk
1
kbk
: (2:22)
Mutliplying Equation (2.21) with (2.22), we obtain
kΔxk
kAkkxk
kA
1
kkΔbk
kbk
:
Rearranging,
kΔxk
kxk
kAkkA
1
k
kΔbk
kbk
: (2:23)
Here, we have a relationship between the relative change in the solution and the
relative change in b. Note that kΔxk=kxk is dependent on two factors:
(1) kΔbk=kbk, and
(2) kAkkA
1
k.
The latter term defines the condition number, and is denoted as cond(A). The condition
number is a measure of the conditioning of a system of linear equations. The minimum
value that a condition number can have is one. This can be proven as follows:
kA A
1
k¼kIk¼1:
Now,
kA A
1
kkAkkA
1
k¼condðAÞ:
Thus,
condðAÞ1:
The condition number can take on values from one to infinity. A well-conditioned
matrix will have low condition numbers close to one, while large condition numbers
99
2.7 Ill-conditioned problems and the condition number
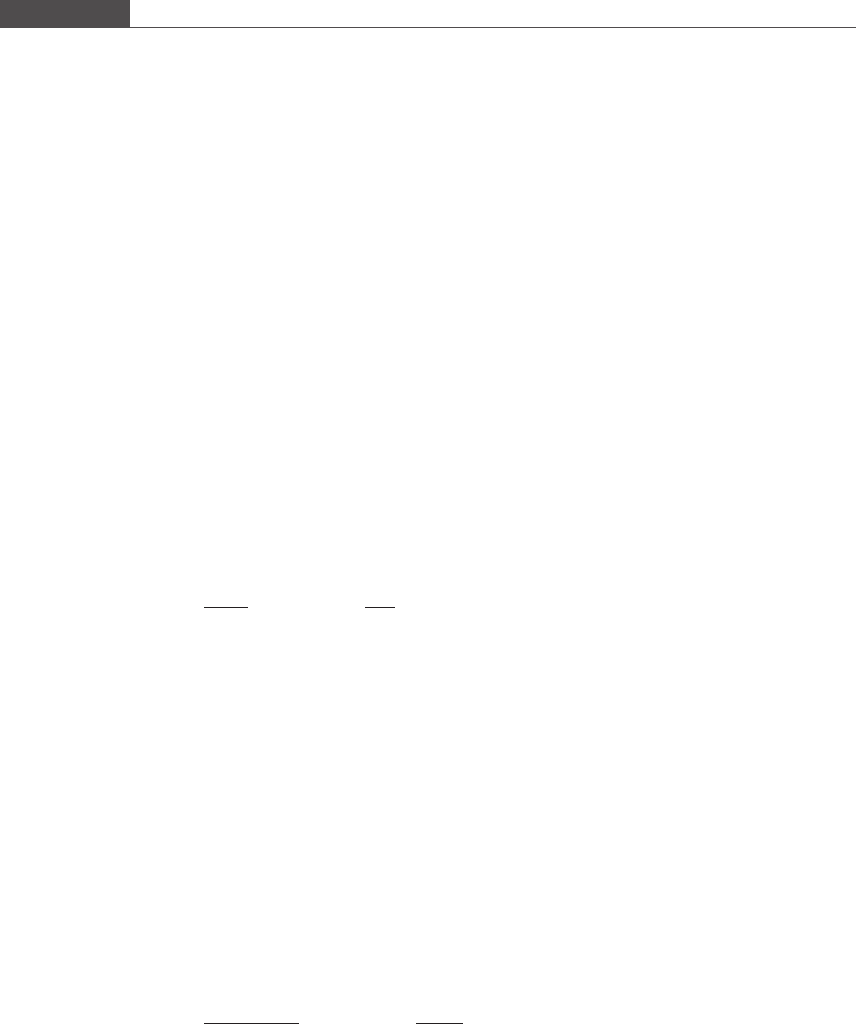
indicate an ill-conditioned problem. The error in the solution kΔxk=kxk will be of the
order of kΔbk=kbk, only if cond(A)isofO(1). Knowledge of the condition number of a
system enables one to interpret the accuracy of the solution to a perturbed system of
equations. The exact value of the condition number depends on the type of norm used
(p =1,2,or∞), but ill-conditioning will be evident regardless of the type of norm.
A fixed cut-off condition number that defines an ill-conditioned system does not exist
partly because the ability to resolve an ill-conditioned problem is dependent on the
level of precision used to perform the arithmetic operations. It is always recommended
that you calculate the condition number when solving systems of equations.
The goal of the Gaussian elimination method of solution (and LU decomposition
method) is to minimize the residual krk¼kb Axk. When numerical procedures are
carried out, round-off errors creep in and can corrupt the calculated values. Such
errors are carried forward to the final solution. If x + Δx is the numerically
calculated solution to the system of equations Ax = b, and x is the exact solution
to Ax = b, then the residual is given by
r ¼ b AxþΔxðÞ¼AΔx
or
kΔxk¼kA
1
rkkA
1
kkrk: (2:24)
Multiplying Equation (2.22) by Equation (2.24) and rearranging, we arrive at
kΔxk
kxk
kAkkA
1
k
krk
kbk
: (2:25)
The magnitude of error in the solution obtained using the Gaussian elimination
method also depends on the condition number of the coefficient matrix.
Let’s look now at the effect of perturbing the coefficient matrix A.Ifx is the exact
solution to Ax = b, and perturbations of magnitude ΔA change the solution x by an
amount Δx, then our corresponding matrix equation is given by
A þ ΔAðÞx þ ΔxðÞ¼b:
After some rearrangement,
Δx ¼A
1
ΔAxþ ΔxðÞ:
Taking norms,
kΔxkkA
1
kkΔAkk x þ ΔxðÞk:
Multiplying and dividing the right-hand side by kAk, we obtain
kΔxk
k x þ ΔxðÞk
kA
1
kkAk
kΔAk
kAk
: (2:26)
Again we observe that the magnitude of error in the solution that results from
modification to the elements of A is dependent on the condition number. Note
that the left-hand side denominator is the solution to the perturbed set of equations.
Using MATLAB
MATLAB provides the cond function to calculate the condition number of a matrix
using either the 1, 2, or ∞ norms (the default norm that MATLAB uses is p = 2).
100
Systems of linear equations

Let’s use this function to calculate the condition numbers of the coefficient matrices
in Example 2.6:
44
A=[23;41];
44
C1 = cond(A,1)
C1 =
3
44
Cinf = cond(A,inf)
Cinf =
3.0000
44
A = [4 2; 2.001 1];
44
C1 = cond(A,1)
C1 =
1.8003e+004
44
Cinf = cond(A,inf)
Cinf =
1.8003e+004
The calcul ation of the condition number involv es determining the inverse of the
matrix which requires O(n
3
) floating-point operations or flops. Calculating A
−1
is an
inefficient way to determine cond(A), and computers use specially devised O(n
2
)
algorithms to estimate the condition number.
2.8 Linear regression
Experimental observations from laboratories, pilot-plant studies, mathematical modeling
of natural phenomena, and outdoor field studies provide us with a host of biomedical,
biological, and engineering data, which often specify a variable of interest that exhibits
certain trends in behavior as a result of its dependency on another variable. Finding a “best-
fit” function that reasonably fits the given data is valuable. In some cases, an established
theory provides a functional relationship between the dependent and independent varia-
bles. In other cases, when a guiding theory is lacking, the data itself may suggest a functional
form for the relationship. Sometimes, we may wish to use simple or uncomplicated
functions, such as a linear or quadratic equation to represent the data, as advised by
Ockham’s (Occam’s) razor – a heuristic principle posited byWilliamOckham, a fourteenth
century logician. Occam’s razor dictates the use of economy and simplicity when develop-
ing scientific theories and explanations and the elimination of superfluous or “non-key”
assumptions that make little difference to the characteristics of the observed phenomena.
The technique used to derive an empirical relationship between two or more varia-
bles based on data obtained from experimental observations is called regression.The
purpose of any regression analysis is to determine the parameters or coefficients of the
function that best relate the dependent variable to one or more independent variables. A
regression of the data to a “best-fit” curve produces a function that only approximates
the given data to within a certain degree, but does not usually predict the exact values
specified by the data. Experimentally obtained data contain not only inherent errors
due to inaccuracies in measurements and underlying assumptions, but also inherent
variability or randomness that is characteristic of all natural processes (see Section 3.1
for a discussion on “variability in nature and biology”). Hence, it is desirable to obtain
an approximate function that agrees with the data in an overall fashion rather than
forcing the function to pass through every experiment ally derived data point since the
exact magnitude of error in the data is usually unknown. The parameters of the fitted
curve are adjusted according to an error minimization criterion to produce a best-fit.
101
2.8 Linear regression
Linear regression models involve a strict linear dependency of the functional form
on the parameters to be determined. For example, if y depends on the value of x, the
independent variable, then a linear regression problem is set up as
y ¼ β
1
f
1
xðÞþβ
2
f
2
xð Þþþβ
n
f
n
xðÞ;
where f can be any function of x,andβ
1
, β
2
, ..., β
n
are constants of the equation that
are to be determined using a suitable regression technique. Note that the ind ividual
functions f
i
(x)(1≤ i ≤ n) may depend nonlinearly on x, but the unknown parameter
β
i
must be separable from the functional form specified by f
i
(x). The linear least-
squares method of regression is a popul ar curve-fitting method used to approximate a
line or a curve to the given data. The functional relationship of the dependent
variable on one or more independent variables can be linear, exponential, quadratic,
cubic, or any other nonlinear dependency.
In any linear regression problem, the number of linear equations m is greater than
the number of coefficients n whose values we seek. As a result, the problem most
often constitutes an overdetermined system of linear equations, i.e. a set of equations
whose coefficient matrix has a rank equal to the number of columns n, but unequal
to the rank of the augmented matrix, which is equal to n + 1, resulting in incon-
sistency of the equation set and therefore the lack of an exact solution. Given a
functional form that relates the variables under consideration, the principle of the
least-squares method is to minimize the error or “scatter” of the data points about
the fitted curve. Before proceeding, let’s consider a few examples of biomedical
problems that can be solved using linear regression techniques.
Box 2.2A Using hemoglobin as a blood substitute: hemoglobin–oxygen binding
Hemoglobin (Hb) is a protein present in red blood cells that is responsible for the transport of oxygen
(O
2
) from lungs to individual tissues throughout the body and removal of carbon dioxide (CO
2
) from the
tissue spaces for transport to the lungs. The hemoglobin molecule is a tetramer and consists of four
subunits, two α chains, and two β chains. Each α or β polypeptide chain contains a single iron atom-
containing heme group that can bind to one O
2
molecule. Thus, a hemoglobin molecule can bind up to
four O
2
molecules. The subunits work cooperatively with each other (allosteric binding), such that the
binding of one O
2
molecule to one of the four subunits produces a conformational change within the
protein that makes O
2
binding to the other subunits more favorable. The binding equation between
hemoglobin and oxygen is as follows:
HbðO
2
Þ
n
$ Hb þ nO
2
;
where n =1, ...,4.
The exchange of O
2
and CO
2
gases between the lungs and tissue spaces via the blood occurs due to
prevailing differences in the partial pressures of pO
2
and pCO
2
, respectively. The atmospheric air
contains 21% O
2
. The O
2
in inspired air exerts a partial pressure of 158 mm Hg (millimeters of mercury),
which then reduces to 100 mm Hg when the inspired air mixes with the alveolar air, which is rich in CO
2
.
Venous blood that contacts the alveoli contains O
2
at a partial pressure of 40 mm Hg. The large
difference in partial pressure drives diffusion of O
2
across the alveolar membranes into blood. Most of
the oxygen in blood enters into the red blood cells and binds to hemoglobin molecules to form
oxyhemoglobin. The oxygenated blood travels to various parts of the body and releases oxygen from
oxyhemoglobin. The partial pressure of oxygen in the tissue spaces depends on the activity level of the
tissues and is lower in more active tissues. The high levels of CO
2
in the surrounding tissue drive entry
of CO
2
into blood and subsequent reactions with water to produce bicarbonates (HCO
3
−
). Some of the
bicarbonates enter the red blood cells and bind to hemoglobin to form carbaminohemoglobin. At the
lungs, the oxygenation of blood is responsible for the transformation of carbaminohemoglobin to
102
Systems of linear equations
oxyhemoglobin or the dissociation of CO
2
from hemoglobin, and conversion of bicarbonates into CO
2
and H
2
O, resulting in CO
2
leaving the blood and entering into the lungs.
The Hill equation describes a mathematical relationship between the extent of oxygen saturation of
hemoglobin and the partial pressure of O
2
in blood. This equation is derived from the application of the
law of mass action to the state of chemical equilibrium of the reaction
HbðO
2
Þ
n
$ Hb þ nO
2
;
S ¼
HbðO
2
Þ
n
HbðO
2
Þ
n
þ Hb½
¼
pO
2
ðÞ
n
P
n
50
þ pO
2
ðÞ
n
: (2:27)
Equation (2.27) is the Hill equation, and accounts for the observed cooperative binding that
produces the characteristic sigmoidal shape of the hemoglobin–oxygen dissociation curve.
The partial pressure of oxygen that results in occupancy of half of the oxygen binding sites of
hemoglobin is denoted by P
50
.
Biochemical engineers at several universities have explored the use of polymerized bovine hemo-
globin as a possible blood substitute (Levy et al., 2002). Monomeric hemoglobin is small enough to
leak out of capillary pores; however, by chemically attaching several hemoglobin units together, the end
product is large enough to be retained within the circulatory system. Data collected for tetrameric bovine
hemoglobin binding to oxygen are given in Table 2.1.
We first examine the shape of the oxygen dissociation curve by plotting the data in Figure 2.5.
We wish to determine the best-fit values of P
50
and n in the Hill equation for this data set and
compare with values measured for human hemoglobin. Equation (2.27) is nonlinear but can be
converted to linear form by first rearranging to
Figure 2.5
A plot of the fractional saturation of hemoglobin S as a function of the partial pressure of oxygen in plasma.
0 20 40 60 80 100 120
0
0.2
0.4
0.6
0.8
1
pO
2
(mm Hg)
S
Hemoglobin−oxygen dissociation curve
Table 2.1. Fractional saturation of hemoglobin as a function of partial pressure of
oxygen in blood
pO
2
(mm Hg)
10 20 30 40 50 60 70 80 90 100 110 120
S 0.18 0.40 0.65 0.80 0.87 0.92 0.94 0.95 0.95 0.96 0.96 0.97
103
2.8 Linear regression

S
1 S
¼
ðpO
2
Þ
n
P
n
50
and then taking the logarithm of both sides to obtain
ln
S
1 S
¼ n ln pO
2
ðÞn ln P
50
: (2:28)
Plotting lnðS=1 SÞ as a function of ln pO
2
ðÞproduces a line with slope n and intercept n ln P
50
.
Equation (2.28) is the functional form for the dependency of oxygen saturation of hemoglobin as a
function of the oxygen par tial pressure, and is linear in the regression parameters.
We will be in a position to evaluate the data once we learn how to fit the line described by Equation
(2.28) to the data presented above.
Box 2.3A Time course of drug concentration in the body
Drugs can be delivered into the body by many different routes, a few examples of which are ingestion of
drug and subsequent absorption into blood via the gastrointestinal tract, inhalation, topical application
on the skin, subcutaneous penetration, and intravenous injection of drug directly into the circulatory
system. The rate at which the drug enters into the vascular system, the extravascular space, and/or
subsequently into cells, depends on a variety of factors, namely the mode of introduction of the drug into
the body, resistance to mass transfer based on the chemical properties of the drug and iner t material
(such as capsules) associated with the drug, and the barriers offered by the body’s interior. Once the
drug enters into the blood, it is transported to all parts of the body and may enter into the tissue spaces
surrounding the capillaries. The amount of drug that succeeds in entering into the extravascular space
depends on the resistance faced by the drug while diffusing across the capillary membranes. While the
active form of the drug distributes throughout the body, it is simultaneously removed from the body or
chemically decomposed and rendered inactive either by liver metabolism, removal by kidney filtration,
excretion of feces, exhalation of air, and/or perspiration. Thus, a number of processes govern the time-
dependent concentrations of the drug in blood and various parts of the body. The study of the processes
by which drugs are absorbed in, distributed in, metabolized by, and eliminated from the body is called
pharmacokinetics, and is a branch of the field of pharmacology. In other words, pharmacokinetics is
the study of how the body affects the transport and efficacy of the drug. A parallel field of study called
pharmacodynamics, which is another branch of pharmacology, deals with the time course of action
and effect of drugs on the body.
Pharmacokinetic studies of drug distribution and elimination aim at developing mathematical
models that predict concentration profiles of a particular drug as a function of time. A simplified
model of the time-dependent concentration of a drug that is injected all at once (bolus injection) and is
cleared from the blood by one or more elimination processes is given by
Ct
ðÞ
¼ C
0
e
kt
; (2:29)
where C
0
is the initial well-mixed blood plasma concentration of the drug immediately following
intravenous injection and is calculated as C
0
¼ D=V
app
, where D is the dosage and V
app
is the apparent
distribution volume. Note that k is the elimination rate constant with units of time
−1
and is equal to the
volumetric flowrate of plasma that is completely cleared of drug, per unit of the distribution volume of
the drug in the body.
Voriconazole is a drug manufactured by Pfizer used to treat invasive fungal infections generally
observed in patients with compromised immune systems. The use of this drug to treat fungal
endophthalmitis is currently being explored. Shen et al.(2007) studied the clearance of this drug
following injection into the vitreous humor (also known as “the vitreous”) of the eyes of rabbits. The
vitreous is a stagnant clear gel situated between the lens and the retina of the eyeball. The rate of
104
Systems of linear equations

exchange of compounds between the vitreous and the systemic circulation is very slow. The aqueous
humor is contained between the lens and the cornea and is continuously produced and drained from the
eye at an equal rate.
The time-dependent concentration of Voriconazole in the vitreous and aqueous humor is given in
Table 2.2 following intravitreal injection (Shen et al., 2007).
We use Equation (2.29) to model the clearance of the drug from rabbit eyes following a bolus
injection of the drug. If we take the logarithm of both sides of the equation, we get
ln CtðÞ¼ln C
0
kt: (2:30)
Equation (2.30) is linear when ln C(t) is plotted against t, and has slope −k and intercept equal to
ln C
0
.
We want to determine the following:
(a) the initial concentration C
0
,
(b) the elimination rate constant k,
(c) the half life of the drug, and
(d) the drug clearance rate from the vitreous of the eye relative to the clearance rate of drug from the
aqueous fluid surrounding the eye.
Table 2.2. Time-dependent concentrations of a drug called Voriconazole
in the vitreous and aqueous humor of rabbit eyes following intravitreal
bolus injection of the drug
Time, t (hr)
Vitreous concentration,
C
v
(t)(μg/ml)
Aqueous concentration,
C
a
(t)(μg/ml)
1 18.912 ± 2.058 0.240 ± 0.051
2 13.702 ± 1.519 0.187 ± 0.066
4 7.406 ± 1.783 0.127 ± 0.008
8 2.351 ± 0.680 0.000 ± 0.000
16 0.292 ± 0.090 0.000 ± 0.000
24 0.000 ± 0.000 0.000 ± 0.000
48 0.000 ± 0.000 0.000 ± 0.000
Box 2.4A Platelet flow rheology
A computational three-dimensional numerical model called “multiparticle adhesive dynamics” (MAD)
uses state-of-the-art fluid mechanical theory to predict the nature of flow of blood cells (e.g. white blood
cells and platelets) in a blood vessel (King and Hammer, 2001). White blood cells play an indispen-
sable role in the inflammatory response and the immune system, and one of their main roles is to search
for and destroy harmful foreign molecules that have entered the body. Platelets patrol the blood vessel
wall for lesions. They aggregate to form clots to curtail blood loss and promote healing at injured sites.
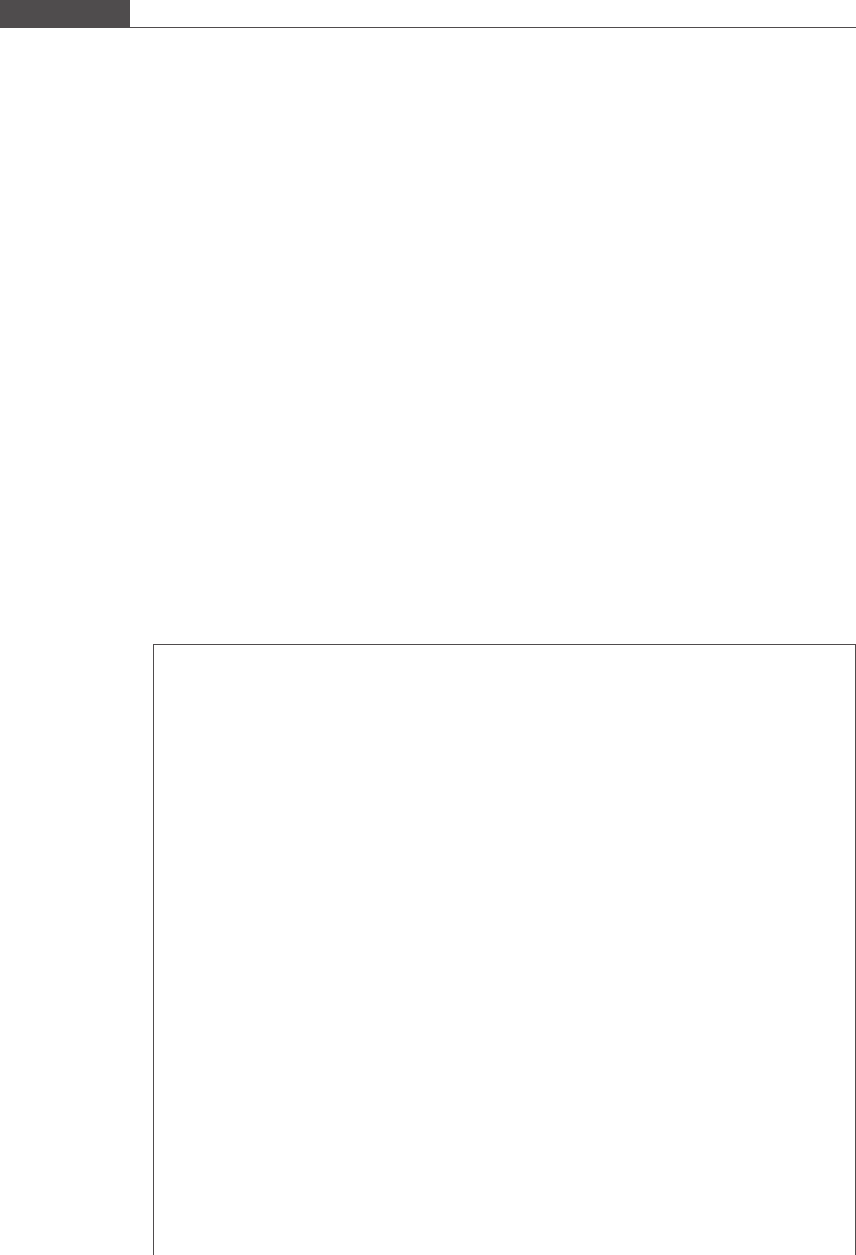
The flow behavior of a platelet-shaped cell (flattened ellipsoid) is dramatically different from that of a
spherical-shaped cell (Mody and King, 2005). It was determined from MAD studies that platelets flowing
in linear shear flow demonstrate three distinct regimes of flow near a plane wall. Platelets of dimension
2 × 2 × 0.5 μm
3
(see Figure 2.6) are observed to rotate periodically while moving in the direction of flow
at flow distances greater than 0.75 μm from the wall. Platelets at these heights also contact the surface at
regular intervals. When the platelet centroid height is less than 0.75 μm, the platelet cell simply glides or
cruises over the surface in the direction of flow without rotating, and does not contact the wall.
105
2.8 Linear regression
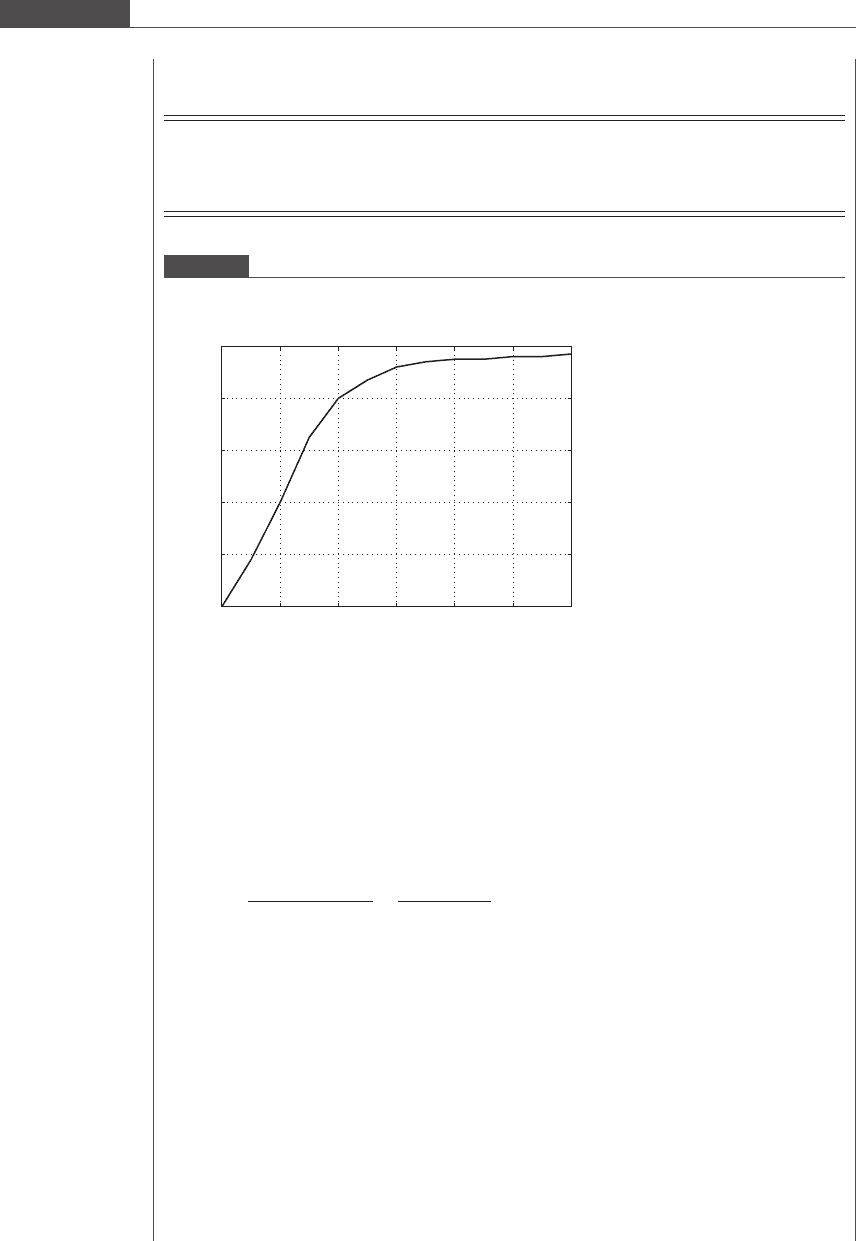
If a platelet flowing near a wall is subjected to linear shear flow, the relevant flow geometry is
depicted in Figure 2.7.
A platelet-shaped cell dramatically changes its flow behavior from a non-tumbling, non-wall-
contacting regime to a tumbling, wall-contacting flow regime under specific conditions of initial cell
orientation and distance of cell from the vessel wall (Mody and King, 2005). The starting orientation of a
platelet that forces a qualitative change in motion depends on the platelet proximity to the wall. The
initial platelet orientations α that produce a regime shift are given in Table 2.3 as a function of the platelet
height H from the surface and plotted in Figure 2.8.
If we wish to relate the initial orientation angle of the platelet α that produces a regime change with
initial platelet height H using a quadratic function, we require only three equations to determine the three
unknown parameters. The data in Table 2.3 contain five sets of (H, α) data points. Linear regression must
be employed to obtain a quadratic function that relates the dependency of α on H as prescribed by these
five data points.
Figure 2.6
Three-dimensional view of a simulated platelet-shaped cell of dimension 2 × 2 × 0.5 μm
3
.
Figure 2.7
Platelet modeled as an oblate spheroid (flattened ellipsoid) that is flowing (translating and rotating) in linear shear
flow over an infinite planar surface.
Linear shear flow
Platelet major axis
H
x
y
z
α
Table 2.3.
Initial tilt α (degrees) 10.5 10.0 8.5 7 4.5
Height H (μm) 0.50 0.55 0.60 0.65 0.70
106
Systems of linear equations
